From the Triage to the Intermediate Area: A Simple and Fast Model for COVID-19 in the Emergency Department
Abstract
1. Introduction
2. Materials and Methods
2.1. Organization of the Emergency Room: Pre-Triage and Triage Area
- isolated fever (body temperature > 37 °C)
- fever and respiratory symptoms
- fever and gastrointestinal symptoms
- vomiting or diarrhea
- general malaise
- myalgias
- anosmia and dysgeusia
- respiratory failure, defined as peripheral oxygen saturation (SpO2) less than 95% at room ambient, or 92% for patients with chronic obstructive pulmonary diseases (COPD).
- a close contact in the last 14 days with a subject with a positive nasopharyngeal (NP) swab for SARS-CoV-2, defined as follows: for at least 15 min at a distance less than 2 m without proper personal protective equipment
- home isolation for suspected SARS-CoV-2 infection
- a NP swab positive for SARS-CoV-2
- reported death of a close family member for unknown causes
- all the patients living in a retirement home.
- diffuse alveolar-interstitial syndrome (AIS) defined as three or more separate or coalescent B-lines in at least two fields for each hemithorax [34]
- focal AIS defined as three or more B-lines in a field
- normal pulmonary pattern (A pattern)
- pleural effusion.
2.2. Classification of the Patients and Data Collection
- a RT-PCR NP swab positive for SARS-CoV-2 in the past 14 days, at admission in the ED or within 72 h, or
- a clinical criterion and signs of interstitial pneumonia at chest X-ray or high-resolution CT-scan even with negative RT-PCR NP swab.
- neither clinical nor epidemiological criteria nor radiological signs of COVID-19 pneumonia, or
- the absence of antibodies (IgG) against SARS-CoV-2 in a period between 1 and 3 months after discharge or
- an alternative diagnosis to SARS-CoV-2 infection as justification for symptoms, after hospitalization of at least one week.
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO Coronavirus Disease (COVID-19) Pandemic. Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019 (accessed on 1 March 2022).
- Singh, J.; Pandit, P.; McArthur, A.G.; Banerjee, A.; Mossman, K. Evolutionary trajectory of SARS-CoV-2 and emerging variants. Virol. J. 2021, 18, 166. [Google Scholar] [CrossRef] [PubMed]
