Seroprevalence Rates against West Nile, Usutu, and Tick-Borne Encephalitis Viruses in Blood-Donors from North-Western Romania
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Ethical Statement
2.3. Serological Analysis
2.3.1. ELISA
2.3.2. Serum-Neutralization Tests
WNV
TBEV
USUV
2.4. Statistical Analysis
3. Results
3.1. Study Group
3.2. ELISA
3.3. SNT
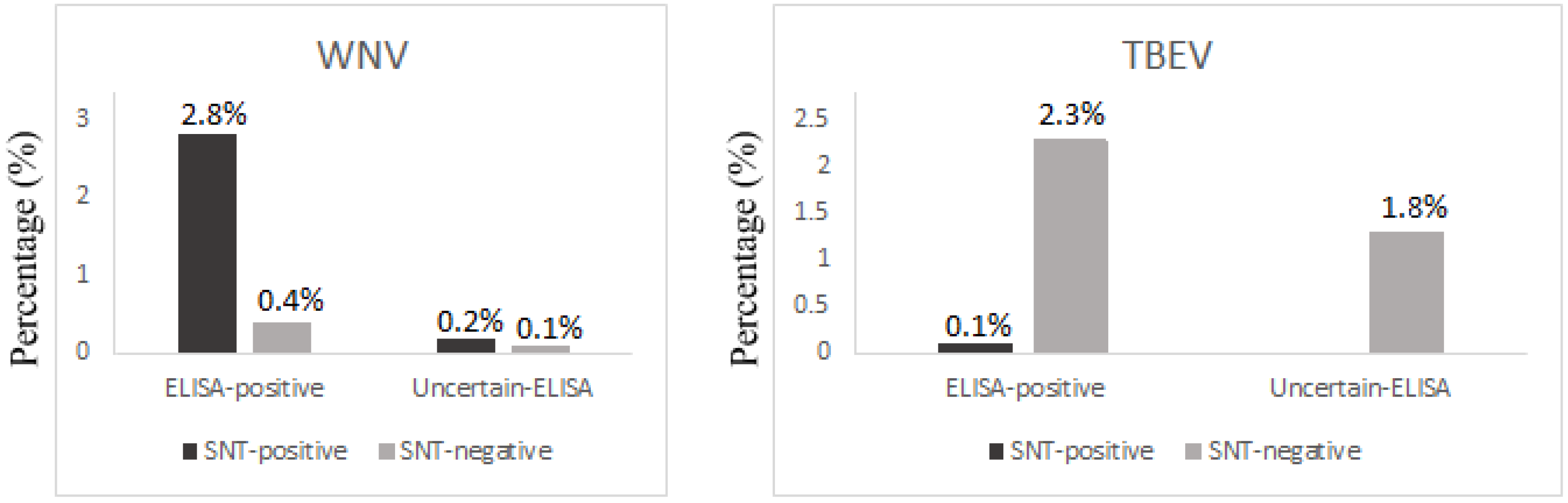
3.4. WNV
3.5. TBEV
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barzon, L. Ongoing and emerging arbovirus threats in Europe. J. Clin. Virol. 2018, 107, 38–47. [Google Scholar] [CrossRef] [PubMed]
- West Nile Virus Infection Outbreak in Humans in Romania; European Center for Disease Prevention and Control: Stockholm, Sweden, 2010; Available online: https://www.ecdc.europa.eu/sites/default/files/media/en/publications/Publications/1104_MIR_West_Nile_outbreak_Romania.pdf (accessed on 25 May 2021).
