Influence of Walking as Physiological Training to Improve Respiratory Parameters in the Elderly Population
Abstract
1. Introduction
2. Materials and Methods
2.1. PICOS and Eligibility Criteria
2.2. Search Strategy
2.3. Study Selection
2.4. Inclusion Criteria
- -
- Studies working with seniors without a specific comorbid disease;
- -
- Studies analysing different types of walking (also as a part of a more comprehensive physical intervention) in seniors;
- -
- Studies working with seniors aged 60 and above;
- -
- Studies designed as a randomized controlled trial (RCT) or randomized cross-over trial;
- -
- Studies working with probands that do not suffer from any other disease that could affect the results of the study;
- -
- Studies working with probands that were able to provide written consent to participate in the study;
- -
- Studies published in the English language;
- -
- Studies published between 2002 and 2022.
2.5. Exclusion Criteria
2.6. Data Extraction
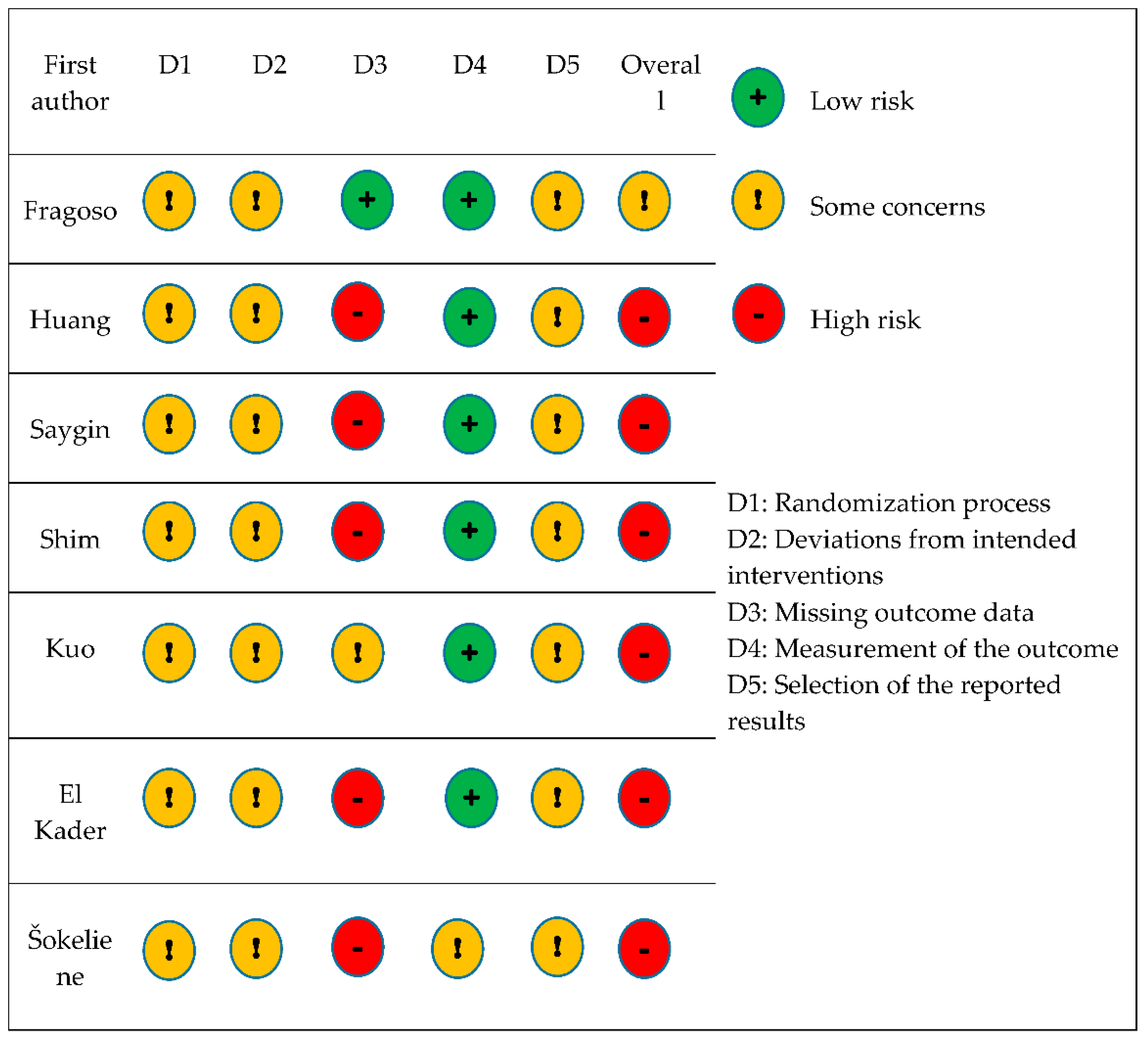
2.7. Assessing the Risk of Bias
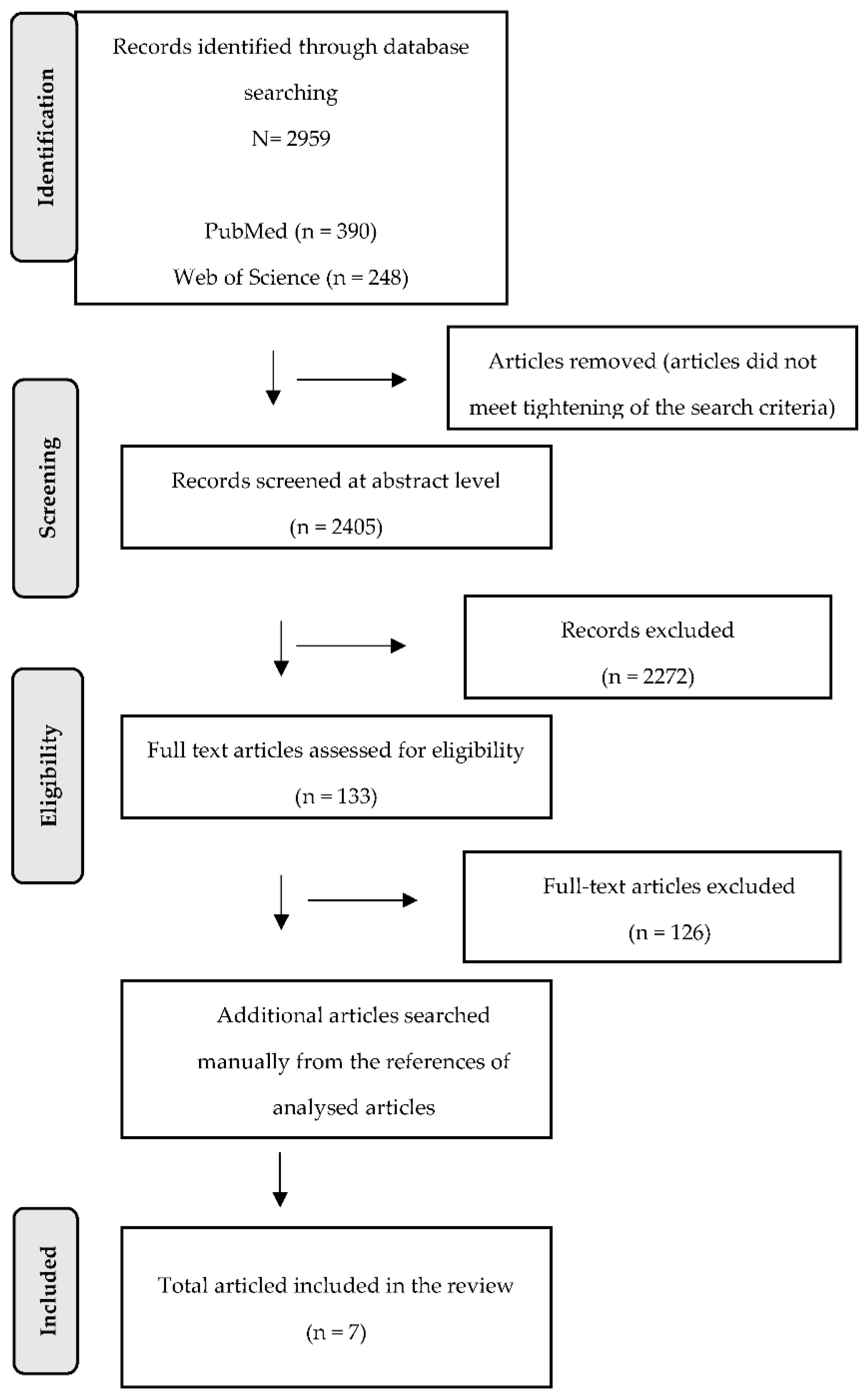
3. Results
3.1. Study Characteristics
3.2. The Outcome Measures
- -
- Lung function: forced vital capacity (FVC), forced expiratory volume in 1 s (FEV1), vital capacity (VC), maximal voluntary ventilation (MVV), maximum inspiratory mouth pressure (PImax. or MIP), maximum expiratory mouth pressure (PEmax.);
- -
- Cardiac function: resting heart rate, systolic blood pressure, diastolic blood pressure;
- -
- General information: weight, height, body mass index (BMI), waist/hip ratio, body fat percentage;
- -
- Muscle strength: handgrip strength, handgrip/body weight ratio.