- Raman, R.; Patel, K.J.; Ranjan, K. COVID-19: Unmasking Emerging SARS-CoV-2 Variants, Vaccines and Therapeutic Strategies. Biomolecules 2021, 11, 993. [Google Scholar] [CrossRef] [PubMed]
- Papanikolaou, V.; Chrysovergis, A.; Ragos, V.; Tsiambas, E.; Katsinis, S.; Manoli, A.; Papouliakos, S.; Roukas, D.; Mastronikolis, S.; Peschos, D.; et al. From delta to Omicron: S1-RBD/S2 mutation/deletion equilibrium in SARS-CoV-2 defined variants. Gene 2022, 814, 146134. [Google Scholar] [CrossRef] [PubMed]
- Poggiali, E.; Vercelli, A.; Mazzoni, S.; Bastoni, D.; Iannicelli, T.; Demichele, E.; Ioannilli, E.; Magnacavallo, A. COVID-19 pandemic, Piacenza calling. The survival strategy of an Italian Emergency Department. Acta Biomed. 2020, 91, 2020045. [Google Scholar] [CrossRef]
- Maniscalco, P.; Poggiali, E.; Quattrini, F.; Ciatti, C.; Magnacavallo, A.; Caprioli, S.; Vadacca, G.; Cavanna, L.; Capelli, P. The deep impact of novel CoVID-19 infection in an Orthopedics and Traumatology Department: The experience of the Piacenza Hospital. Acta Biomed. 2020, 91, 97–105. [Google Scholar] [CrossRef]
- Erika, P.; Andrea, V.; Cillis, M.G.; Ioannilli, E.; Iannicelli, T.; Andrea, M. Triage decision-making at the time of COVID-19 infection: The Piacenza strategy. Intern. Emerg. Med. 2020, 15, 879–882. [Google Scholar] [CrossRef]
- Murgante, B.; Borruso, G.; Balletto, G.; Castiglia, P.; Dettori, M. Why Italy First? Health, Geographical and Planning Aspects of the COVID-19 Outbreak. Sustainability 2020, 12, 5064. [Google Scholar] [CrossRef]
- Comelli, I.; Scioscioli, F.; Cervellin, G. Impact of the COVID-19 epidemic on census, organization and activity of a large urban Emergency Department. Acta Biomed. 2020, 91, 45–49. [Google Scholar] [CrossRef]
- Giostra, F.; Mirarchi, M.G.; Farina, G.; Paolillo, C.; Sepe, C.; Benedusi, F.; Bellone, A.; Ghiadoni, L.; Barbieri, G.; Santini, M.; et al. Impact of COVID-19 pandemic and lockdown on emergency room access in Northern and Central Italy. Emerg. Care J. 2020, 17, 9705. [Google Scholar] [CrossRef]
