Biodecolorization and Ecotoxicity Abatement of Disperse Dye-Production Wastewater Treatment with Pycnoporus Laccase
Abstract
:1. Introduction
2. Materials and Methods
2.1. Laccase, Chemicals, Wheat Seed, and Activated Sludge
2.2. Measurement of Laccase Activity
2.3. Decolorization of Disperse Dyes
2.4. Seed Germination and Growth Experiment
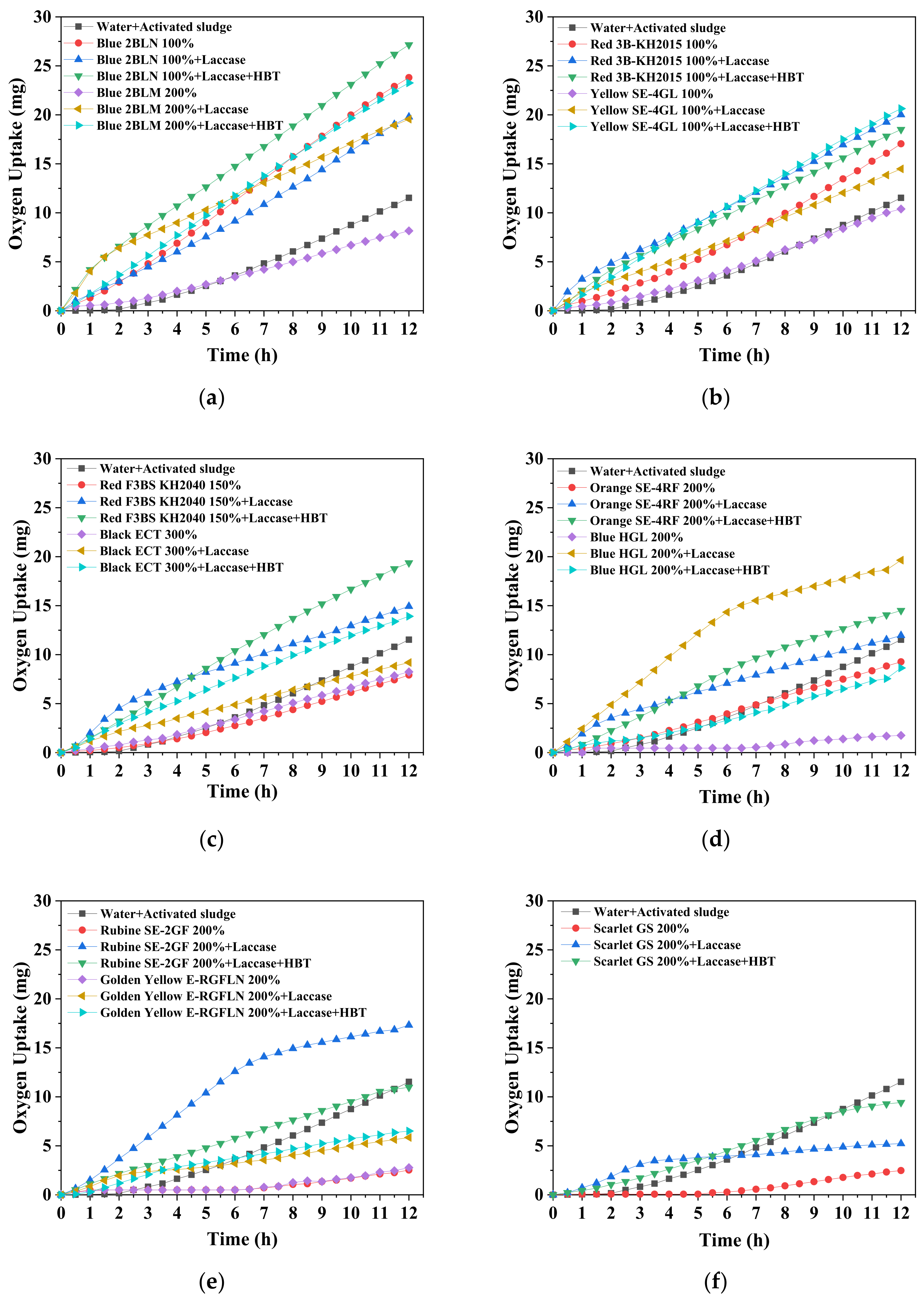
2.5. Oxygen Uptake by Aerobic Activated Sludge (AS)
2.6. Statistical Analyses
3. Results
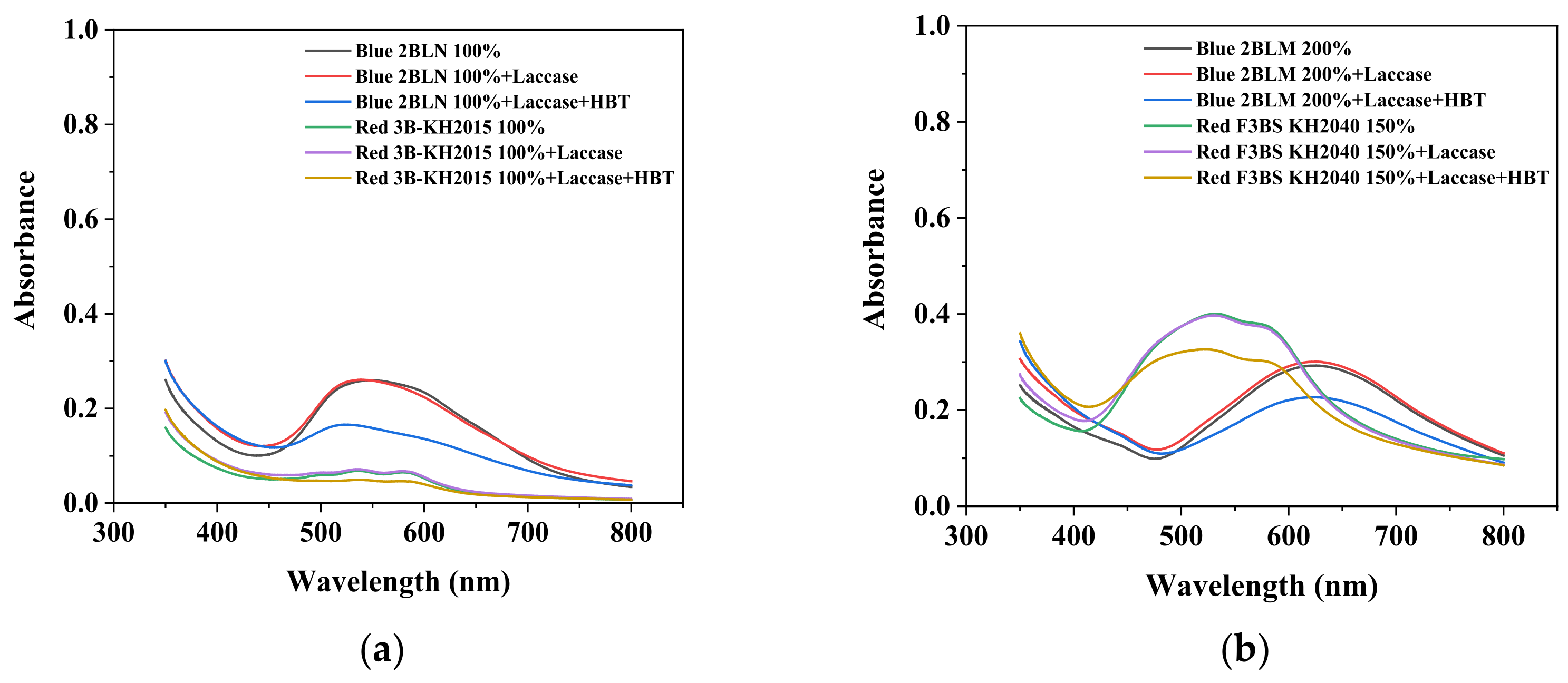
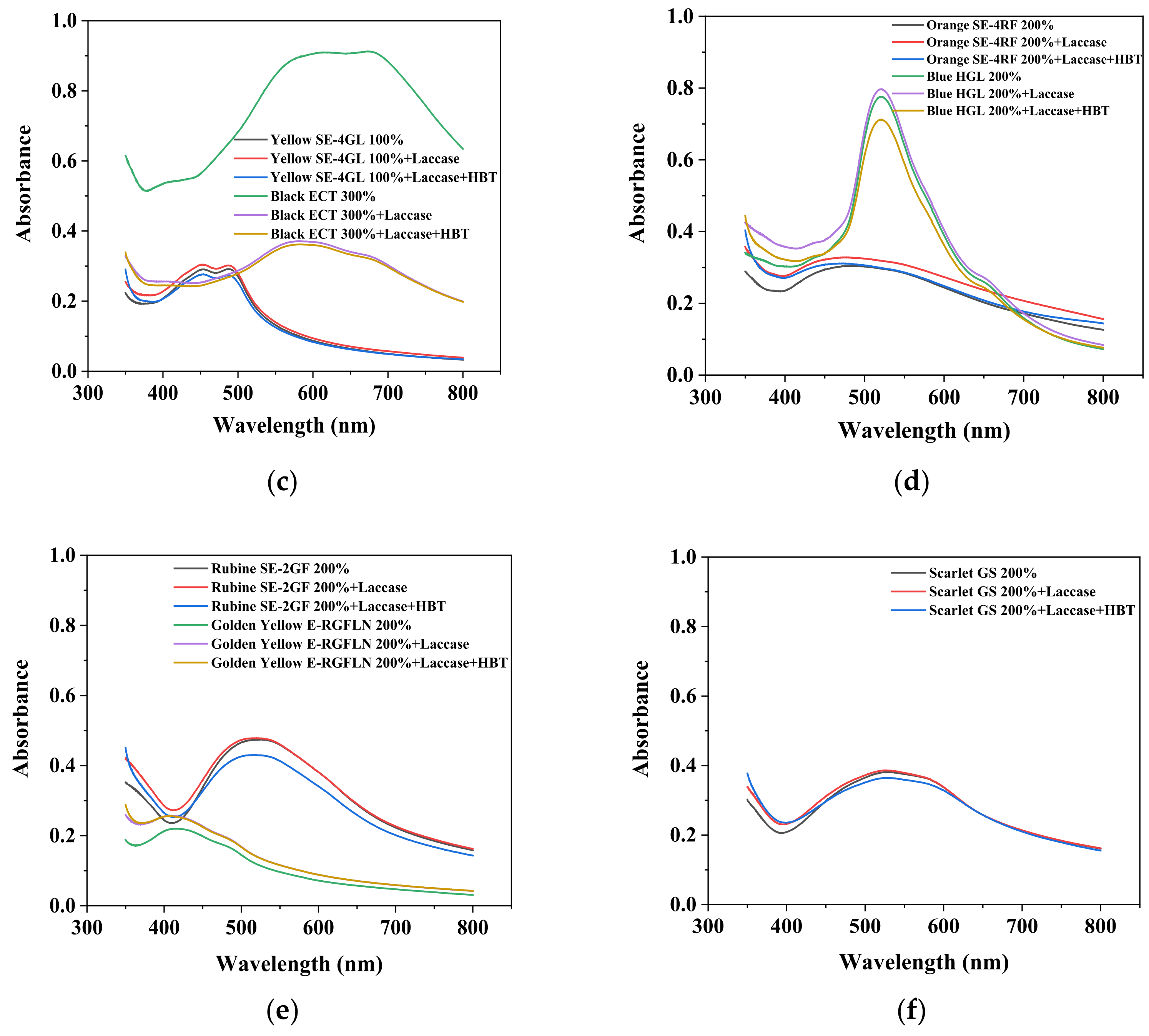
3.1. Visible Absorption Spectrum of Disperse Dyes
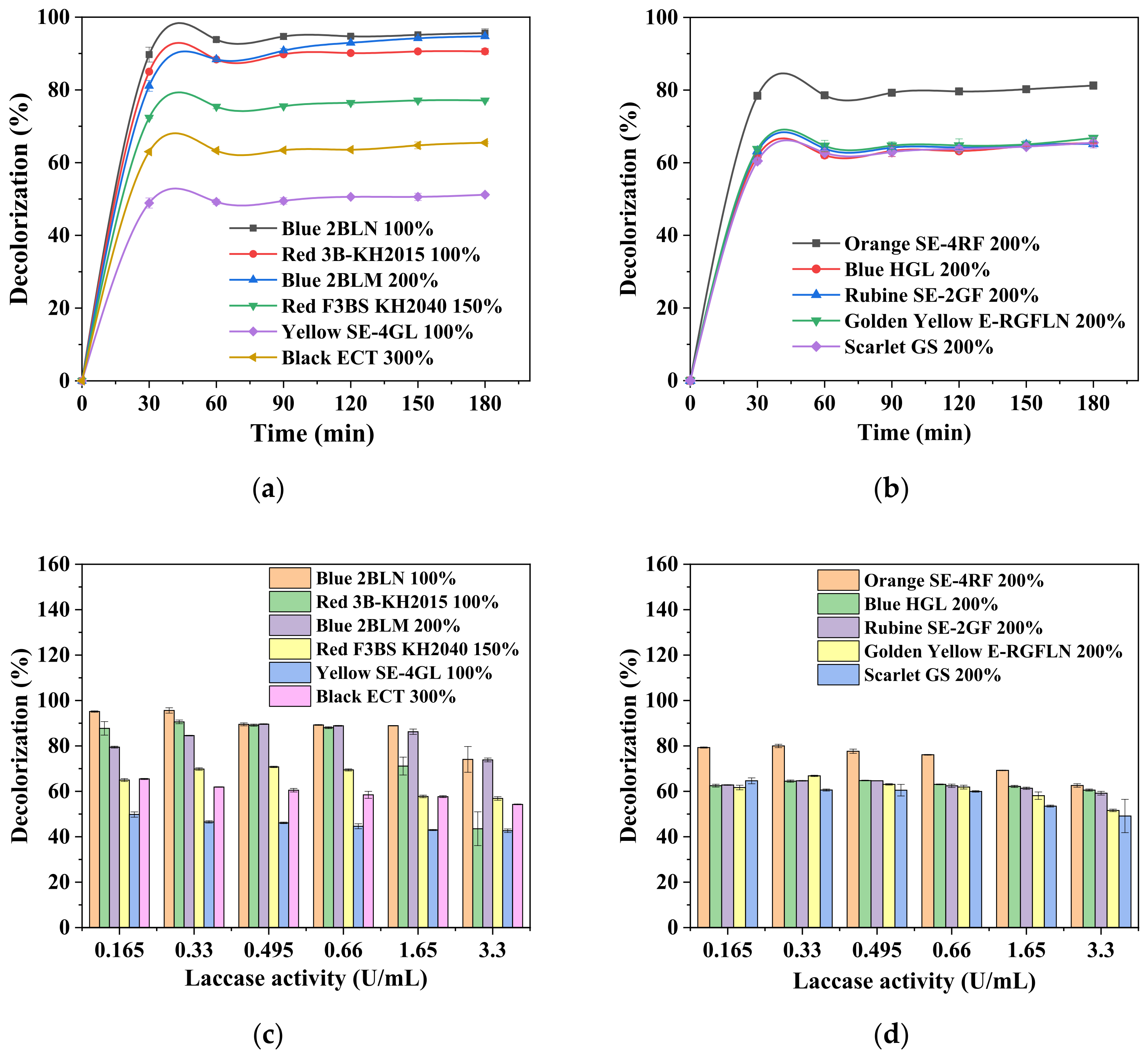
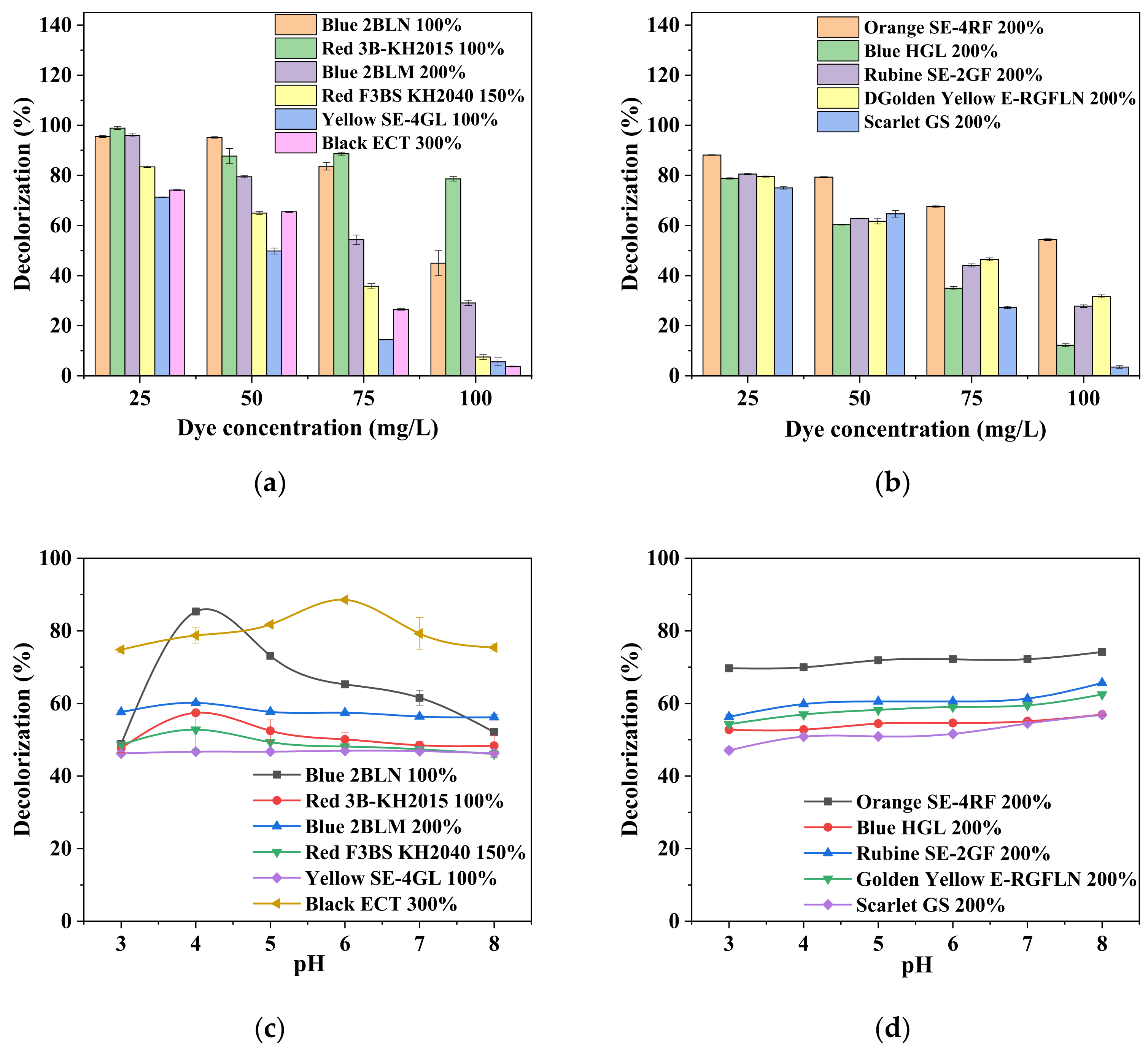
3.2. Effect of Different Parameters on Decolorization Rate
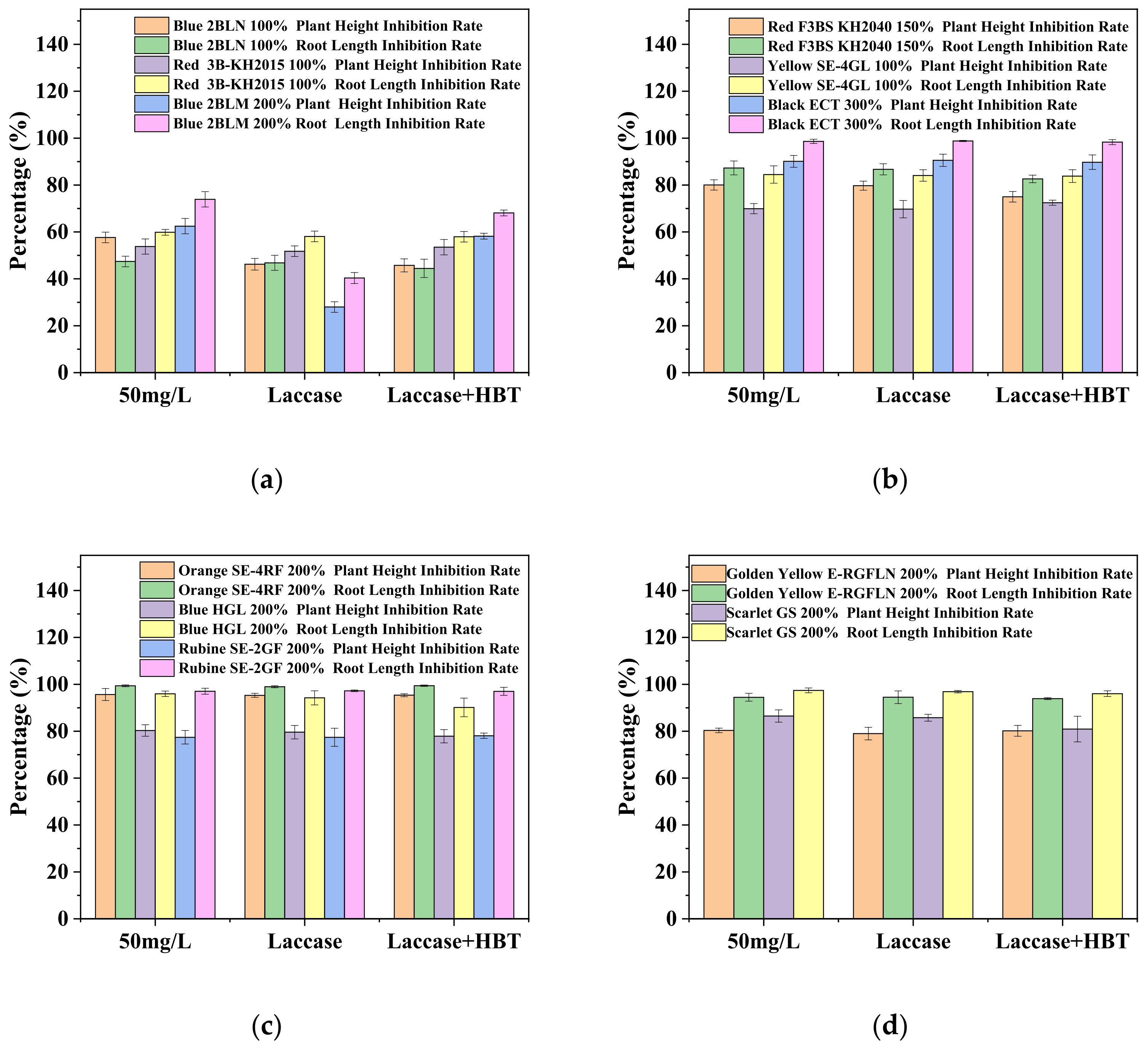
3.3. Wheat Seed Germination and Growth in Different Dye Solution
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tkaczyk, A.; Mitrowska, K.; Posyniak, A. Synthetic organic dyes as contaminants of the aquatic environment and their implications for ecosystems: A review. Sci. Total Environ. 2020, 717, 137222. [Google Scholar] [CrossRef] [PubMed]
- Das, A.; Mishra, S. Removal of textile dye reactive green-19 using bacterial consortium: Process optimization using response surface methodology and kinetics study. J. Environ. Chem. Eng. 2017, 5, 612–627. [Google Scholar] [CrossRef]
- Croce, R.; Cinà, F.; Lombardo, A.; Crispeyn, G.; Cappelli, C.I.; Vian, M.; Maiorana, S.; Benfenati, E.; Baderna, D. Aquatic toxicity of several textile dye formulations: Acute and chronic assays with Daphnia magna and Raphidocelis subcapitata. Ecotoxicol. Environ. Saf. 2017, 144, 79–87. [Google Scholar] [CrossRef]
- Cui, M.-H.; Cui, D.; Gao, L.; Wang, A.-J.; Cheng, H.-Y. Azo dye decolorization in an up-flow bioelectrochemical reactor with domestic wastewater as a cost-effective yet highly efficient electron donor source. Water Res. 2016, 105, 520–526. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Ning, X.