- Tsai, T.; Popovici, F.; Cernescu, C.; Campbell, G.; Nedelcu, N. West Nile encephalitis epidemic in southeastern Romania. Lancet 1998, 352, 767–771. [Google Scholar] [CrossRef]
- Cernescu, C.; Nedelcu, N.; Tardei, G.; Ruta, S.; Tsai, T.F. Continued transmission of West Nile Virus to humans in southeastern Romania, 1997–1998. J. Infect. Dis. 2000, 181, 710–712. [Google Scholar] [CrossRef] [PubMed]
- Sîrbu, A.; Ceianu, C.S.; Panculescu-Gatej, R.I.; Vazquez, A.; Tenorio, A.; Rebreanu, R.; Niedrig, M.; Nicolescu, G.; Pistol, A. Outbreak of West Nile virus infection in humans, Romania, July to October 2010. Eurosurveillance 2011, 16, 19762. [Google Scholar] [CrossRef]
- Dinu, S.; Cotar, A.I.; Pănculescu-Gătej, I.R.; Fălcuţă, E.; Prioteasa, F.L.; Sîrbu, A.; Oprişan, G.; Bădescu, D.; Reiter, P.; Ceianu, C.S. West Nile virus circulation in south-eastern Romania, 2011 to 2013. Eurosurveillance 2015, 20, 21130. [Google Scholar] [CrossRef] [Green Version]
- Cotar, A.I.; Fălcuță, E.; Dinu, S.; Necula, A.; Bîrluțiu, V.; Ceianu, C.S.; Prioteasa, F.L. West Nile virus lineage 2 in Romania, 2015–2016: Co-circulation and strain replacement. Parasites Vectors 2018, 11, 562. [Google Scholar] [CrossRef] [Green Version]
- Transmission of West Nile Virus, June to December 2018–Table of Cases, 2018 Transmission Season; European Centre for Disease Prevention and Control: Stockholm, Sweden, 2018; Available online: https://www.ecdc.europa.eu/en/publications-data/transmission-west-nile-virus-june-december-2018-table-cases-2018-transmission (accessed on 20 May 2021).
- West Nile Virus Infection. Annual Epidemiological Report for 2019; European Center for Disease Prevention and Control: Stockholm, Sweden, 2021; Available online: https://www.ecdc.europa.eu/sites/default/files/documents/AER-WNV-infection-2019.pdf (accessed on 20 May 2021).
- Epidemiological Update: West Nile Virus Transmission Season in Europe; European Center for Disease Prevention and Control: Stockholm, Sweden, 2021; Available online: https://www.ecdc.europa.eu/en/news-events/epidemiological-update-west-nile-virus-transmission-season-europe-2020 (accessed on 20 May 2021).
- Weekly Updates: 2021 West Nile virus Transmission Season; European Centre for Disease Prevention and Control: Stockholm, Sweden, 2021; Available online: https://www.ecdc.europa.eu/en/west-nile-fever/surveillance-and-disease-data/disease-data-ecdc (accessed on 20 May 2021).
- WNV Infection Surveillance and Control System June–November 2020; National Center for Surveillance and Control of Transmissible Diseases Romania: Bucharest, Romania, 2020; (In Romanian). Available online: https://www.cnscbt.ro/index.php/metodologii/west-nile/1760-metodologie-supraveghere-neuroinfectie-wn-sezon-2020/file (accessed on 20 May 2021).
- Popescu, C.P.; Cotar, A.I.; Dinu, S.; Zaharia, M.; Tardei, G.; Ceausu, E.; Badescu, D.; Ruta, S.; Ceianu, C.S.; Florescu, S.A. Emergence of Toscana virus, Romania, 2017–2018. Emerg. Infect. Dis. 2021, 27, 1482–1485. [Google Scholar] [CrossRef]
- Crivei, L.A. Arboviroze cu Caracter Zoonotic Transmise de Ţânţari în România. Ph.D. Thesis, Iași University of Life Sciences, Iași, Romania, 2021. [Google Scholar]
- Gould, E.; Solomon, T. Pathogenic flaviviruses. Lancet 2008, 371, 500–509. [Google Scholar] [CrossRef]
- Clé, M.; Beck, C.; Salinas, S.; Lecollinet, S.; Gutierrez, S.; Van de Perre, P.; Baldet, T.; Foulongne, V.; Simonin, Y. Usutu virus: A new threat? Epidemiol. Infect. 2019, 147, e232. [Google Scholar] [CrossRef] [Green Version]