- Turcato, G.; Zaboli, A.; Pfeifer, N. The COVID-19 epidemic and reorganisation of triage, an observational study. Intern. Emerg. Med. 2020, 15, 1517–1524. [Google Scholar] [CrossRef]
- Carenzo, L.; Costantini, E.; Greco, M.; Barra, F.L.; Rendiniello, V.; Mainetti, M.; Bui, R.; Zanella, A.; Grasselli, G.; Lagioia, M.; et al. Hospital surge capacity in a tertiary emergency referral centre during the COVID-19 outbreak in Italy. Anaesthesia 2020, 75, 928–934, Erratum in Anaesthesia 2020, 75, 1540. [Google Scholar] [CrossRef] [PubMed]
- Coen, D.; Paolillo, C.; Cavazza, M.; Cervellin, G.; Bellone, A.; Perlini, S.; Casagranda, I. Changing Emergency Department and hospital organization in response to a changing epidemic. Emerg. Care J. 2020, 16, 8969. [Google Scholar] [CrossRef]
- Barbieri, G.; Spinelli, S.; Filippi, M.; Foltran, F.; Giraldi, M.; Martino, M.C.; Cipriano, A.; Cinotti, F.; Santini, M.; Ghiadoni, L. COVID-19 pandemic management at the Emergency Department: The changing scenario at the University Hospital of Pisa. Emerg. Care J. 2020, 16, 9146. [Google Scholar] [CrossRef]
- Cerqua, A.; Letta, M. Local inequalities of the COVID-19 crisis. Reg. Sci. Urban Econ. 2022, 92, 103752. [Google Scholar] [CrossRef] [PubMed]
- Dettori, M.; Deiana, G.; Balletto, G.; Borruso, G.; Murgante, B.; Arghittu, A.; Azara, A.; Castiglia, P. Air pollutants and risk of death due to COVID-19 in Italy. Environ. Res. 2021, 192, 110459. [Google Scholar] [CrossRef]
- Giwa, A.L.; Desai, A. Novel 2019 coronavirus SARS-CoV-2 (COVID-19): An overview for emergency clinicians. Emerg. Med. Pract. 2020, 17, 1–24. [Google Scholar]
- Jeffery, M.M.; D’Onofrio, G.; Paek, H.; Platts-Mills, T.F.; Soares, W.E.; Hoppe, J.A.; Genes, N.; Nath, B.; Melnick, E.R. Trends in Emergency Department Visits and Hospital Admissions in Health Care Systems in 5 States in the First Months of the COVID-19 Pandemic in the US. JAMA Intern. Med. 2020, 180, 1328–1333. [Google Scholar] [CrossRef]
- Roberge, D.; Pineault, R.; Larouche, D.; Poirier, L.R. The continuing saga of emergency room overcrowding: Are we aiming at the right target? Healthc. Policy 2010, 5, 27–39. [Google Scholar] [CrossRef][Green Version]