-A.; Sun, J.; Song, J.; Lu, J.; Cai, H.; Hong, Y. Toxicity evaluation of textile dyeing effluent and its possible relationship with chemical oxygen demand. Ecotoxicol. Environ. Saf. 2018, 166, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Coria-Oriundo, L.L.; Battaglini, F.; Wirth, S.A. Efficient decolorization of recalcitrant dyes at neutral/alkaline pH by a new bacterial laccase-mediator system. Ecotoxicol. Environ. Saf. 2021, 217, 112237. [Google Scholar] [CrossRef] [PubMed]
- Criado, S.P.; Gonçalves, M.J.; Tavares, L.B.B.; Bertoli, S.L. Optimization of electrocoagulation process for disperse and reactive dyes using the response surface method with reuse application. J. Clean. Prod. 2020, 275, 122690. [Google Scholar] [CrossRef]
- Kishor, R.; Purchase, D.; Saratale, G.D.; Saratale, R.G.; Ferreira, L.F.R.; Bilal, M.; Chandra, R.; Bharagava, R.N. Ecotoxicological and health concerns of persistent coloring pollutants of textile industry wastewater and treatment approaches for environmental safety. J. Environ. Chem. Eng. 2021, 9, 105012. [Google Scholar] [CrossRef]
- Iark, D.; Buzzo, A.J.D.R.; Garcia, J.A.A.; Côrrea, V.G.; Helm, C.V.; Corrêa, R.C.G.; Peralta, R.A.; Moreira, R.D.F.P.M.; Bracht, A.; Peralta, R.M. Enzymatic degradation and detoxification of azo dye congo red by a new laccase from Oudemansiella canarii. Bioresour. Technol. 2019, 289, 121655. [Google Scholar] [CrossRef]
- Ali, H. Biodegradation of synthetic dyes—A review. Water Air Soil Pollut. 2010, 213, 251–273. [Google Scholar] [CrossRef]
- Choi, K.-Y. Discoloration of indigo dyes by eco-friendly biocatalysts. Dye. Pigment. 2020, 184, 108749. [Google Scholar] [CrossRef]
- Baldrian, P. Fungal laccases—Occurrence and properties. FEMS Microbiol. Rev. 2006, 30, 215–242. [Google Scholar] [CrossRef] [Green Version]
- Vilar, D.D.S.; Bilal, M.; Bharagava, R.N.; Kumar, A.; Nadda, A.K.; Salazar-Banda, G.R.; Eguiluz, K.I.B.; Ferreira, L.F.R. Lignin-modifying enzymes: A green and environmental responsive technology for organic compound degradation. J. Chem. Technol. Biotechnol. 2021, 97, 327–342. [Google Scholar] [CrossRef]
- Claus, H.; Faber, G.; König, H. Redox-mediated decolorization of synthetic dyes by fungal laccases. Appl. Microbiol. Biotechnol. 2002, 59, 672–678. [Google Scholar] [CrossRef] [PubMed]