- -
- Endurance: aerobic endurance, inspiratory muscle endurance, calf endurance.
- -
- Physical activity: physical activity level, fatigue intensity in performing ADL and IADL;
- -
- Other outcome measures: six-minute walk test (6MWT), exacerbation of obstructive airway disease (EOAD), reaction time, flexibility, Sit and Reach test, Get Up and Go test, the functional movement screen (FMS), quality of life (SF-36), Mini mental state exam (MMSE), Barthel index, Instrumental Activities of Daily Living (IADL), Geriatric Depression Scale (GDS).
3.2.1. Lung Function
3.2.2. Cardiac Function
3.2.3. General Information
3.2.4. Muscle Strength
3.2.5. Endurance
3.2.6. Physical Activity
3.2.7. Other Outcome Measures
3.3. Methodological Quality Assessment
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organisation. Ageing and Health. 2021. Available online: https://www.who.int/news-room/fact-sheets/detail/ageing-and-health (accessed on 4 February 2022).
- Mazzeo, R.S.; Tanaka, H. Exercise Prescription for the Elderly. Sports Med. 2001, 31, 809–818. [Google Scholar] [CrossRef]
- Sharma, G.; Goodwin, J. Effect of aging on respiratory systém physiology and imunology. Clin. Interv. Aging 2006, 1, 253–260. [Google Scholar] [CrossRef]
- Venosa, A. Senescence in Pulmonary Fibrosis: Between Aging and Exposure. Front. Med. 2020, 7, 606462. [Google Scholar] [CrossRef]
- Zadák, Z. Prevence a terapie sarkopenie ve stáří. Vnitr. Lek. 2016, 62, 671–677. [Google Scholar]
- Visser, M.; Schaap, L.A. Consequences of Sarcopenia. Clin. Geriatr. Med. 2011, 27, 387–399. [Google Scholar] [CrossRef]
- Bulow, J.; Ulijaszek, S.J.; Holm, L. Rejuvenation of the term sarcopenia. J. Appl. Physiol. 2019, 126, 255–256. [Google Scholar] [CrossRef]
- Marzetti, E.; Calvani, R.; Tosato, M. Sarcopenia: An overview. Aging Clin. Exp. Res. 2017, 29, 11–17. [Google Scholar] [CrossRef]
- Kim, M.S.; Cha, Y.J.; Choi, J.D. Correlation between forward head posture, respiratory functions, and respiratory accessory muscles in young adults. J. Back. Musculoskelet. Rehabil. 2017, 30, 711–715. [Google Scholar] [CrossRef]
- Zaugg, M.; Lucchinetii, E. Respiratory function in the elderly. Anesthesiol. Clin. N. Am. 2000, 18, 47–58. [Google Scholar] [CrossRef]
- Lee, N.K.; Jung, S.I.; Lee, D.Y. Effects of exercises on cervical angle and respiratory function in smartphone users. Osong Public Health Res. Perspect. 2017, 8, 271–274. [Google Scholar] [CrossRef]
- Klevetová, D. Motivační Prvky Při Práci se Seniory, 2nd ed.; Grada: Praha, Czech Republic, 2008; pp. 143–144. [Google Scholar]