- Weissenböck, H.; Kolodziejek, J.; Url, A.; Lussy, H.; Rebel-Bauder, B.; Nowotny, N. Emergence of Usutu virus, an African mosquito-borne flavivirus of the Japanese encephalitis virus group, central Europe. Emerg. Infect. Dis. 2002, 8, 652–656. [Google Scholar] [CrossRef]
- Weissenböck, H.; Bakonyi, T.; Rossi, G.; Mani, P.; Nowotny, N. Usutu virus, Italy, 1996. Emerg. Infect. Dis. 2013, 19, 274–277. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, U.; Ye, J.; Ruan, X.; Wan, S.; Zhu, B.; Cao, S. Usutu virus: An emerging flavivirus in Europe. Viruses 2015, 7, 219–238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vilibic-Cavlek, T.; Petrovic, T.; Savic, V.; Barbic, L.; Tabain, I.; Stevanovic, V.; Barbic, L.; Tabain, I.; Stevanovic, V.; Klobucar, A.; et al. Epidemiology of Usutu virus: The European scenario. Pathogens 2020, 9, 699. [Google Scholar] [CrossRef]
- Pecorari, M.; Longo, G.; Gennari, W.; Grottola, A.; Sabbatini, A.; Tagliazucchi, S.; Savini, G.; Monaco, F.; Simone, M.L.; Lelli, R.; et al. First human case of Usutu virus neuroinvasive infection, Italy, August–September 2009. Eurosurveillance 2009, 14, 19446. [Google Scholar] [CrossRef] [PubMed]
- Santini, M.; Vilibic-Cavlek, T.; Barsic, B.; Barbic, L.; Savic, V.; Stevanovic, V.; Listes, E.; Gennaro, A.D.; Savini, G. First cases of human Usutu virus neuroinvasive infection in Croatia, August–September 2013: Clinical and laboratory features. J. Neurovirol. 2015, 21, 92–97. [Google Scholar] [CrossRef]
- Simonin, Y.; Sillam, O.; Carles, M.J.; Gutierrez, S.; Gil, P.; Constant, O.; Martin, M.F.; Girard, G.; Van de Perre, P.; Salinas, S.; et al. Human Usutu virus infection with atypical neurologic presentation, Montpellier, France, 2016. Emerg. Infect. Dis. 2018, 24, 875–878. [Google Scholar] [CrossRef] [Green Version]
- Pacenti, M.; Sinigaglia, A.; Martello, T.; De Rui, M.E.; Franchin, E.; Pagni, S.; Peta, E.; Riccetti, S.; Milani, A.; Montarsi, F.; et al. Clinical and virological findings in patients with Usutu virus infection, northern Italy, 2018. Eurosurveillance 2019, 24, 1900180. [Google Scholar] [CrossRef]
- Gaibani, P.; Pierro, A.; Alicino, R.; Rossini, G.; Cavrini, F.; Landini, M.P.; Sambri, V. Detection of Usutu-virus-specific IgG in blood donors from northern Italy. Vector Borne Zoonotic Dis. 2012, 12, 431–433. [Google Scholar] [CrossRef]
- Allering, L.; Jöst, H.; Emmerich, P.; Günther, S.; Lattwein, E.; Schmidt, M.; Seifried, E.; Sambri, V.; Hourfar, K.; Schmidt-Chanasit, J. Detection of Usutu virus infection in a healthy blood donor from south-west Germany, 2012. Eurosurveillance 2012, 17, 20341. [Google Scholar] [CrossRef]
- Bakonyi, T.; Jungbauer, C.; Aberle, S.W.; Kolodziejek, J.; Dimmel, K.; Stiasny, K.; Allerberger, F.; Nowotny, N. Usutu virus infections among blood donors, Austria, July and August 2017–raising awareness for diagnostic challenges. Eurosurveillance 2017, 22, 17–00644. [Google Scholar] [CrossRef]
- Zaaijer, H.L.; Slot, E.; Molier, M. Reusken CBEM, Koppelman MHGM. Usutu virus infection in Dutch blood donors. Transfusion 2019, 59, 2931–2937. [Google Scholar] [CrossRef] [PubMed]
- Stefanoff, P.; Pfeffer, M.; Hellenbrand, W.; Rogalska, J.; Rühe, F.; Makówka, A.; Michalik, J.; Wodecka, B.; Rymaszewska, A.; Kiewra, D.; et al. Virus detection in questing ticks is not a sensitive indicator for risk assessment of tick-borne encephalitis in humans. Zoonoses Public Health 2013, 60, 215–226. [Google Scholar] [CrossRef] [PubMed]
- Tick-Borne Encephalitis-Annual Epidemiological Report for 2017; European Centre for Disease Prevention and Control: Stockholm, Sweden, 2019; Available online: https://www.ecdc.europa.eu/en/publications-data/tick-borne-encephalitis-annual-epidemiological-report-2017 (accessed on 14 June 2021).