- Pesaresi, C.; Migliara, G.; Pavia, D.; De Vito, C. Emergency Department Overcrowding: A Retrospective Spatial Analysis and the Geocoding of Accesses. A Pilot Study in Rome. ISPRS Int. J. Geo-Inf. 2020, 9, 579. [Google Scholar] [CrossRef]
- Yarmohammadian, M.H.; Rezaei, F.; Haghshenas, A.; Tavakoli, N. Overcrowding in Emergency Departments: A Review of Strategies to Decrease Future Challenges. J. Res. Med. Sci. 2017, 22, 23. [Google Scholar]
- Poggiali, E.; Dacrema, A.; Bastoni, D.; Tinelli, V.; Demichele, E.; Ramos, P.M.; Marcianò, T.; Silva, M.; Vercelli, A.; Magnacavallo, A. Can Lung US Help Critical Care Clinicians in the Early Diagnosis of Novel Coronavirus (COVID-19) Pneumonia? Radiology 2020, 295, E6. [Google Scholar] [CrossRef] [PubMed]
- Dacrema, A.; Silva, M.; Rovero, L.; Vertemati, V.; Losi, G.; Piepoli, M.F.; Sacchi, R.; Mangiacotti, M.; Nazerian, P.; Pagani, L.; et al. A simple lung ultrasound protocol for the screening of COVID-19 pneumonia in the emergency department. Intern. Emerg. Med. 2021, 16, 1297–1305. [Google Scholar] [CrossRef] [PubMed]
- Volpicelli, G.; Gargani, L.; Perlini, S.; Spinelli, S.; Barbieri, G.; Lanotte, A.; Casasola, G.G.; Nogué-Bou, R.; Lamorte, A.; Agricola, E.; et al. Lung ultrasound for the early diagnosis of COVID-19 pneumonia: An international multicenter study. Intensiv. Care Med. 2021, 47, 444–454. [Google Scholar] [CrossRef]
- Caroselli, C.; Blaivas, M.; Marcosignori, M.; Chen, Y.T.; Falzetti, S.; Mariz, J.; Fiorentino, R.; Silva, R.P.; Cochicho, J.G.; Sebastiani, S.; et al. Early Lung Ultrasound Findings in Patients with COVID-19 Pneumonia: A Retrospective Multicenter Study of 479 Patients. J. Ultrasound Med. 2022. [Google Scholar] [CrossRef] [PubMed]
- Abhilash, K.P.; David, S.; Joseph, E.S.; Peter, J. Acute management of COVID-19 in the emergency department: An evidence-based review. J. Fam. Med. Prim. Care 2022, 11, 424. [Google Scholar] [CrossRef]
- Jackson, K.; Butler, R.; Aujayeb, A. Lung ultrasound in the COVID-19 pandemic. Postgrad. Med. J. 2021, 97, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xue, H.; Wang, M.; He, N.; Lv, Z.; Cui, L. Lung Ultrasound Findings in Patients With Coronavirus Disease (COVID-19). Am. J. Roentgenol. 2021, 216, 80–84. [Google Scholar] [CrossRef]