- Belo, J.; Palmeiro, T.; Caires, I.; Papoila, A.L.; Alves, M.; Carreiro-Martins, P.; Botelho, M.A.; Neuparth, N. Reference values for spirometry in elderl individuals: A cross-sectional study of different reference equations. Multidiscip Respir. Med. 2018, 13, 4. [Google Scholar] [CrossRef]
- Pawlicka-Lisowska, A.; Motylewski, S.; Lisowski, S.; Michalak, K.; Poziomska-Piatkowska, E. Faulty posture and selected respiratory indicators. Pol. Merkur. Lek. 2013, 35, 67–71. [Google Scholar]
- Luoto, J.; Pihlsgard, M.; Wollmer, P.; Elmstahl, E. Relative and absolute lung function change in a general population aged 60–102 years. Eur. Respir. J. 2019, 53, 1701812. [Google Scholar] [CrossRef]
- Egidi, V. Health status of older people. Aging 2003, 59, 169–200. [Google Scholar]
- Fletcher, P.C.; Hirdes, J.P. Restriction in activity associated with fear of falling among community-based seniors using home care services. Age Aging 2004, 33, 273–279. [Google Scholar] [CrossRef]
- Gijsen, R.; Hoeymans, N.; Schellevis, F.G.; Ruwaard, D.; Satariano, W.A.; van den Bos, G.A. Causes and consequences of comorbidity: A review. J. Clin. Epidemiol. 2001, 54, 661–674. [Google Scholar] [CrossRef]
- Visser, M.; Schaap, L.A.; Wijnhoven, H.A.H. Self-reported impact of the COVID-19 pandemic on nutrition and physical activity behaviour in dutch older adults living independently. Nutrients 2020, 12, 3708. [Google Scholar] [CrossRef]
- Haykowsky, M.J.; Brubaker, P.H.; Stewart, K.P.; Morgan, T.M.; Eggebeen, J.; Kitzman, D.W. Effect of endurance training on the determinants of peak exercise oxygen consumption in elderly patients with stable compensated heart failure and preserved ejection fraction. J. Am. Coll. Cardiol. 2012, 60, 120–128. [Google Scholar] [CrossRef]
- Della Gatta, P.A.; Garnham, A.P.; Peake, J.M.; Cameron-Smith, D. Effect of exercise trainind on skeletal muscle cytokine expression in the elderly. Brain Behav. Immun. 2014, 39, 80–86. [Google Scholar] [CrossRef]
- Shahindi, M.; Mojtahed, A.; Modabbernia, A.; Mojtahed, M.; Shafiabady, A.; Delavar, A.; Honari, H. Laughter yoga versus group exercise program in elderly depressed women: A randomized controlled trial. Int. J. Geriatr. Psychiatry 2011, 26, 322–327. [Google Scholar] [CrossRef]
- Kosmidou, K.V.; Eleftheriadou, A.I.; Volaklis, K.A. The effect of a 3-yrs combined exercise program on body compostition and lipid profile in elderly women. Ach. Hell. Med. 2014, 31, 191–199. [Google Scholar]
- World Health Organisation. Mental Health of Older Adults. 2017. Available online: https://www.who.int/news-room/fact-sheets/detail/mental-health-of-older-adults (accessed on 10 May 2022).