- Chitimia-Dobler, L.; Hristea, A.; Erber, W.; Vuković-Janković, T. TBE in Romania, 3rd ed.; Dobler, G., Erber, W., Bröker, M., Schmitt, H.J., Eds.; Chapter 12b; The TBE Book; Global Health Press: Singapore, 2020; p. 290. [Google Scholar]
- Ionescu, L.; Alexse, A.; Ceianu, C.; Necsulescu, M.; Popescu, D.; Bicheru, S.; Dumitrescu, G.; Cumpănăsoiu, C.E.; Cumpănăsoiu, C.; Pasat, L.; et al. Investigation methods used for identifying the presence of tick-borne encephalitis virus (TBEV) in vector arthropods. Sci. Pap. Vet. Med. 2009, 42, 288–293. [Google Scholar]
- Ionescu, L.; Necsulescu, M.; Alexse, A.; Ceianu, C.; Popescu, D.; Bicheru, S.; Ordeanu, V.; Nicolescu, G.; Vladimirescu, A.L.; Postoarca, A. Infection with tick-borne encephalitis virus in Romania. Rev. Rom. Med. Vet. 2008, 3, 69–79. [Google Scholar]
- Molnár, G.B.; Persecă, T.; Feder, A.; Păcuraru, D.; Marialaki, E.; Cojan, A. Epidemiological assessment of morbidity and natural foci of TBE-CEE virus infection in Transylvania. Rev. Med. Chir. Soc. Med. Nat. Iasi 2008, 112, 471–477. [Google Scholar]
- Salat, J.; Mihalca, A.D.; Mihaiu, M.; Modrý, D.; Ruzek, D. Tick-borne encephalitis in sheep, Romania. Emerg. Infect. Dis. 2017, 23, 2065–2067. [Google Scholar] [CrossRef] [Green Version]
- Surveillance System of TBEV Infection; National Center for Surveillance and Control of Transmissible Diseases Romania: Bucharest, Romania, 2016; (In Romanian). Available online: https://www.cnscbt.ro/index.php/metodologii/tbe/477-tbe-metodologie/file (accessed on 20 December 2021).
- Steffen, R. Epidemiology of tick-borne encephalitis (TBE) in international travellers to Western/Central Europe and conclusions on vaccination recommendations. J. Travel Med. 2016, 23, 4. [Google Scholar]
- Kalmár, Z.; Briciu, V.; Coroian, M.; Flonta, M.; Rădulescu, A.-L.; Topan, A.; Mihalca, A.D.; Lupșe, M. Seroprevalence of antibodies against Borrelia burgdorferi sensu lato in healthy blood donors in Romania: An update. Parasites Vectors 2021, 14, 596. [Google Scholar] [CrossRef]
- Van Maanen, C.; Terpstra, C. Comparison of a liquid-phase blocking sandwich ELISA and a serum neutralization test to evaluate immunity in potency tests of foot-and-mouth disease vaccines. J. Immunol. Methods 1989, 124, 111–119. [Google Scholar] [CrossRef]
- Haut, M.; Girl, P.; Oswald, B.; Romig, T.; Obiegala, A.; Dobler, G.; Pfeffer, M. The Red Fox (Vulpes vulpes) as sentinel for tick-borne encephalitis virus in endemic and non-endemic areas. Microorganisms 2020, 8, 1817. [Google Scholar] [CrossRef]
- Calisher, C.H.; Karabatsos, N.; Dalrymple, J.M.; Shope, R.E.; Porterfield, J.S.; Westaway, E.G.; Brandt, W.E. Antigenic relationship between flaviviruses as detemrined by cross-neutralization tests with polyclonal antisera. J. Gen. Virol. 1989, 70, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Lustig, Y.; Sofer, D.; Bucris, E.D.; Mendelson, E. Surveillance and diagnosis of West Nile Virus in the face of flavivirus cross-reactivity. Front Microbiol. 2018, 9, 2421. [Google Scholar] [CrossRef] [PubMed]
- Vector Control Practices and Strategies against West Nile virus; European Centre for Disease Prevention and Control: Stockholm, Sweden, 2020; Available online: https://www.ecdc.europa.eu/sites/default/files/documents/Vector-control-practices-and-strategies-against-West-Nile-virus.pdf (accessed on 22 December 2021).