- Sorlini, C.; Femia, M.; Nattino, G.; Bellone, P.; Gesu, E.; Francione, P.; Paternò, M.; Grillo, P.; Ruffino, A.; Bertolini, G.; et al. The role of lung ultrasound as a frontline diagnostic tool in the era of COVID-19 outbreak. Intern. Emerg. Med. 2021, 16, 749–756. [Google Scholar] [CrossRef]
- Boccatonda, A.; Cocco, G.; Ianniello, E.; Montanari, M.; D’Ardes, D.; Borghi, C.; Giostra, F.; Copetti, R.; Schiavone, C. One year of SARS-CoV-2 and lung ultrasound: What has been learned and future perspectives. J. Ultrasound 2022, 24, 115–123. [Google Scholar] [CrossRef]
- Smith, M.; Hayward, S.; Innes, S.; Miller, A. Point-of-care lung ultrasound in patients with COVID-19—A narrative review. Anaesthesia 2020, 75, 1096–1104. [Google Scholar] [CrossRef]
- Soldati, G.; Smargiassi, A.; Inchingolo, R.; Buonsenso, D.; Perrone, T.; Briganti, D.F.; Perlini, S.; Torri, E.; Mariani, A.; Mossolani, E.E.; et al. Is There a Role for Lung Ultrasound During the COVID-19 Pandemic? J. Ultrasound Med. 2020, 39, 1459–1462. [Google Scholar] [CrossRef]
- Poggiali, E.; Bastoni, D.; Ferrari, M.; Moretto, D.; Buttafava, F.; Ramos, P.M.; Burzio, V.; Petri, J.; Magnacavallo, A.; Vercelli, A. COVID-19 or not COVID-19, that is the question. The role of screening criteria and point-of-care lung ultrasound in the triage decision-making process in an Emergency Department during the phase 2 of the COVID-19 Italian epidemic. Emerg. Care J. 2021, 17, 9708. [Google Scholar] [CrossRef]
- Volpicelli, G.; Elbarbary, M.; Blaivas, M.; Lichtenstein, D.A.; Mathis, G.; Kirkpatrick, A.W.; Melniker, L.; Gargani, L.; Noble, V.E.; Via, G.; et al. International evidence-based recommendations for point-of-care lung ultrasound. Intensive Care Med. 2012, 38, 577–591. [Google Scholar] [CrossRef] [PubMed]
- Poggiali, E.; Vercelli, A.; Vadacca, G.B.; Schiavo, R.; Mazzoni, S.; Ioannilli, E.; Demichele, E.; Magnacavallo, A. Negative nasopharyngeal swabs in COVID-19 pneumonia: The experience of an Italian Emergengy Department (Piacenza) during the first month of the Italian epidemic. Acta BioMed. Atenei Parm. 2020, 91, e2020024. [Google Scholar] [CrossRef]
