Intermittent Exercise at Lactate Threshold Induces Lower Acute Stress than Its Continuous Counterpart in Middle-to-Older Aged Men
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Baseline Test
2.3. Experimental Tests
2.4. Measurements
2.5. Blood Analyses
2.6. Statistical Analyses
3. Results
3.1. Physiological Characteristics
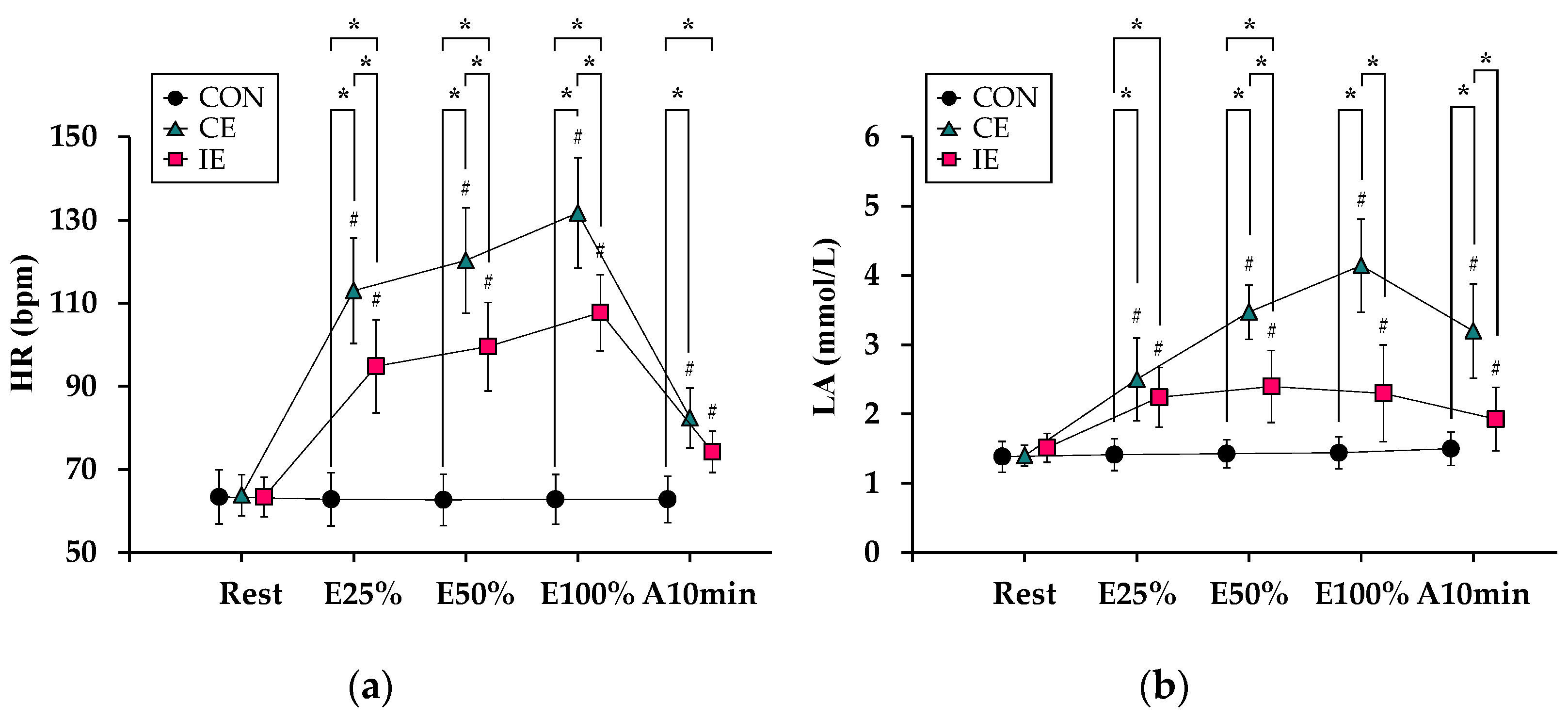
3.2. Comparison of a Degree of Exhaustion (with Conventional Indices)
3.3. Comparison of the Degree of Exhaustion (with Additional Indices Derived from Blood Analyses)
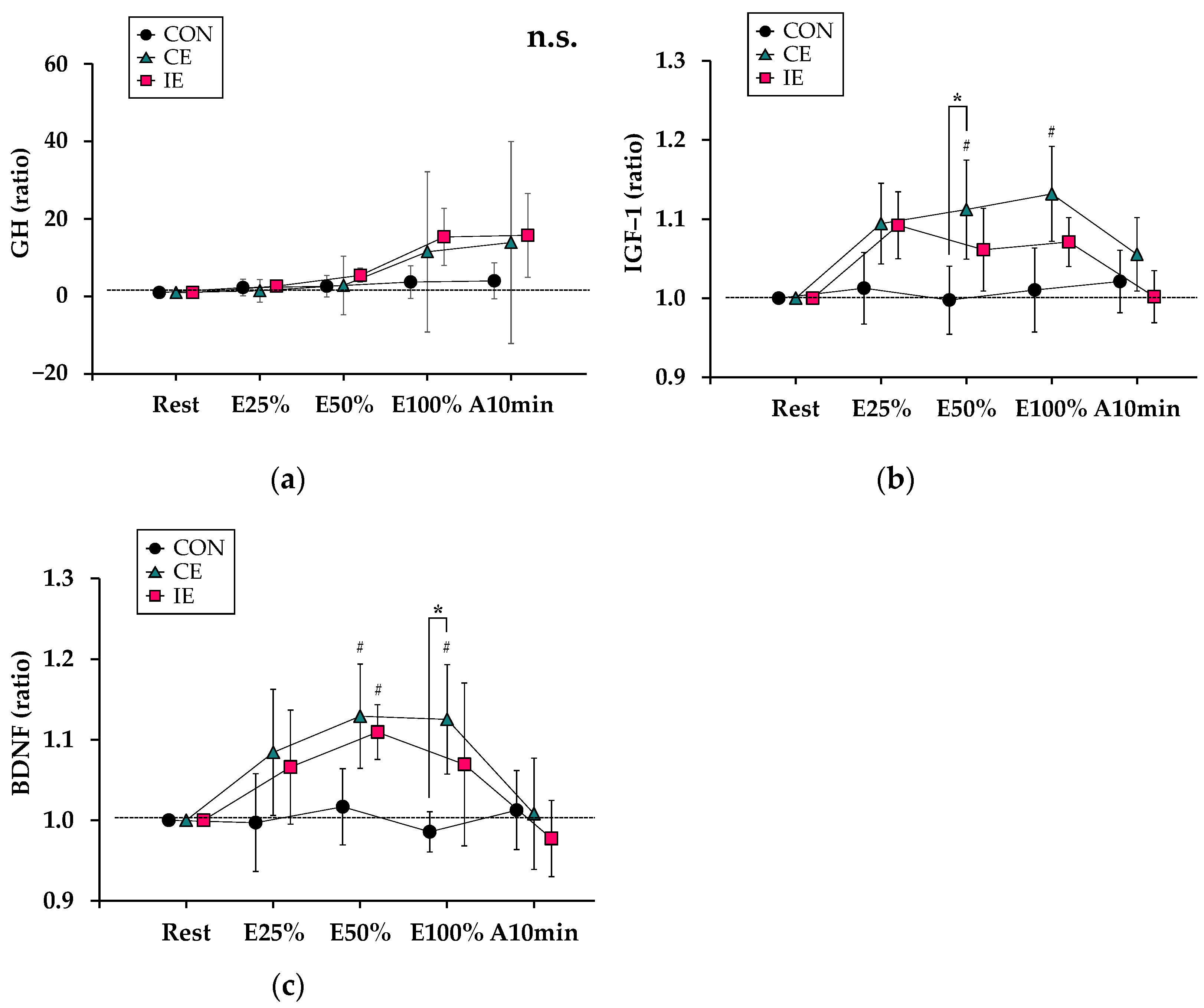
3.4. Comparison of Myotrophic and Neurotrophic Hormones
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Okely, A.D.; Kontsevaya, A.; Ng, J.; Abdeta, C. 2020 WHO guidelines on physical activity and sedentary behavior. Sports Med. Health Sci. 2021, 3, 115–118. [Google Scholar] [CrossRef]
- Janssen, I.; Heymsfield, S.B.; Ross, R. Low Relative Skeletal Muscle Mass (Sarcopenia) in Older Persons Is Associated with Functional Impairment and Physical Disability. J. Am. Geriatr. Soc. 2002, 50, 889–896. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.Y.; Tung, H.H.; Liu, C.Y.; Chen, L.K. Physical Activity and Sarcopenia in the Geriatric Population: A Systematic Review. J. Am. Med. Dir. Assoc. 2018, 19, 378–383. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.-Y.; Huang, K.-S.; Chen, K.-M.; Chou, C.-P.; Tu, Y.-K. Exercise, Nutrition, and Combined Exercise and Nutrition in Older Adults with Sarcopenia: A Systematic Review and Network Meta-analysis. Maturitas 2021, 145, 38–48. [Google Scholar] [CrossRef] [PubMed]
- Alty, J.; Farrow, M.; Lawler, K. Exercise and dementia prevention. Pract. Neurol. 2020, 20, 234–240. [Google Scholar] [CrossRef]
- US Department of Health and Human Services. Physical Activity Guidelines for Americans, 2nd ed.; US Department of Health and Human Services: Wasington, DC, USA, 2018.