- Husain, M.; Husain, Q. Applications of redox mediators in the treatment of organic pollutants by using oxidoreductive enzymes: A review. Crit. Rev. Environ. Sci. Technol. 2007, 38, 1–42. [Google Scholar] [CrossRef]
- Rybczyńska-Tkaczyk, K.; Korniłłowicz-Kowalska, T.; Szychowski, K.; Gmiński, J. Biotransformation and toxicity effect of monoanthraquinone dyes during Bjerkandera adusta CCBAS 930 cultures. Ecotoxicol. Environ. Saf. 2020, 191, 110203. [Google Scholar] [CrossRef]
- Yagub, M.T.; Sen, T.K.; Afroze, S.; Ang, H.M. Dye and its removal from aqueous solution by adsorption: A review. Adv. Colloid Interface Sci. 2014, 209, 172–184. [Google Scholar] [CrossRef]
- Becker, D.; Della Giustina, S.V.; Rodriguez-Mozaz, S.; Schoevaart, R.; Barceló, D.; De Cazes, M.; Belleville, M.-P.; Sanchez-Marcano, J.; De Gunzburg, J.; Couillerot, O.; et al. Removal of antibiotics in wastewater by enzymatic treatment with fungal laccase—Degradation of compounds does not always eliminate toxicity. Bioresour. Technol. 2016, 219, 500–509. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, M.; Shah, K.; Kalra, S.S.; Rome, L.H.; Mahendra, S. Decolorization and detoxification of synthetic dye compounds by laccase immobilized in vault nanoparticles. Bioresour. Technol. 2022, 351, 127040. [Google Scholar] [CrossRef]
- Novotný, C.; Dias, N.; Kapanen, A.; Malachová, K.; Vandrovcova, M.; Itävaara, M.; Lima, N. Comparative use of bacterial, algal and protozoan tests to study toxicity of azo- and anthraquinone dyes. Chemosphere 2006, 63, 1436–1442. [Google Scholar] [CrossRef] [Green Version]
- Routoula, E.; Patwardhan, S.V. Degradation of anthraquinone dyes from effluents: A review focusing on enzymatic dye degradation with industrial potential. Environ. Sci. Technol. 2020, 54, 647–664. [Google Scholar] [CrossRef]
- Penthala, R.; Park, S.H.; Oh, H.; Lee, I.Y.; Ko, E.H.; Son, Y.-A. An ecofriendly dyeing of nylon and cotton fabrics in supercritical CO2 with novel tricyanopyrrolidone reactive disperse dye. J. CO2 Util. 2022, 60, 102004. [Google Scholar] [CrossRef]