- Historical Data by Year-West Nile virus Seasonal Surveillance; European Centre for Disease Prevention and Control: Stockholm, Sweden, 2019; Available online: https://www.ecdc.europa.eu/en/west-nile-fever/surveillance-and-disease-data/historical (accessed on 22 December 2021).
- Coroian, M.; Petrić, M.; Pistol, A.; Sirbu, A.; Domșa, C.; Mihalca, A.D. Human West Nile Meningo-Encephalitis in a Highly Endemic Country: A Complex Epidemiological Analysis on Biotic and Abiotic Risk Factors. IJERPH 2020, 17, 8250. [Google Scholar] [CrossRef] [PubMed]
- Campbell, G.L.; Ceianu, C.S.; Savage, H.M. Epidemic West Nile encephalitis in Romania: Waiting for history to repeat itself. Ann. N. Y. Acad. Sci. 2001, 951, 94–101. [Google Scholar] [CrossRef]
- Pezzotti, P.; Piovesan, C.; Barzon, L.; Cusinato, R.; Cattai, M.; Pacenti, M.; Piazza, A.; Franchin, E.; Pagni, S.; Bressan, S.; et al. Prevalence of IgM and IgG antibodies to West Nile virus among blood donors in an affected area of north-eastern Italy, summer 2009. Eurosurveillance 2011, 16, 19814. [Google Scholar] [CrossRef] [Green Version]
- Pierro, A.; Gaibani, P.; Manisera, C.; Dirani, G.; Rossini, G.; Cavrini, F.; Ghinelli, F.; Ghinelli, P.; Finarelli, A.C.; Mattivi, A. Seroprevalence of West Nile virus-specific antibodies in a cohort of blood donors in Northeastern Italy. Vector-Borne Zoonotic Dis. 2011, 11, 1605–1607. [Google Scholar] [CrossRef]
- Pierro, A.; Gaibani, P.; Spadafora, C.; Ruggeri, D.; Randi, V.; Parenti, S.; Finarelli, A.C.; Rossini, G.; Landini, M.P.; Sambri, V. Detection of specific antibodies against West Nile and Usutu viruses in healthy blood donors in northern Italy, 2010–2011. Clin. Microbiol. Infect. 2013, 19, 451–453. [Google Scholar] [CrossRef] [Green Version]
- Nagy, A.; Szöllősi, T.; Takács, M.; Magyar, N.; Barabás, É. West Nile virus seroprevalence among blood donors in Hungary. Vector-Borne Zoonotic Dis. 2019, 19, 844–850. [Google Scholar] [CrossRef]
- Hadjichristodoulou, C.; Pournaras, S.; Mavrouli, M.; Marka, A.; Tserkezou, P.; Baka, A.; Billinis, C.; Katsioulis, A.; Psaroulaki, A.; Papa, A. West Nile Virus seroprevalence in the Greek population in 2013: A nationwide cross-sectional survey. PLoS ONE 2015, 10, e0143803. [Google Scholar] [CrossRef] [Green Version]
- Han, L.L.; Popovici, F.; Alexander, J.P., Jr.; Laurentia, V.; Tengelsen, L.A.; Cernescu, C.; Gary, E.H., Jr.; Ion-Nedelcu, N.; Campbell, G.L.; Tsai, T.F. Risk factors for West Nile virus infection and meningoencephalitis, Romania, 1996. J. Infect. Dis. 1999, 179, 230–233. [Google Scholar] [CrossRef]