- Faude, O.; Kindermann, W.; Meyer, T. Lactate threshold concepts: How valid are they? Sports Med. 2009, 39, 469–490. [Google Scholar] [CrossRef]
- American College of Sports Medicine. ACSM’s Guidelines for Exercise Testing and Prescription, 10th ed.; Lippincott Williams and Wilkins: Philadelphia, PA, USA, 2017; ISBN 149633907X. [Google Scholar]
- Michishita, R.; Tanaka, H.; Kumahara, H.; Ayabe, M.; Tobina, T.; Yoshimura, E.; Matsuda, T.; Higaki, Y.; Kiyonaga, A. Effects of Lifestyle Modifications on Improvement in the Blood Lipid Profiles in Patients with Dyslipidemia. J. Metab. Syndr. 2014, 3, 150. [Google Scholar] [CrossRef]
- Fujita, S.; Rasmussen, B.B.; Cadenas, J.G.; Drummond, M.J.; Glynn, E.L.; Sattler, F.R.; Volpi, E. Aerobic Exercise Overcomes the Age-Related Insulin Resistance of Muscle Protein Metabolism by Improving Endothelial Function and Akt/Mammalian Target of Rapamycin Signaling. Diabetes 2007, 56, 1615–1622. [Google Scholar] [CrossRef] [Green Version]
- Frystyk, J. Exercise and the Growth Hormone–Insulin-Like Growth Factor Axis. Med. Sci. Sports Exerc. 2009, 42, 58–66. [Google Scholar] [CrossRef]
- Vijayakumar, A.; Novosyadlyy, R.; Wu, Y.J.; Yakar, S.; LeRoith, D. Biological effects of growth hormone on carbohydrate and lipid metabolism. Growth Horm. IGF Res. 2010, 20, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Gibney, J.; Healy, M.L.; Sönksen, P.H. The growth hormone/insulin-like growth factor-I axis in exercise and sport. Endocr. Rev. 2007, 28, 603–624. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Numakawa, T.; Suzuki, S.; Kumamaru, E.; Adachi, N.; Richards, M.; Kunugi, H. BDNF function and intracellular signaling in neurons. Histol. Histopathol. 2010, 25, 237–258. [Google Scholar] [CrossRef] [PubMed]
- Dinoff, A.; Herrmann, N.; Swardfager, W.; Lanctôt, K.L. The effect of acute exercise on blood concentrations of brain-derived neurotrophic factor in healthy adults: A meta-analysis. Eur. J. Neurosci. 2017, 46, 1635–1646. [Google Scholar] [CrossRef] [PubMed]
- Máderová, D.; Krumpolec, P.; Slobodová, L.; Schön, M.; Tirpáková, V.; Kovaničová, Z.; Klepochová, R.; Vajda, M.; Šutovský, S.; Cvečka, J.; et al. Acute and regular exercise distinctly modulate serum, plasma and skeletal muscle BDNF in the elderly. Neuropeptides 2019, 78, 101961. [Google Scholar] [CrossRef] [PubMed]
- Walsh, J.J.; Scribbans, T.D.; Bentley, R.F.; Mikhail Kellawan, J.; Gurd, B.; Tschakovsky, M.E. Neurotrophic growth factor responses to lower body resistance training in older adults. Appl. Physiol. Nutr. Metab. 2015, 41, 315–323. [Google Scholar] [CrossRef]
- Beneke, R.; Leithäuser, R.M.; Ochentel, O. Blood lactate diagnostics in exercise testing and training. Int. J. Sports Physiol. Perform. 2011, 6, 8–24. [Google Scholar] [CrossRef] [Green Version]
- Kraemer, W.J.; Noble, B.J.; Clark, M.J.; Culver, B.W. Physiologic responses to heavy-resistance exercise with very short rest periods. Int. J. Sports Med. 1987, 8, 247–252. [Google Scholar] [CrossRef]