- Liu, J.; Yu, Z.; Liao, X.; Liu, J.; Mao, F.; Huang, Q. Scalable production, fast purification, and spray drying of native Pycnoporus laccase and circular dichroism characterization. J. Clean. Prod. 2016, 127, 600–609. [Google Scholar] [CrossRef]
- Abadulla, E.; Tzanov, T.; Costa, S.; Robra, K.-H.; Cavaco-Paulo, A.; Gübitz, G.M. Decolorization and detoxification of textile dyes with a laccase from Trametes hirsuta. Appl. Environ. Microbiol. 2000, 66, 3357–3362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsu, C.-A.; Wen, T.-N.; Su, Y.-C.; Jiang, Z.-B.; Chen, C.-W.; Shyur, L.-F. Biological degradation of anthroquinone and azo dyes by a novel laccase from Lentinus sp. Environ. Sci. Technol. 2012, 46, 5109–5117. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Huang, F.; Yu, Y.; Jiang, Y.; Zhao, K.; He, Y.; Xu, Y.; Zhang, Y. Determination and toxicity evaluation of the generated byproducts from sulfamethazine degradation during catalytic oxidation process. Chemosphere 2019, 226, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.; He, M.; Song, L.; Fu, X.; Shi, S. Aerobic decolorization, degradation and detoxification of azo dyes by a newly isolated salt-tolerant yeast Scheffersomyces spartinae TLHS-SF1. Bioresour. Technol. 2016, 203, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.L.; Singh, P.K.; Singh, R.P. Enzymatic decolorization and degradation of azo dyes—A review. Int. Biodeterior. Biodegrad. 2015, 104, 21–31. [Google Scholar] [CrossRef]
- Debnath, R.; Mistry, P.; Roy, P.; Roy, B.; Saha, T. Partial purification and characterization of a thermophilic and alkali-stable laccase of Phoma herbarum isolate KU4 with dye-decolorization efficiency. Prep. Biochem. Biotechnol. 2021, 51, 901–918. [Google Scholar] [CrossRef] [PubMed]
- Espina, G.; Cáceres-Moreno, P.; Mejías-Navarrete, G.; Ji, M.; Sun, J.; Blamey, J.M. A novel and highly active recombinant spore-coat bacterial laccase, able to rapidly biodecolorize azo, triarylmethane and anthraquinonic dyestuffs. Int. J. Biol. Macromol. 2020, 170, 298–306. [Google Scholar] [CrossRef]