- Hosmer, D.W., Jr.; Lemeshow, S.; Sturdivant, R.X. Applied Logistic Regression; John Wiley & Sons: Hoboken, NJ, USA, 2013; Volume 398. [Google Scholar]
- Tjur, T. Coefficients of determination in logistic regression models—A new proposal: The coefficient of discrimination. Am. Stat. 2009, 63, 366–372. [Google Scholar] [CrossRef]
- Steyerberg, E.W.; Harrell, F.E.; Borsboom, G.J.; Eijkemans, M.; Vergouwe, Y.; Habbema, J.F. Internal validation of predictive models: Efficiency of some procedures for logistic regression analysis. J. Clin. Epidemiol. 2001, 54, 774–781. [Google Scholar] [CrossRef]
- Green, M.S. Evaluating the discriminatory power of a multiple logistic regression model. Stat. Med. 1988, 17, 519–524. [Google Scholar] [CrossRef]
- Kosmidis, I.; Kenne Pagui, E.C.; Sartori, N. Mean and median bias reduction in generalized linear models. Stat. Comput. 2020, 30, 43–59. [Google Scholar] [CrossRef]
- Lüdecke, D.; Ben-Shachar, M.S.; Patil, I.; Waggoner, P.; Makowski, D. Performance: An R package for assessment, comparison and testing of statistical models. J. Open Source Softw. 2021, 6, 3139. [Google Scholar] [CrossRef]
- Quarato, C.; Mirijello, A.; Lacedonia, D.; Russo, R.; Maggi, M.; Rea, G.; Simeone, A.; Borelli, C.; Feragalli, B.; Scioscia, G.; et al. Low Sensitivity of Admission Lung US Compared to Chest CT for Diagnosis of Lung Involvement in a Cohort of 82 Patients with COVID-19 Pneumonia. Medicina (Kaunas, Lithuania) 2021, 57, 236. [Google Scholar] [CrossRef]
- Zanforlin, A.; Strapazzon, G.; Falk, M.; Gallina, V.; Viteritti, A.; Valzolgher, L.; La Guardia, M.; Ferro, F.; Pagani, L.; Vezzali, N. Lung Ultrasound in the Emergency Department for Early Identification of COVID-19 Pneumonia. Respiration 2021, 100, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Bosso, G.; Allegorico, E.; Pagano, A.; Porta, G.; Serra, C.; Minerva, V.; Mercurio, V.; Russo, T.; Altruda, C.; Arbo, P.; et al. Lung ultrasound as diagnostic tool for SARS-CoV-2 infection. Intern. Emerg. Med. 2021, 16, 471–476. [Google Scholar] [CrossRef] [PubMed]

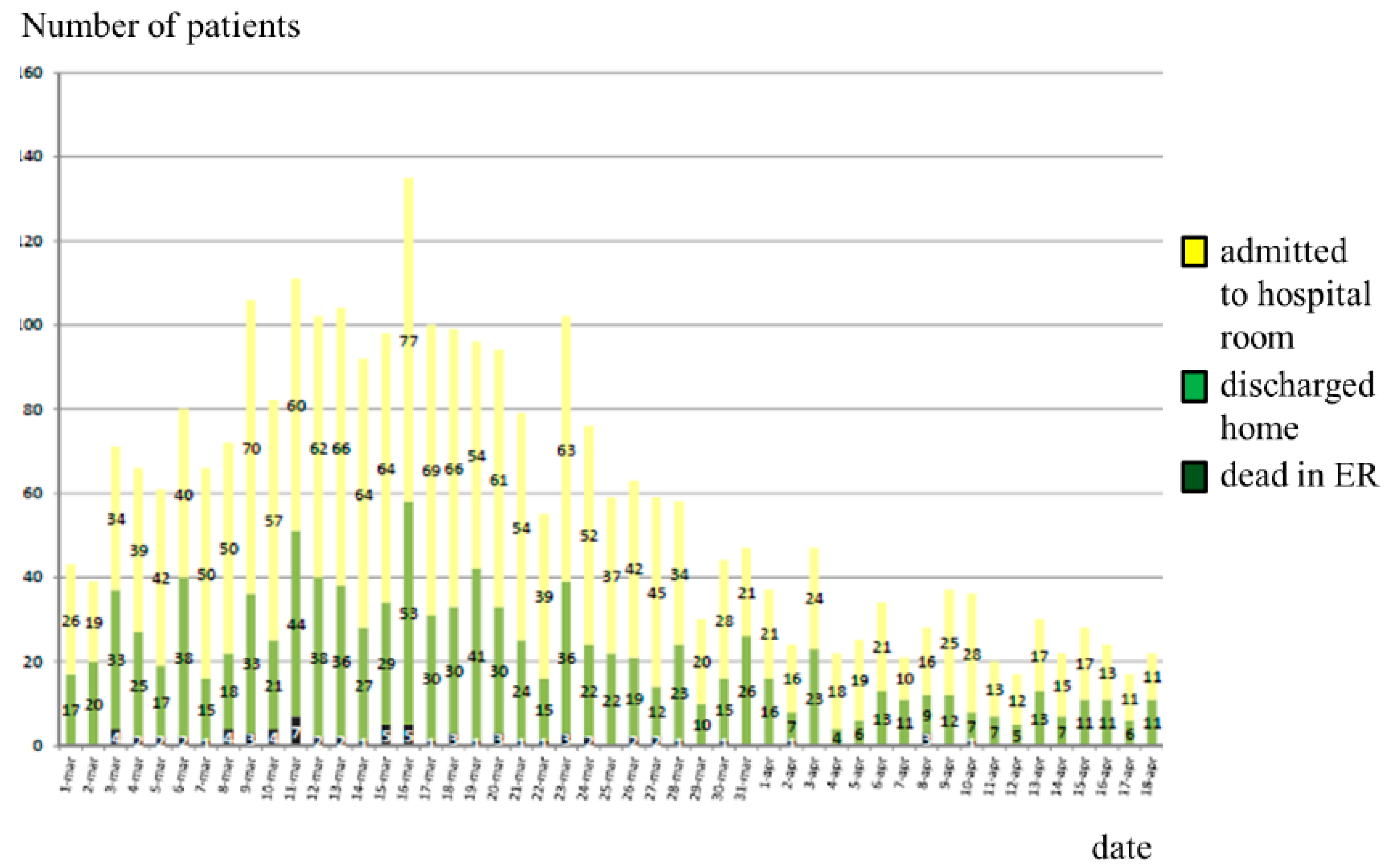
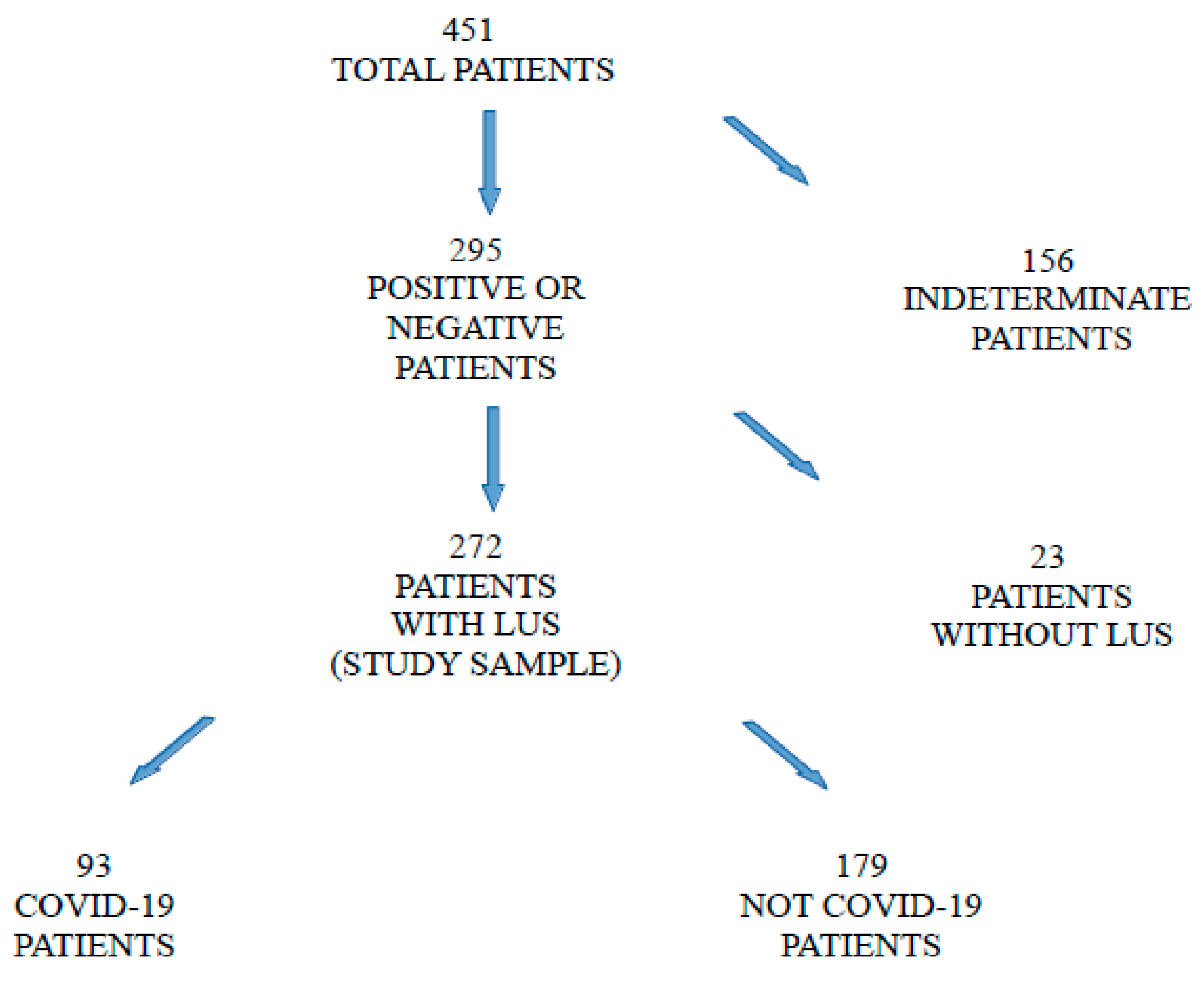
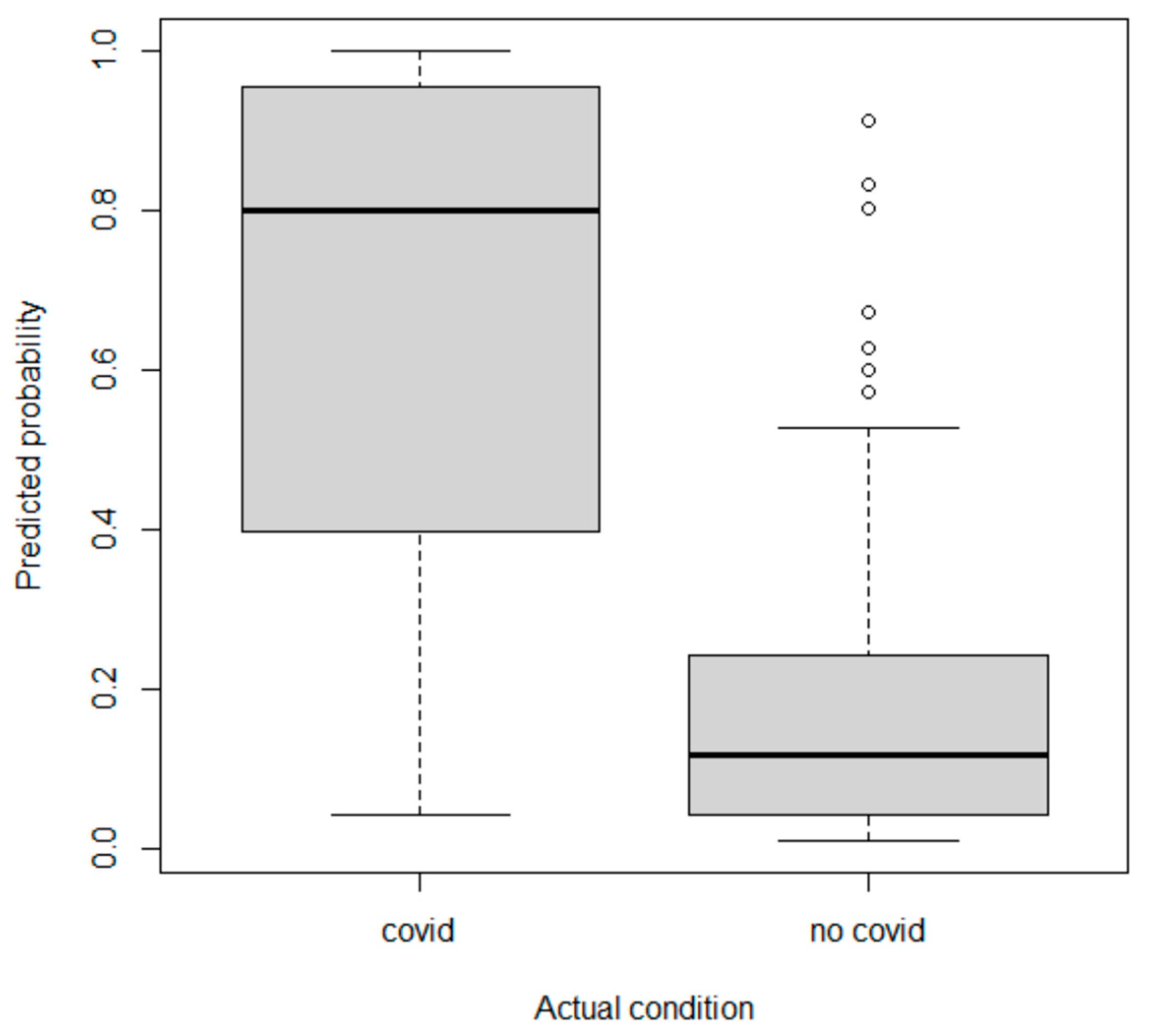