- Battaglia, G.; Giustino, V.; Messina, G.; Faraone, M.; Brusa, J.; Bordonali, A.; Barbagallo, M.; Palma, A.; Dominguez, L. Walking in natural environments as geriatrician’s recommendation for fall prevention: Preliminary outcomes from the “Passiata Day“ model. Sustainability 2020, 12, 2684. [Google Scholar] [CrossRef]
- Donath, L.; Roth, R.; Zahner, L.; Faude, O. Effects of stair-climbing on balance, gait, strength, resting heart rate, and submaximal endurance in healthy seniors. Scand. J. Med. Sci. Sports 2014, 24, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Xiujie, M.; Wang, L.; Zhang, C.; Song, Q.; Gu, H.; Mao, D. Effects of Tai Chi Chuan and brisk walking exercise on balance ability in elderly women: A randomized controlled trial. Motor. Control. 2018, 23, 100–114. [Google Scholar] [CrossRef] [PubMed]
- Costa Branco, J.; Jansen, K.; Teixeira Sobrinho, J.; Carrapatoso, S.; Spessato, B.; Carvalho, J.; Mota, J.; Azevedo da Silva, R. Physical benefits and reduction of depressive symptoms among the elderly: Results from the Portuguese “National Walking Program”. Cien Saude Colet 2015, 20, 789–795. [Google Scholar] [CrossRef]
- Gomeňuka, N.A.; Bianchi Oliviera, H.; Soares Silva, E.; Rocha Costa, R.; Kanitz, A.C.; Veiga Liedtke, G.; Barreto Schuch, F.; Peyré-Tartaruga, L.A. Effects of Nordic walking training on quality of life, balance, functional mobility in elderly: A randomized clinical trial. PLoS ONE 2019, 14, 3. [Google Scholar] [CrossRef]
- Li, Q.; Morimoto, K.; Nakadai, A.; Inagaki, H.; Katsumata, M.; Shimizu, T.; Hirata, Y.; Hirata, K.; Suzuki, H.; Miyazaki, Y.; et al. Forest bathing enhances human natural killer activity and expression of anti-cancer proteins. Int. J. Immunopathol. Pharmacol. 2007, 20, 3–8. [Google Scholar] [CrossRef]
- Ishii, H.; Tsunetsugu, Y.; Park, B.J.; Hirano, H.; Kagawa, T.; Miyzaki, Y. Physiological effects of Shinrin-yoku (taking in the atmosphere of the forest) in a mixed forest in Shinano Town, Japan. Scand. J. For. Res. 2007, 26, 135–142. [Google Scholar]
- Li, Q.; Otsuka, T.; Kobayashi, M.; Wakayama, Y.; Inagaki, H.; Katsumata, M.; Hirata, Y.; Li, Y.; Hirata, K.; Shimizu, T.; et al. Acute effects of walking in forest environments on cardiovascular and metabolic parameters. Eur. J. Appl. Physiol. 2011, 111, 2845–2853. [Google Scholar] [CrossRef]
- Melzer, I.; Benjuya, N.; Kaplanski, J. Effects of regular walking on postural stability in the elderly. Gerontology 2003, 49, 240–245. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Prisma, G. Preferred reporting itemsfor systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, 889–896. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, 89. [Google Scholar]
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. Cochrane Handbook for Systematic Reviews of Interventions version 6.3 (updated February 2022); Cochrane Training: Cochrane, AB, Canda, 2022. [Google Scholar]
- Park, B.J.; Tsunetsugu, Y.; Kasetani, T.; Kagawa, T.; Miyazaki, Y. The physiological effects of Shinrin-yoku (taking in the forest atmosphere or forestbathing): Evidence from field experiments in 24 forests across Japan. Environ. Health Prev. Med. 2010, 15, 18–26. [Google Scholar] [CrossRef] [PubMed]
- El-Kader, M.S.A.; El-Den Ashmawi, E.M.S. Aerobic exercise training and incentive spirometry can control age-related respiratory muscle performance changes in elderly. J. Gen. Med. 2013, 10, 14–19. [Google Scholar] [CrossRef]
- Kuo, M.; Chen, M.; Jeng, C. A randomized controlled trial of the prescribed Stepper walking program in preventing frailty among the dwelling elderly. Top. Geriatr. Rehabil. 2018, 34, 223–233. [Google Scholar] [CrossRef]
- Kaczmarczyk, K.; Wiszomirska, I.; Magiera, A.; Ilnicka, L.; Blazkiewicz, M. Changes in lung function and anthropometric parameters post training in older women. Int. J. Gerontol. 2015, 9, 123–125. [Google Scholar] [CrossRef][Green Version]
- Saygin, O. Long-term walking exercise may affect some physical functions in the elderly. Stud. EthnoMed. 2015, 9, 379–384. [Google Scholar] [CrossRef]
- Huang, G.; Osness, W.H. Changes in pulmonary function response to a 10-week controlled exercise program in sedentary elderly adults. Percept. Mot. Ski. 2005, 100, 394–402. [Google Scholar] [CrossRef]
- Shim, Y.J.; Choi, H.S.; Shin, W.S. Aerobic training with rhytmic functional movement: Influence on cardiopulmonary function, functional movment and quality of life in the elderly women. J. Hum. Sport 2019, 14, 748–756. [Google Scholar]