- Zimbardi, A.L.R.L.; Camargo, P.F.; Carli, S.; Neto, S.A.; Meleiro, L.P.; Rosa, J.C.; De Andrade, A.R.; Jorge, J.A.; Furriel, R.P.M. A high redox potential laccase from Pycnoporus sanguineus RP15: Potential application for dye decolorization. Int. J. Mol. Sci. 2016, 17, 672. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mechichi, T.; Mhiri, N.; Sayadi, S. Remazol brilliant blue R decolourization by the laccase from Trametes trogii. Chemosphere 2006, 64, 998–1005. [Google Scholar] [CrossRef] [PubMed]
- Sen, S.K.; Raut, S.; Bandyopadhyay, P.; Raut, S. Fungal decolouration and degradation of azo dyes: A review. Fungal Biol. Rev. 2016, 30, 112–133. [Google Scholar] [CrossRef]
- Sayahi, E.; Ladhari, N.; Mechichi, T.; Sakli, F. Azo dyes decolourization by the laccase from Trametes trogii. J. Text. Inst. 2015, 107, 1478–1482. [Google Scholar] [CrossRef]
- Morsi, R.; Bilal, M.; Iqbal, H.M.; Ashraf, S.S. Laccases and peroxidases: The smart, greener and futuristic biocatalytic tools to mitigate recalcitrant emerging pollutants. Sci. Total Environ. 2020, 714, 136572. [Google Scholar] [CrossRef]
- Zeng, X.; Cai, Y.; Liao, X.; Zeng, X.; Li, W.; Zhang, D. Decolorization of synthetic dyes by crude laccase from a newly isolated Trametes trogii strain cultivated on solid agro-industrial residue. J. Hazard. Mater. 2011, 187, 517–525. [Google Scholar] [CrossRef]
- Nakamura, Y.; Mtui, G. Biodegradation of endocrine-disrupting phenolic compounds using laccase followed by activated sludge treatment. Biotechnol. Bioprocess Eng. 2003, 8, 294–298. [Google Scholar] [CrossRef]
- Manai, I.; Miladi, B.; El Mselmi, A.; Hamdi, M.; Bouallagui, H. Improvement of activated sludge resistance to shock loading by fungal enzyme addition during textile wastewater treatment. Environ. Technol. 2016, 38, 880–890. [Google Scholar] [CrossRef]
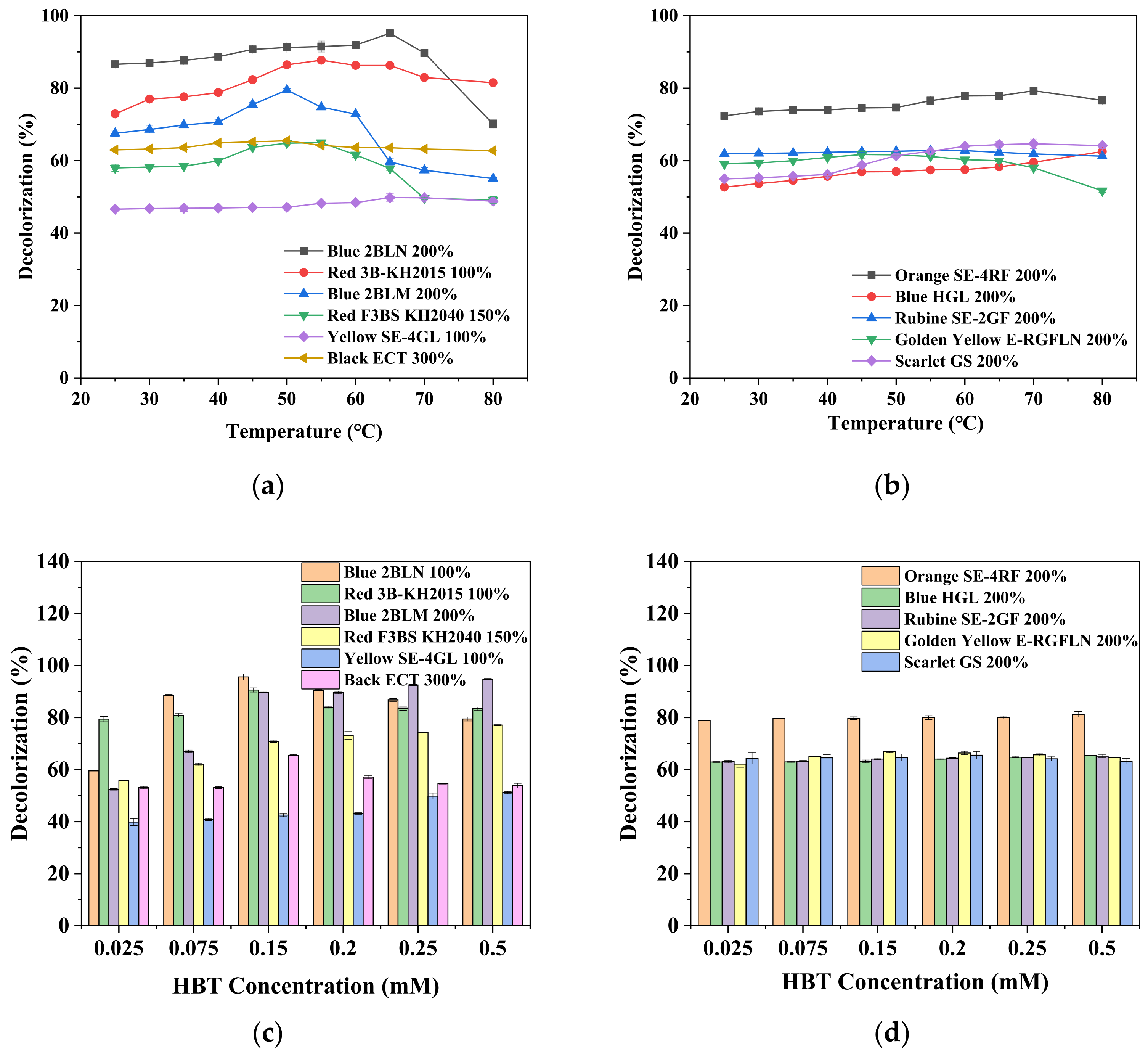
| Dye Name | λmax (nm) | MW (g/mol) | CAS | Chemical Formula | Structure | Classification | Optimal Condition for Decolorization |
|---|---|---|---|---|---|---|---|
| Blue 2BLN 100% | 547 | 304.69 | 12217-79-7 | C14H9ClN2O4 | 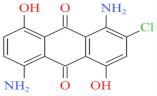 | Anthraquinone | pH 4, 65 °C, laccase 0.33 U/mL, HBT 0.15 mM, decolorization 95.6% |
| Red 3B-KH2015 100% | 583 | / | / | / | / | / | pH 4, 55 °C, laccase 0.33 U/mL, HBT 0.15 mM, decolorization 90.6% |