| COVID-19 Patients (n = 93) | Not-COVID-19 Patients (n = 179) | |
|---|---|---|
| Sex (M/F) | 39 (42%)/54 (58%) | 89 (50%)/90 (50%) |
| Mean age (years) (min-max) | 70 (27–96) | 61 (15–99) |
| Fever alone | 24 (26%) | 21 (12%) |
| Fever and respiratory symptoms | 34 (36%) | 20 (11%) |
| Fever and gastrointestinal symptoms | 19 (20%) | 20 (11%) |
| Vomiting or diarrhea | 14 (15%) | 35 (20%) |
| General malaise | 25 (27%) | 27 (15%) |
| Myalgias | 18 (19%) | 18 (10%) |
| Anosmia or dysgeusia | 11 (12%) | 12 (7%) |
| Acute respiratory failure | 35 (38%) | 26 (14%) |
| Chest X-ray | 20 (21%) | 61 (34%) |
| HRCT | 67 (72%) | 81 (45%) |
| COVID-19 Patients (n = 93) | Not-COVID-19 Patients (n = 179) | |
|---|---|---|
| A pattern | 16 (17%) | 91 (51%) |
| B-lines | 31 (33%) | 56 (31%) |
| Diffuse AIS | 45 (48%) | 24 (13%) |
| Pleural effusion | 9 (9%) | 21 (12%) |
| COVID-19 Patients (n = 35) | Not-COVID-19 Patients (n = 26) | |
|---|---|---|
| A pattern | 1 (3%) | 5 (19%) |
| B-lines | 6 (17%) | 12 (46%) |
| Diffuse AIS | 28 (80%) | 7 (27%) |
| Pleural effusion | 3 (9%) | 7 (27%) |
| Estimate Std. | Error | z Value | Pr(>|z|) | |
|---|---|---|---|---|
| (Intercept) | −1.5128 | 0.3280 | −4.612 | 0.00000399 *** |
| NP swab | 5.3990 | 1.5824 | 3.412 | 0.000645 *** |
| Close contact | 2.4112 | 0.5455 | 4.420 | 0.00000986 *** |
| Fever alone | 0.4959 | 0.4548 | 1.090 | 0.275509 |
| Fever and gastrointestinal symptoms | −0.1680 | 0.5268 | −0.319 | 0.749807 |
| Fever and respiratory symptoms | 1.2985 | 0.4620 | 2.811 | 0.004943 ** |
| Respiratory failure | 0.6819 | 0.4163 | 1.638 | 0.101401 |
| Anosmia and dysgeusia | 0.9937 | 0.5752 | 1.728 | 0.084056 . |
| Diffuse AIS | 0.841 | 0.4151 | 2.027 | 0.042671 * |
| A pattern | −1.6403 | 0.4911 | −3.340 | 0.000838 *** |
| Pleural effusion | −0.7286 | 0.5538 | −1.316 | 0.188257 |
| Score | |
|---|---|
| Known positive NP swab | 5.4 |
| Close contact | 2.4 |
| Fever alone | 0.5 |
| Fever and gastrointestinal symptoms | 0.1 |
| Fever and respiratory symptoms | 1.3 |
| Respiratory failure | 0.7 |
| Anosmia and dysgeusia | 1.0 |
| Diffuse AIS | 0.8 |
| A pattern | −1.6 |
| Pleural effusion | −0.7 |