- Kraemer, W.J.; Gordon, S.E.; Fleck, S.J.; Marchitelli, L.J.; Melloo, R.; Dziados, J.E.; Friedl, K.; Harman, E.; Maresh, C.; Fry, A.C. Endogenous anabolic hormonal and growth factor responses to heavy resistance exercise in males and females. Int. J. Sports Med. 1991, 12, 228–235. [Google Scholar] [CrossRef]
- Kraemer, W.J.; Ratamess, N.A. Hormonal responses and adaptations to resistance exercise and training. Sports Med. 2005, 35, 339–361. [Google Scholar] [CrossRef]
- Davis, J.A.; Storer, T.W.; Caiozzo, V.J. Prediction of normal values for lactate threshold estimated by gas exchange in men and women. Eur. J. Appl. Physiol. Occup. Physiol. 1997, 76, 157–164. [Google Scholar] [CrossRef]
- Beaver, W.L.; Wasserman, K.; Whipp, B.J. Improved detection of lactate threshold during exercise using a log-log transformation. J. Appl. Physiol. 1985, 59, 1936–1940. [Google Scholar] [CrossRef] [PubMed]
- Hatamoto, Y.; Goya, R.; Yamada, Y.; Yoshimura, E.; Nishimura, S.; Higaki, Y.; Tanaka, H. Effect of exercise timing on elevated postprandial glucose levels. J. Appl. Physiol. 2017, 123, 278–284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ikenaga, M.; Yamada, Y.; Kose, Y.; Morimura, K.; Higaki, Y.; Kiyonaga, A.; Tanaka, H. Nakagawa Study Group Effects of a 12-week, short-interval, intermittent, low-intensity, slow-jogging program on skeletal muscle, fat infiltration, and fitness in older adults: Randomized controlled trial. Eur. J. Appl. Physiol. 2017, 117, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Borg, G.A. Psychophysical bases of perceived exertion. Med. Sci. Sports Exerc. 1982, 14, 377–381. [Google Scholar] [CrossRef] [PubMed]
- Kopin, I.J.; Lake, R.C.; Ziegler, M. Plasma levels of norepinephrine. Ann. Intern. Med. 1978, 88, 671–680. [Google Scholar] [CrossRef]
- Isojima, T.; Shimatsu, A.; Yokoya, S.; Chihara, K.; Tanaka, T.; Hizuka, N.; Teramoto, A.; Tatsumi, K.; Tachibana, K.; Katsumata, N.; et al. Standardized centile curves and reference intervals of serum insulin-like growth factor-I (IGF-I) levels in a normal Japanese population using the LMS method. Endocr. J. 2012, 59, 771–780. [Google Scholar] [CrossRef] [Green Version]
- Meeusen, R. Commentaries on viewpoint: Perception of effort during exercise is independent of afferent feedback from skeletal muscles, heart, and lungs. J. Appl. Physiol. 2009, 106, 2063–2066. [Google Scholar] [CrossRef]
- Phillips, E.M.; Schneider, J.C.; Mercer, G.R. Motivating elders to initiate and maintain exercise. Arch. Phys. Med. Rehabil. 2004, 85, 52–57. [Google Scholar] [CrossRef]
- Baron, B.; Dekerle, J.; Robin, S.; Neviere, R.; Dupont, L.; Matran, R.; Vanvelcenaher, J.; Robin, H.; Pelayo, P. Maximal Lactate Steady State Does Not Correspond to a Complete Physiological Steady State. Int. J. Sports Med. 2003, 24, 582–587. [Google Scholar] [CrossRef]
- Mazzeo, R.S.; Marshall, P. Influence of plasma catecholamines on the lactate threshold during graded exercise. J. Appl. Physiol. 1989, 67, 1319–1322. [Google Scholar] [CrossRef]