- Ladbury, G.A.F.; Gavana, M.; Danis, K.; Papa, A.; Papamichail, D.; Mourelatos, S.; Gewehr, S.; Theocharopoulos, G.; Bonovas, S.; Benos, A.; et al. Population seroprevalence study after a West Nile virus lineage 2 epidemic, Greece, 2010. PLoS ONE 2013, 8, e80432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nikolay, B. A review of West Nile and Usutu virus co-circulation in Europe: How much do transmission cycles overlap? Trans. R. Soc. Trop. Med. Hyg. 2015, 109, 609–618. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vilibic-Cavlek, T.; Savic, V.; Petrovic, T.; Toplak, I.; Barbic, L.; Petric, D.; Tabain, I.; Hrnjakovic-Cvjetkovic, I.; Bogdanic, M.; Klobucar, A.; et al. Emerging trends in the epidemiology of West Nile and Usutu virus infections in Southern Europe. Front. Veter. Sci. 2019, 6, 437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grottola, A.; Marcacci, M.; Tagliazucchi, S.; Gennari, W.; Di Gennaro, A.; Orsini, M.; Monaco, F.; Marchegiano, P.; Marini, V.; Meacci, M.; et al. Usutu virus infections in humans: A retrospective analysis in the municipality of Modena, Italy. Clin. Microbiol. Infect. 2017, 23, 33–37. [Google Scholar] [CrossRef] [Green Version]
- Pugliese, A.; Beltramo, T.; Torre, D. Seroprevalence study of tick-borne encephalitis, Borrelia burgdorferi, Dengue and Toscana virus in Turin province. Cell. Biochem. Funct. 2007, 25, 185–188. [Google Scholar] [CrossRef]
- Tokarevich, N.; Tronin, A.; Gnativ, B.; Revich, B.; Blinova, O.; Evengard, B. Impact of air temperature variation on the ixodid ticks habitat and tick-borne encephalitis incidence in the Russian Arctic: The case of the Komi Republic. Int. J. Circumpolar. Health 2017, 76, 1298882. [Google Scholar] [CrossRef]
- Kotrbova, K.; Lunackova, J. Seroprevalence of tick-borne encephalitis and Lyme borreliosis in a defined Czech population. Int. J. Infect. Dis. 2019, 79, 135. [Google Scholar] [CrossRef] [Green Version]
- Christova, I.; Panayotova, E.; Tchakarova, S.; Taseva, E.; Trifonova, I.; Gladnishka, T. A nationwide seroprevalence screening for West Nile virus and tick-borne encephalitis virus in the population of Bulgaria. J. Med. Virol. 2017, 89, 1875–1878. [Google Scholar] [CrossRef]
- Larsen, A.L.; Kanestrøm, A.; Bjørland, M.; Andreassen, Å.; Soleng, A.; Vene, S.; Dudman, S.G. Detection of specific IgG antibodies in blood donors and tick-borne encephalitis virus in ticks within a non-endemic area in southeast Norway. Scand. J. Infect. Dis. 2014, 46, 181–184. [Google Scholar] [CrossRef]
- Marvik, Å.; Tveten, Y.; Pedersen, A.-B.; Stiasny, K.; Andreassen, Å.K.; Grude, N. Low prevalence of tick-borne encephalitis virus antibodies in Norwegian blood donors. Infect. Dis. 2021, 53, 44–51. [Google Scholar] [CrossRef]
- Litzba, N.; Zelena, H.; Kreil, T.R.; Niklasson, B.; Kühlmann-Rabens, I.; Remoli, M.E.; Niedrig, M. Evaluation of different serological diagnostic methods for tick-borne encephalitis virus: Enzyme-linked immunosorbent, immunofluorescence, and neutralization assay. Vector-Borne Zoonotic Dis. 2014, 14, 149–159. [Google Scholar] [CrossRef] [PubMed]