- Fragoso, C.A.V.; Beavers, D.P.; Anton, S.D.; Liu, C.K.; McDermott, M.M.; Newman, A.B.; Pahor, M.; Stafford, R.S.; Gill, T.M. Effect of structured physical activity on respiratory outcomes in sedentary elderly adults with mobility limitations. J. Am. Geriatr. Soc. 2016, 64, 501–509. [Google Scholar] [CrossRef]
- Šokeliene, V.; Česnaitiene, V.J. The influence of Nordic walking on physical fitness of elderly people. Sportas 2011, 82, 45–51. [Google Scholar] [CrossRef]
- Bai, X.; Soh, K.G.; Omar Dev, R.D.; Talib, O.; Xiao, W.; Cai, H. Effect of brisk walking on health-related physical fitness balance and life satisfaction among the elderly: A systematic review. Front. Public Health 2022, 9, 829367. [Google Scholar] [CrossRef]
- Hanson, S.; Jones, A. Is there evidence that waking groups have health benefits? A systematic review and meta-analysis. Br. J. Sports Med. 2015, 49, 710–715. [Google Scholar] [CrossRef]
- Nelson, M.E.; Folta, S.C. Further evidence for the benefits of walking. AJCN 2009, 89, 15–16. [Google Scholar] [CrossRef]
- Dogra, S.; Good, J.; Gardiner, A.; Copeland, J.L.; Stickland, M.K.; Rudoler, D.; Buman, M.P. Effects of replacing sitting time with physical activity on lung function: An analysis of the Canadian longitudinal study on aging. Health Rep. 2019, 30, 12–23. [Google Scholar]
- Lee, J.Y.; Lee, D.C. Cardiac and pulmonary benefits of forest walking versus city walking in elderly women: A randomised, controlled, open-label trial. Eur. J. Integr. Med. 2014, 14, 5–11. [Google Scholar] [CrossRef]
- Spruit, M.A. An official American Thoracic Society/European Respiratory Society Statement: Key concepts and advances in pulmonary rehabilitation. Am. J. Respir. Crit. Care Med. 2013, 188, e13–e64. [Google Scholar] [CrossRef]
- Burtin, C.; Decramer, M.; Gosselink, R.; Janssens, W.; Troosters, T. Rehabilitation and acute exacerbations. Eur. Respir. J. 2011, 38, 702–712. [Google Scholar] [CrossRef]
- Ries, A.L.; Bauldoff, G.S.; Carlin, B.W.; Casaburi, R.; Emery, C.F.; Mahler, D.A.; Make, B.; Rochester, C.L.; Zuwallack, R.; Herrerias, C. Pulmonary Rehabilitation: Joint ACCP/AACVPR evidence-based clinical practice guidelines. Chest 2007, 131, 4S–42S. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos Pascotini, F.; de Castro Ramos, M.; da Silva, A.M.V.; Trevisan, M.E. Volume-oriented versus flow-oriented incentive spirometry over respiratory parameters among the elderly. Fisioter Pesq 2013, 20, 355–360. [Google Scholar]
- Shin, Y.H. The effects of a walking exercise program on physical function and emotional state of elderly Korean women. Public Health Nurs. 1999, 16, 146–154. [Google Scholar] [CrossRef]
- Sum, S.K.; Peng, Y.C.; Yin, S.Y.; Huang, P.F.; Wang, Y.C.; Chen, T.P.; Tung, H.H.; Yeh, C.H. Using an incentive spirometer reduces pulmonary complications in patients with traumatic rib fractures: A randomized controlled trial. Trials 2019, 20, 797. [Google Scholar] [CrossRef]
- Weiner, P.; Weiner, M.; Rabner, M.; Waizman, J.; Magadle, R.; Zamir, D.; Greiff, Y. The effect of incentive spirometer and inspiratory muscle training on pulmonary function after lung resection. J. Thorac. Cardiovasc. Surg. 1997, 113, 552–557. [Google Scholar] [CrossRef]
- Church, T.S.; Earnest, C.P.; Morss, G.M. Field testing of physiological responses to Nordic walking. Res. Q. Exerc. Sport 2002, 73, 296–300. [Google Scholar] [CrossRef]
- Narcis, G.; Hernández-Mocholí, M.A.; Olivares, P.R. Changes in HRQoL after 12 months of exercise linked to primary care are associated with fitness effect in older adults. Eur. J. Public Health 2015, 25, 873–879. [Google Scholar]
- Irez, G.B. The effects of different exercises on balance, fear and risk of falling among adults aged 65 and over. Anthropologist 2014, 8, 129–134. [Google Scholar] [CrossRef]
- Awick, E.A.; Wójcicki, T.R.; Olson, E.A.; Fanning, J.; Chung, H.D.; Zuniga, K.; Mackenzie, M.; Kramer, A.F. McAuley, E. Differential exercises effects on quality of life and health-related quality of life in older adults: A randomized controlled trial. Qual. Life Res. 2015, 24, 455–462. [Google Scholar] [CrossRef]
- United Nations Department of Economic and Social Affairs. Population Division. World Population Prospects: The 2017 Revision: Key Findings and Advance Tables. 2017. Available online: https://population.un.org/wpp/publications/files/wpp2017_keyfindings.pdf (accessed on 10 May 2022).