- Kirschbaum, C.; Hellhammer, D.H. Salivary cortisol in psychoneuroendocrine research: Recent developments and applications. Psychoneuroendocrinology 1994, 19, 313–333. [Google Scholar] [CrossRef]
- Zouhal, H.; Jacob, C.; Delamarche, P.; Gratas-Delamarche, A. Catecholamines and the effects of exercise, training and gender. Sports Med. 2008, 38, 401–423. [Google Scholar] [CrossRef] [PubMed]
- Fisher, J.P.; Young, C.N.; Fadel, P.J. Autonomic adjustments to exercise in humans. Compr. Physiol. 2015, 5, 475–512. [Google Scholar] [CrossRef] [PubMed]
- Copeland, J.L.; Heggie, L. IGF-I and IGFBP-3 during continuous and interval exercise. Int. J. Sports Med. 2008, 29, 182–187. [Google Scholar] [CrossRef]
- Goldspink, G.; Harridge, S.D.R. Growth factors and muscle ageing. Exp. Gerontol. 2004, 39, 1433–1438. [Google Scholar] [CrossRef]
 ) and open arrow (
) and open arrow (  ) indicate the timing of HR measurement and blood sampling and the timing of RPE measurement, respectively.
) indicate the timing of HR measurement and blood sampling and the timing of RPE measurement, respectively.
 ) and open arrow (
) and open arrow (  ) indicate the timing of HR measurement and blood sampling and the timing of RPE measurement, respectively.
) indicate the timing of HR measurement and blood sampling and the timing of RPE measurement, respectively.


| Means ± SD | |
|---|---|
| Age (years) | 59.3 ± 10.4 |
| Height (cm) | 168.5 ± 4.2 |
| Weight (kg) | 68.9 ± 5.1 |
| Body mass index (kg/m2) | 24.3 ± 2.3 |
| Cycling load at LT intensity (kp) | 1.8 ± 0.4 |
| Cycling METs at LT intensity | 6.3 ± 1.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamamoto, T.; Tanoue, Y.; Uehara, Y.; Higaki, Y.; Tanaka, H.; Narazaki, K. Intermittent Exercise at Lactate Threshold Induces Lower Acute Stress than Its Continuous Counterpart in Middle-to-Older Aged Men. Int. J. Environ. Res. Public Health 2022, 19, 7503. https://doi.org/10.3390/ijerph19127503
Yamamoto T, Tanoue Y, Uehara Y, Higaki Y, Tanaka H, Narazaki K. Intermittent Exercise at Lactate Threshold Induces Lower Acute Stress than Its Continuous Counterpart in Middle-to-Older Aged Men. International Journal of Environmental Research and Public Health. 2022; 19(12):7503. https://doi.org/10.3390/ijerph19127503
Chicago/Turabian StyleYamamoto, Taiki, Yukiya Tanoue, Yoshinari Uehara, Yasuki Higaki, Hiroaki Tanaka, and Kenji Narazaki. 2022. "Intermittent Exercise at Lactate Threshold Induces Lower Acute Stress than Its Continuous Counterpart in Middle-to-Older Aged Men" International Journal of Environmental Research and Public Health 19, no. 12: 7503. https://doi.org/10.3390/ijerph19127503
APA StyleYamamoto, T., Tanoue, Y., Uehara, Y., Higaki, Y., Tanaka, H., & Narazaki, K. (2022). Intermittent Exercise at Lactate Threshold Induces Lower Acute Stress than Its Continuous Counterpart in Middle-to-Older Aged Men. International Journal of Environmental Research and Public Health, 19(12), 7503. https://doi.org/10.3390/ijerph19127503