| Variable | % (n; 95% CI) | |
|---|---|---|
| Gender | Males | 66.2 (794; 63.4–68.8) |
| Females | 33.8 (406; 31.2–36.6) | |
| Age category | Young adults | 47.4 (569; 44.6–50.3) |
| Middle-aged | 48.4 (581; 45.6–51.2) | |
| Old adults | 4.2 (50; 3.2–5.5) | |
| Education level | Higher | 45.1 (541; 42.3–47.9) |
| Secondary | 54.9 (659; 52.1–57.7) | |
| Activities | Outdoor | 11.9 (143; 10.2–13.9) |
| Indoor | 88.1 (1057; 86.1–89.8) | |
| Environment | Urban | 67.4 (809; 64.7–70.0) |
| Rural | 32.6 (391; 30.0–35.3) | |
| ELISA | TBE | ||
|---|---|---|---|
| WNV | Positive | Equivocal | Negative |
| % (n; 95% CI) | |||
| Positive | 2.2 (26; 1.5–3.2) | 0.4 (5; 0.2–1.0) | 0.7 (8; 0.3–1.3) |
| Equivocal | 0.0 | 0.1 (1; 0.1–0.5) | 0.2 (2; 0.1–0.6) |
| Negative | 0.2 (2; 0.1–0.6) | 0.8 (10; 0.5–1.5) | 95.5 (1146; 94.2–96.5) |
| Variables | WNV | TBEV | ||
|---|---|---|---|---|
| % (+/n; 95% CI) | p | % (+/n; 95% CI) | p | |
| Gender | ||||
| Females | 1.5 (6/406; 0.7–3.2) | 0.0267 | 0 (0/406) | 0.4743 |
| Males | 4.0 (32/794; 2.9–5.6) | 0.1 (1/794; 0.1–0.7) | ||
| Environment | ||||
| Urban | 2.7 (22/809; 1.8–4.1) | 0.2727 | 0 (0/809) | 0.7100 |
| Rural | 4.1 (16/391; 2.5–6.5) | 0.3 (1/391; 0.1–1.4) | ||
| Education | ||||
| Higher | 1.9 (10/541; 1.1–3.4) | 0.0280 | 0 (0/541) | 0.3647 |
| Secondary | 4.3 (28/659; 3.0–6.1) | 0.2 (1/659; 0.1–0.9) | ||
| Activities | ||||
| Outdoor | 4.9 (7/143; 2.0–9.8) | 0.3157 | 0 (0/143) | 0.7128 |
| Indoor | 2.9 (31/1057; 212–4.1) | 0.1 (1/1057; 1.5–3.4) | ||
| Age group | ||||
| Young adults | 2.8 (16/569; 1.7–4.5) | 0.4578 | 0 (0/569) | 0.5868 |
| Middle-aged | 3.3 (19/581; 2.1–5.1) | 0.2 (1/581; 1.3–3.8) | ||
| Old adults | 6.0 (3/50; 1.3–16.6) | 0 (0/50) | ||
| Total | 3.2 (38/1200; 2.3–4.3) | 0.1 (1/1200; 0.0–0.5) | ||
| Age Group | Gender | WNV | TBEV | ||
|---|---|---|---|---|---|
| % (+/n; 95% CI) | p | % (+/n; 95% CI) | p | ||
| Young adults | Males | 2.9 (11/384; 1.6–5.1) | 1.000 | 0.0 (0/384) | 1.000 |
| Females | 2.7 (5/185; 0.9–6. 2) | 0.0 (0/185) | |||
| Total | 2.8 (16/569; 1.7–4.5) | 0.0 (0/569) | |||
| Middle-aged | Males | 4.8 (18/373; 3.1–7.5) | 0.0098 | 0.3 (1/373; 0.1–1.5) | 1.000 |
| Females | 0.5 (1/208; 0.1–2.7) | 0.0 (0/208) | |||
| Total | 3.3 (19/581; 2.1–5.1) | 0.2 (1/581; 0.1–1.0) | |||
| Old adults | Males | 8.1 (3/37; 1.7–21.9) | 0.7038 | 0.0 (0/37) | 1.000 |
| Females | 0 (0/13) | 0.0 (0/13) | |||
| Total | 6.0 (3/50; 1.3–16.6) | 0.0 (0/50) | |||
| Total | 3.2 (38/1200; 2.3–4.3) | 0.4578 | 0.1 (1/1200; 0.1–0.5) | 0.5868 | |
| County | Age Group | WNV | TBEV | ||
|---|---|---|---|---|---|
| % (+/n; 95% CI) | p | % (+/n; 95% CI) | p | ||
| Alba | Young | 4.5 (4/89; 1.2–11.1) | 0.7294 | 0.0 (0/89) | 1.0000 |
| Middle | 2.9 (3/104; 0.6–8.2) | 0.0 (0/104) | |||
| Old | 0.0 (0/7) | 0.0 (0/7) | |||
| Total | 3.5 (7/200; 1.4–7.1) | 0.0 (0/200) | |||