| Blue 2BLM 200% | 629 | / | / | / | / | / | pH 4, 50 °C, laccase 0.50 U/mL, HBT 0.50 mM, decolorization 94.74% |
| Red F3BS KH2040 150% | 526 | / | / | / | / | / | pH 4, 55 °C, laccase 0.50 U/mL, HBT 0.50 mM, decolorization 77.1% |
| Yellow SE-4GL 100% | 486 | / | / | / | / | / | pH 6, 65 °C, laccase 0.17 U/mL, HBT 0.50 mM, decolorization 51.2% |
| Black ECT 300% | 580 | / | / | / | / | / | pH 6, 50 °C, laccase 0.17 U/mL, HBT 0.15 mM, decolorization 65.5% |
| Orange SE-4RF 200% | 488 | / | / | / | / | / | pH 8, 70 °C, laccase 0.33 U/mL, HBT 0.50 mM, decolorization 81.2% |
| Blue HGL 200% | 520 | 639.41 | 12239-34-8 | C24H27BrN6O10 | 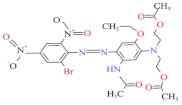 | Azo | pH 8, 80 °C, laccase 0.50 U/mL, HBT 0.50 mM, decolorization 65.4% |
| Rubine SE-2GF 200% | 522 | / | / | / | / | / | pH 8, 55 °C, laccase 0.50 U/mL, HBT 0.50 mM, decolorization 65.2% |
| Golden Yellow E-RGFLN 200% | 417 | 302.33 | 6250-23-3 | C18H14N4O | 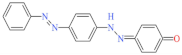 | Azo | pH 8, 45 °C, laccase 0.33 U/mL, HBT 0.15 mM, decolorization 66.8% |
| Scarlet GS 200% | 527 | 404.32 | 78564-87-1 | C18H15Cl2N5S | 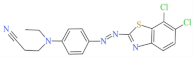 | Azo | pH 8, 70 °C, laccase 0.17 U/mL, HBT 0.20 mM, decolorization 65.5% |
| Dye | Germination Rate (%) | Root Length (cm) | Shoot Length (cm) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Water | Un-Treated | Laccase + HBT | Laccase | Water | Un-Treated | Laccase + HBT | Laccase | Water | Un-Treated | Laccase + HBT | Laccase | |
| Blue 2BLN 100% | 93.3 ± 9.4 | 76.7 ± 4.7 | 80 ± 16.3 | 76.7 ± 4.7 | 3.24 ± 1.1 | 1.45 ± 0.2 | 1.51 ± 0.3 | 1.47 ± 0.1 | 2.23 ± 0.7 | 0.8 ± 0.1 | 0.9 ± 0.3 | 0.95 ± 0.2 |
| Red 3B-KH2015 100% | 96.7 ± 4.7 | 93.3 ± 4.7 | 93.3 ± 4.7 | 96.7 ± 4.7 | 4.33 ± 0.3 | 1.72 ± 0.3 | 1.82 ± 0.1 | 1.82 ± 0.2 | 3.52 ± 0.7 | 1.56 ± 0.6 | 1.57 ± 0.1 | 1.62 ± 0.2 |
| Blue 2BLM 200% | 93.3 ± 4.7 | 83.8 ± 4.7 | 86.7 ± 4.7 | 96.7 ± 4.7 | 4.16 ± 0.4 | 1.13 ± 0.2 | 1.38 ± 0.4 | 2.6 ± 0.4 | 3.34 ± 0.3 | 1.29 ± 0.2 | 1.45 ± 0.2 | 2.47 ± 0.9 |