- Graham, B.L.; Steenbruggen, I.; Miller, M.R.; Barjaktarevic, I.Z.; Cooper, B.G.; Hall, G.L.; Hallstrand, T.S.; Kaminsky, D.A.; McCarthy, K.; McCormack, M.C.; et al. Standardization of Spirometry 2019 Update. An Official American Thoracic Society and European Respiratory Society Technical Statement. Am. J. Respir. Crit. Care Med. 2019, 200, e70–e88. [Google Scholar] [CrossRef]
- Rous, M.R.G.; Lobato, S.D.; Trigo, G.R.; Vélez, F.M.; San Miguel, M.; Cejudo, P.; Ruiz, F.O.; Munoz, A.; Iturri, J.B.G.; García, A. Pulmonary rehabilitation. Arch. De Bronconeumol. (Engl. Ed.) 2014, 50, 332–344. [Google Scholar] [CrossRef]
| Database | Keywords | Total Number of Results | Number of Articles in English Published between 2002–2022 |
|---|---|---|---|
| Web of Science | ALL = (elderly) OR ALL = (seniors) OR ALL = (aged) OR ALL = (“older adults”) AND ALL = (walking) OR ALL = (“aerobic training”) AND AB = (spirometry) OR AB = (FVC) OR AB = (FEV1) OR AB = (“lung function”) AND ALL = (“randomized controlled trial”) OR ALL = (RCT) OR ALL = (randomized) | 248 | 202 |
| PubMed | (elderly) OR (seniors) OR (aged) OR (“older adults”) AND (walking) OR (“aerobic training”) AND (spirometry [Title/Abstract]) OR (FVC[Title/Abstract]) OR (FEV1[Title/Abstract]) OR (“lung function”[Title/Abstract]) AND (“randomized controlled trial”) OR (RCT) OR (randomized) | 390 | 290 |
| Scopus | ALL (elderly) OR ALL (seniors) OR ALL (aged) OR ALL (“older adults”) AND ALL (walking) OR ALL (“aerobic training”) AND ABS (spirometry) OR ABS (fvc) OR ABD (fev1) OR ABS (“lung function”) AND (ALL (“randomized controlled trial”) OR ALL (rct) OR ALL (randomized) | 1652 | 1279 |
| EBSCO Essentials | “AND elderly OR seniors OR aged OR “older adults” AllFields AND walking OR “aerobic training” AllFields AND spirometry OR FVC OR FEV1 OR “lung function” Abstract AND “randomized controlled trial” OR RCT OR randomized AllFields“ | 669 | 634 |
| PICOS | Inclusion Criteria | Exclusion Criteria |
|---|---|---|
| Population | Studies including seniors aged 60 and above | Studies not including seniors aged 60 and above |
| Intervention | Different types of walking | Studies not including different types of walking as intervention |
| Comparator | Studies including a comparison group (control group, placebo group) | NA |
| Measured outcome | Spirometry | Studies not including spirometry |
| Study design | RCT | Cross-over studies, reviews, non-randomized controlled trials, preliminary studies, studies with no control group |
| PICOS | Keywords |
|---|---|
| P | (“elderly” OR “seniors” OR “aged” OR “older adults”) |
| I | (“walking” OR “aerobic training”) |
| C | NA |
| O | (“spirometry” OR “FVC“ OR “FEV1” OR “lung function”) |
| S | (randomized controlled “trial” OR “RCT” OR “randomized” |
| First Author | Fragoso | Huang | Saygin | Šokeliene | Shim | Kuo | El Kader |
|---|---|---|---|---|---|---|---|
| Year | 2016 | 2005 | 2015 | 2011 | 2019 | 2018 | 2013 |
| N | 1635 | 45 | 30 | 41 | 30 | 36 | 40 |
| Female | 1098 | not stated | 30 | 30 | not stated | 30 | 20 |