| Not COVID-19 Area | COVID-19 Area | |
|---|---|---|
| Not-COVID-19 patients | 145 | 34 |
| COVID-19 patients | 16 | 77 |
| Not COVID-19 Area | Intermediate Area | COVID-19 Area | |
|---|---|---|---|
| Not-COVID-19 patients | 99 | 61 | 19 |
| COVID-19 patients | 8 | 19 | 66 |
| Respiratory Failure | |||
|---|---|---|---|
| COVID-19 | Not COVID-19 | Total | |
| Positive LUS | 26 | 6 | 32 |
| Negative LUS | 9 | 20 | 29 |
| Total | 35 | 26 | |
| Respiratory Failure | |||
|---|---|---|---|
| COVID-19 | Not COVID-19 | Total | |
| Positive LUS | 10 | 1 | 11 |
| Negative LUS | 15 | 34 | 49 |
| Total | 26 | 35 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Poggiali, E.; Fabrizi, E.; Bastoni, D.; Iannicelli, T.; Galluzzo, C.; Canini, C.; Cillis, M.G.; Ponzi, D.G.; Magnacavallo, A.; Vercelli, A. From the Triage to the Intermediate Area: A Simple and Fast Model for COVID-19 in the Emergency Department. Int. J. Environ. Res. Public Health 2022, 19, 8070. https://doi.org/10.3390/ijerph19138070
Poggiali E, Fabrizi E, Bastoni D, Iannicelli T, Galluzzo C, Canini C, Cillis MG, Ponzi DG, Magnacavallo A, Vercelli A. From the Triage to the Intermediate Area: A Simple and Fast Model for COVID-19 in the Emergency Department. International Journal of Environmental Research and Public Health. 2022; 19(13):8070. https://doi.org/10.3390/ijerph19138070
Chicago/Turabian StylePoggiali, Erika, Enrico Fabrizi, Davide Bastoni, Teresa Iannicelli, Claudia Galluzzo, Chiara Canini, Maria Grazia Cillis, Davide Giulio Ponzi, Andrea Magnacavallo, and Andrea Vercelli. 2022. "From the Triage to the Intermediate Area: A Simple and Fast Model for COVID-19 in the Emergency Department" International Journal of Environmental Research and Public Health 19, no. 13: 8070. https://doi.org/10.3390/ijerph19138070
APA StylePoggiali, E., Fabrizi, E., Bastoni, D., Iannicelli, T., Galluzzo, C., Canini, C., Cillis, M. G., Ponzi, D. G., Magnacavallo, A., & Vercelli, A. (2022). From the Triage to the Intermediate Area: A Simple and Fast Model for COVID-19 in the Emergency Department. International Journal of Environmental Research and Public Health, 19(13), 8070. https://doi.org/10.3390/ijerph19138070