| Bistrița-Năsăud | Young | 1.2 (1/87; 0.1–6.2) | 0.9627 | 0.0 (0/87) | 1.0000 |
| Middle | 0.9 (1/108; 0.1–5.1) | 0.0 (1/108) | |||
| Old | 0.0 (0/5) | 0.0 (0/5) | |||
| Total | 1.0 (2/200; 0.1–3.6) | 0.0 (0/200) | |||
| Cluj | Young | 0.7 (1/139; 0.1–3.9) | 0.8021 | 0.0 (0/139) | 1.0000 |
| Middle | 0.0 (0/57) | 0.0 (0/57) | |||
| Old | 0.0 (0/4) | 0.0 (0/4) | |||
| Total | 0.5 (1/200; 0.1–2.8) | 0.0 (0/200) | |||
| Maramureș | Young | 2.0 (2/100; 0.2–7.0) | 0.8022 | 0.0 (0/100) | 1.000 |
| Middle | 3.2 (3/94; 0.7–9.0) | 0.0 (0/94) | |||
| Old | 0.0 (0/6) | 0.0 (0/6) | |||
| Total | 2.5 (5/200; 0.8–5.7) | 0.0 (0/200) | |||
| Satu-Mare | Young | 11.3 (7/62; 4.7–21. 9) | 0.7743 | 0.0 (0/62) | 0.7001 |
| Middle | 9.4 (11/117; 4.8–16.2) | 0.9 (1/117; 0.1–4.7) | |||
| Old | 14.3 (3/21; 3.1–36.3) | 0.0 (0/21) | |||
| Total | 10.5 (21/200; 6.6–15.6) | 0.5 (1/200; 0.1–2.8) | |||
| Sălaj | Young | 1.1 (1/92; 0.1–5.9) | 0.9618 | 0.0 (0/92) | 1.0000 |
| Middle | 1.0 (1/101; 0.1–5.4) | 0.0 (0/101) | |||
| Old | 0.0 (0/7) | 0.0 (0/7) | |||
| Total | 1. 0 (2/200; 0.1–3.6) | 0.0 (0/200) | |||
| Total | 3.2 (38/1200; 2.3–4.3) | 0.1 (1/1200; 0.1–0.5) | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coroian, M.; Mihalca, A.D.; Dobler, G.; Euringer, K.; Girl, P.; Borșan, S.-D.; Kalmár, Z.; Tincuța Briciu, V.; Flonta, M.; Topan, A.; et al. Seroprevalence Rates against West Nile, Usutu, and Tick-Borne Encephalitis Viruses in Blood-Donors from North-Western Romania. Int. J. Environ. Res. Public Health 2022, 19, 8182. https://doi.org/10.3390/ijerph19138182
Coroian M, Mihalca AD, Dobler G, Euringer K, Girl P, Borșan S-D, Kalmár Z, Tincuța Briciu V, Flonta M, Topan A, et al. Seroprevalence Rates against West Nile, Usutu, and Tick-Borne Encephalitis Viruses in Blood-Donors from North-Western Romania. International Journal of Environmental Research and Public Health. 2022; 19(13):8182. https://doi.org/10.3390/ijerph19138182
Chicago/Turabian StyleCoroian, Mircea, Andrei Daniel Mihalca, Gerhard Dobler, Kathrin Euringer, Philipp Girl, Silvia-Diana Borșan, Zsuzsa Kalmár, Violeta Tincuța Briciu, Mirela Flonta, Adriana Topan, and et al. 2022. "Seroprevalence Rates against West Nile, Usutu, and Tick-Borne Encephalitis Viruses in Blood-Donors from North-Western Romania" International Journal of Environmental Research and Public Health 19, no. 13: 8182. https://doi.org/10.3390/ijerph19138182
APA StyleCoroian, M., Mihalca, A. D., Dobler, G., Euringer, K., Girl, P., Borșan, S.-D., Kalmár, Z., Tincuța Briciu, V., Flonta, M., Topan, A., Rădulescu, A. L., Ungur, A., & Lupșe, M. S. (2022). Seroprevalence Rates against West Nile, Usutu, and Tick-Borne Encephalitis Viruses in Blood-Donors from North-Western Romania. International Journal of Environmental Research and Public Health, 19(13), 8182. https://doi.org/10.3390/ijerph19138182