| Male | 537 | not stated | 0 | 11 | not stated | 6 | 20 |
| Mean Age (±) | 78.7 (5.2) I.G. 79.1 (5.2) C.G. | 85.3 (2.5) 82. (3.1) I.G. 82.9 (3.0) C.G. | 74.28 (8.78) I.G. 75.53 (7.21) C.G. | 65 (5) I.G. 65(5) C.G. | 75.33 (5.31) I.G. 74.40 (3.24) C.G. | 68.93 (3.81) I.G. 70.38 (5.22) C.G. | 67.27 (5.05) I.G. 69,18 (4.13) C.G. |
| Intervention | Structured physical activity | High/ moderate intensity exercise program | Long-term regular walking | Nordic walking | Aerobic training with rhythmic functional movement | Prescribed Stepper walking program | Aerobic Exercise Training + Incentive Spirometry |
| Duration | 24–42 months | 10 weeks | 6 months | 12 weeks | 8 weeks | 8 weeks | 3 months |
| Comparison Group | Control group | Control group | Control group | Control group | Control group | Control group | Control group |
| Outcome Measurement | FEV1, maximal inspiratory pressure (MIP), exacerbation of obstructive airways disease (EOAD) | FVC, FEV1, diastolic blood pressure, systolic blood pressure, resting heart rate, flexibility, reaction time, height, weight | FVC, Sit and Reach test, handgrip strength, Get Up and Go test, physical activity level, body fat percentage, height, weight | VC, aerobic endurance, calf endurance, Sit and Reach test, weight, height, waist/hip ratio | FVC, FEV1, maximal voluntary ventilation (MVV), The functional movement screen (FMS), quality of life (SF-36) | FVC, FEV1, 6MWT, fatigue intensity, extremity muscle power, BMI, MMSE, Barthel index, IADL scale, GDS-15, waist/hip ratio, body fat %, handgrip/body weight ratio | VC, FEV1, MVV, PImax., PEmax., inspiratory muscle endurance |
| Study Design | RCT | RCT | RCT | RCT | RCT | RCT | RCT |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Novotová, K.; Pavlů, D.; Dvořáčková, D.; Arnal-Gómez, A.; Espí-López, G.V. Influence of Walking as Physiological Training to Improve Respiratory Parameters in the Elderly Population. Int. J. Environ. Res. Public Health 2022, 19, 7995. https://doi.org/10.3390/ijerph19137995
Novotová K, Pavlů D, Dvořáčková D, Arnal-Gómez A, Espí-López GV. Influence of Walking as Physiological Training to Improve Respiratory Parameters in the Elderly Population. International Journal of Environmental Research and Public Health. 2022; 19(13):7995. https://doi.org/10.3390/ijerph19137995
Chicago/Turabian StyleNovotová, Klára, Dagmar Pavlů, Dominika Dvořáčková, Anna Arnal-Gómez, and Gemma Victoria Espí-López. 2022. "Influence of Walking as Physiological Training to Improve Respiratory Parameters in the Elderly Population" International Journal of Environmental Research and Public Health 19, no. 13: 7995. https://doi.org/10.3390/ijerph19137995
APA StyleNovotová, K., Pavlů, D., Dvořáčková, D., Arnal-Gómez, A., & Espí-López, G. V. (2022). Influence of Walking as Physiological Training to Improve Respiratory Parameters in the Elderly Population. International Journal of Environmental Research and Public Health, 19(13), 7995. https://doi.org/10.3390/ijerph19137995