| Red F3BS KH2040 150% | 100.0 ± 0 | 60.0 ± 8.1 | 66.7 ± 17.0 | 66.3 ± 12.5 | 4.53 ± 1.1 | 0.56 ± 0.1 | 0.72 ± 0.1 | 0.58 ± 0.1 | 4.12 ± 1.5 | 0.71 ± 0.1 | 0.87 ± 0.1 | 0.73 ± 0.1 |
| Yellow SE-4GL 100% | 96.7 ± 4.7 | 90.0 ± 8.2 | 99.3 ± 4.7 | 86.7 ± 4.7 | 4.54 ± 1.2 | 0.66 ± 0.03 | 0.69 ± 0.1 | 0.68 ± 0.03 | 4.36 ± 1.6 | 0.95 ± 0.3 | 0.99 ± 0.2 | 1.12 ± 0.2 |
| Black ECT 300% | 90.0 ± 8.2 | 26.7 ± 9.4 | 36.7 ± 9.4 | 33.3 ± 12.5 | 7.94 ± 2.5 | 0.09 ± 0.03 | 0.11 ± 0.04 | 0.1 ± 0.05 | 8.78 ± 1.0 | 0.83 ± 0.5 | 0.88 ± 0.3 | 0.84 ± 0.3 |
| Orange SE-4RF 200% | 93.3 ± 4.7 | 16.7 ± 4.7 | 23.3 ± 4.7 | 20.0 ± 0.7 | 7.25 ± 2.4 | 0.04 ± 0.02 | 0.04 ± 0.01 | 0.07 ± 0.01 | 8.48 ± 1.7 | 0.39 ± 0.2 | 0.4 ± 0.02 | 0.40 ± 0.03 |
| Blue HGL 200% | 96.7 ± 4.7 | 60.0 ± 0.5 | 66.7 ± 9.3 | 63.3 ± 4.7 | 6.21 ± 1.6 | 0.23 ± 0.03 | 0.55 ± 0.1 | 0.40 ± 0.3 | 6.25 ± 1.2 | 0.95 ± 0.2 | 1.06 ± 0.1 | 1.01 ± 0.2 |
| Rubine SE-2GF 200% | 90.0 ± 8.2 | 50.0 ± 14.1 | 60.0 ± 14.1 | 53.3 ± 18.9 | 6.15 ± 1.0 | 0.16 ± 0.04 | 0.20 ± 0.1 | 0.17 ± 0.05 | 5.10 ± 1.3 | 1.08 ± 0.4 | 1.1 ± 0.5 | 1.09 ± 0.3 |
| Golden Yellow E-RGFLN 200% | 100.0 ± 0 | 70.0 ± 14.1 | 70.0 ± 14.1 | 66.7 ± 20.6 | 7.26 ± 0.1 | 0.42 ± 0.14 | 0.46 ± 0.04 | 0.43 ± 0.2 | 8.01 ± 0.2 | 1.60 ± 0.1 | 1.62 ± 0.2 | 1.73 ± 0.6 |
| Scarlet GS 200% | 100.0 ± 0 | 55.3 ± 12.5 | 63.3 ± 18.9 | 56.7 ± 17.1 | 7.52 ± 0.7 | 0.19 ± 0.07 | 0.29 ± 0.1 | 0.23 ± 0.02 | 8.14 ± 0.4 | 1.09 ± 0.2 | 1.53 ± 0.4 | 1.16 ± 0.2 |
| Dye | Treatment a | Treatment b | Treatment c | |||
|---|---|---|---|---|---|---|
| CODcr (mg/L) Before | CODcr (mg/L) After | CODcr (mg/L) Before | CODcr (mg/L) After | CODcr (mg/L) Before | CODcr (mg/L) After | |
| Blue 2BLN 100% | 112.33 ± 2.05 | 97.67 ± 2.05 | 377 ± 2.94 | 197.67 ± 2.03 | 217 ± 2.16 | 147.67 ± 2.05 |
| Red 3B-KH2015 100% | 416 ± 2.94 | 67.12 ± 2.16 | 77.67 ± 2.06 | 47.67 ± 2.04 | 207 ± 2.45 | 112.33 ± 1.70 |
| Blue 2BLM 200% | 56 ± 2.74 | 77.67 ± 2.05 | 187.67 ± 2.01 | 77.67 ± 2.04 | 289.67 ± 1.25 | 177 ± 2.16 |
| Red F3BS KH2040 150% | 107.67 ± 2.05 | 58 ± 2.16 | 127.66 ± 2.04 | 97.67 ± 1.70 | 292.33 ± 1.70 | 270 ± 1.63 |
| Yellow SE-4GL 100% | 127.33 ± 2.09 | 207.67 ± 1.70 | 177.68 ± 2.09 | 61 ± 0.82 | 270 ± 1.63 | 177 ± 2.16 |
| Black ECT 300% | 147.67 ± 2.07 | 57.33 ± 2.05 | 126.67 ± 2.13 | 102 ± 2.16 | 260.67 ± 1.70 | 138 ± 2.14 |
| Orange SE-4RF 200% | 77.67 ± 2.03 | 67 ± 2.16 | 87.76 ± 2.32 | 77.67 ± 2.05 | 249 ± 2.94 | 188 ± 1.63 |
| Blue HGL 200% | 177.67 ± 2.06 | 70 ± 1.63 | 236.77 ± 2.49 | 108.33 ± 1.70 | 297.67 ± 2.05 | 287.33 ± 2.05 |
| Rubine SE-2GF 200% | 187 ± 2.16 | 56.33 ± 2.62 | 467.32 ± 2.03 | 138.33 ± 1.72 | 279.67 ± 2.87 | 262.33 ± 1.70 |
| Golden Yellow E-RGFLN 200% | 57.67 ± 2.05 | 477.67 ± 2.05 | 137.43 ± 2.07 | 127 ± 2.45 | 116.67 ± 2.87 | 110 ± 1.63 |
| Scarlet GS 200% | 17.67 ± 2.05 | 17.33 ± 1.25 | 87 ± 2.16 | 67 ± 2.16 | 149.33 ± 2.49 | 90.33 ± 1.25 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, B.; Chen, Y.; Guan, J.; Ding, Y.; He, Y.; Zhang, X.; Shukurov, N.; Romanholo Ferreira, L.F.; Liu, J.; Zhu, M. Biodecolorization and Ecotoxicity Abatement of Disperse Dye-Production Wastewater Treatment with Pycnoporus Laccase. Int. J. Environ. Res. Public Health 2022, 19, 7983. https://doi.org/10.3390/ijerph19137983
Wang B, Chen Y, Guan J, Ding Y, He Y, Zhang X, Shukurov N, Romanholo Ferreira LF, Liu J, Zhu M. Biodecolorization and Ecotoxicity Abatement of Disperse Dye-Production Wastewater Treatment with Pycnoporus Laccase. International Journal of Environmental Research and Public Health. 2022; 19(13):7983. https://doi.org/10.3390/ijerph19137983
Chicago/Turabian StyleWang, Bin, Yanjun Chen, Jian Guan, Yiwen Ding, Yide He, Xueying Zhang, Nosir Shukurov, Luiz Fernando Romanholo Ferreira, Jiayang Liu, and Mingxin Zhu. 2022. "Biodecolorization and Ecotoxicity Abatement of Disperse Dye-Production Wastewater Treatment with Pycnoporus Laccase" International Journal of Environmental Research and Public Health 19, no. 13: 7983. https://doi.org/10.3390/ijerph19137983
APA StyleWang, B., Chen, Y., Guan, J., Ding, Y., He, Y., Zhang, X., Shukurov, N., Romanholo Ferreira, L. F., Liu, J., & Zhu, M. (2022). Biodecolorization and Ecotoxicity Abatement of Disperse Dye-Production Wastewater Treatment with Pycnoporus Laccase. International Journal of Environmental Research and Public Health, 19(13), 7983. https://doi.org/10.3390/ijerph19137983








