Barriers and Facilitators of Pharmacoeconomic Studies: A Review of Evidence from the Middle Eastern Countries
Abstract
1. Introduction
2. Materials and Methods
3. Main Findings
3.1. Need for Pharmacoeconomic Studies
- It provides an assessment of adverse drug reactions, thus aiding efficient pharmacovigilance and reducing negative consequences concerning national health systems.
- It gives a broad insight into a drug or disease’s medical and financial implications.
- Optimizing budget utilization is needed to provide the best possible treatments for a particular disease without putting a heavy cost-related burden on the patients.
- It helps to find alternative treatment plans that are cheaper and more effective for diseases.
- It reveals that newer drugs could be more cost-effective and therapeutically efficient than the overused older ones or Standard of Care (SC) treatment methods.
- It aids in good prescription practice, allowing physicians to prescribe more beneficial and cost-effective medicines that are very favorable for patients.
- It helps in including new drugs in reimbursement and insurance schemes.
- It allows drug price evaluation and highlights the need to fix the price of an existing drug or set the price of a new drug in an optimized way.
- At the industrial level, pharmacoeconomics is required to evaluate the cost of drug formulation as an input and its comparison with the output, i.e., how profitable the drug is in terms of therapeutic efficiency.
- Medication prescribing can be optimized concerning Efficacy, Suitability, Price and Safety (ESPS), with the help of cost-analysis studies.
- It allows effective formulary management by aiding the decision-making process of policies by Pharmacy and Therapeutics (P&Ts) Committees.
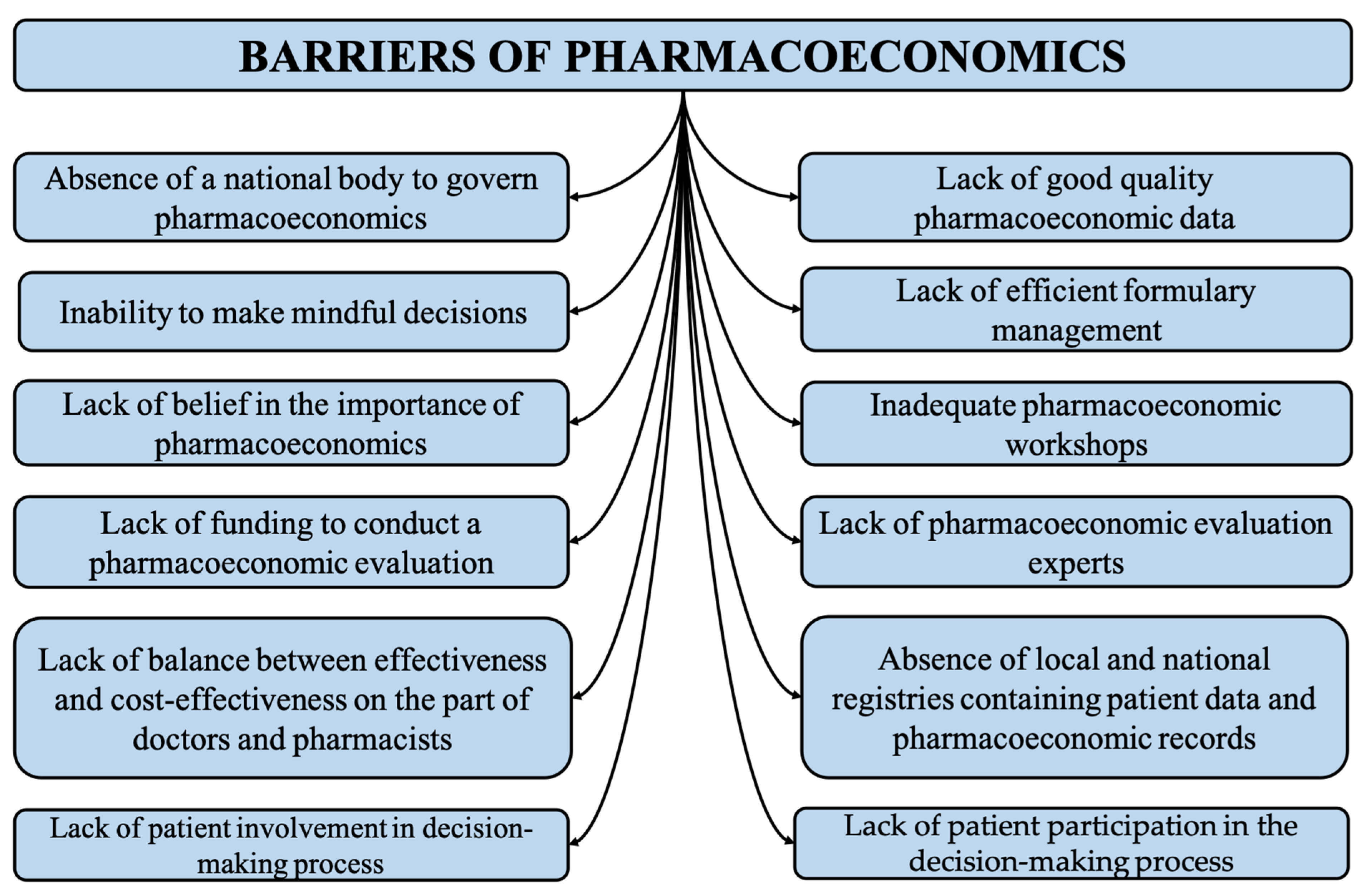
3.2. Barriers to Pharmacoeconomic Studies
3.2.1. Absence of a National Body to Govern Pharmacoeconomics
3.2.2. Lack of Balance between Effectiveness and Cost-Effectiveness on the Part of Doctors and Pharmacists
3.2.3. Absence of Local and National Registries Containing Patient Data and Pharmacoeconomic Records
3.2.4. Lack of Funding to Conduct a Pharmacoeconomic Evaluation
3.2.5. Lack of Good Quality Pharmacoeconomic Data
3.2.6. Inadequate Pharmacoeconomic Workshops
3.2.7. Lack of Belief in the Importance of Pharmacoeconomics
3.2.8. Inability to Make Conscious Decisions
3.2.9. Lack of Pharmacoeconomic Evaluation Experts
3.2.10. Lack of Patient Participation in the Decision-Making Process
3.2.11. Lack of Efficient Formulary Management
3.2.12. Lack of Public Awareness Regarding the Importance of Pharmacoeconomics
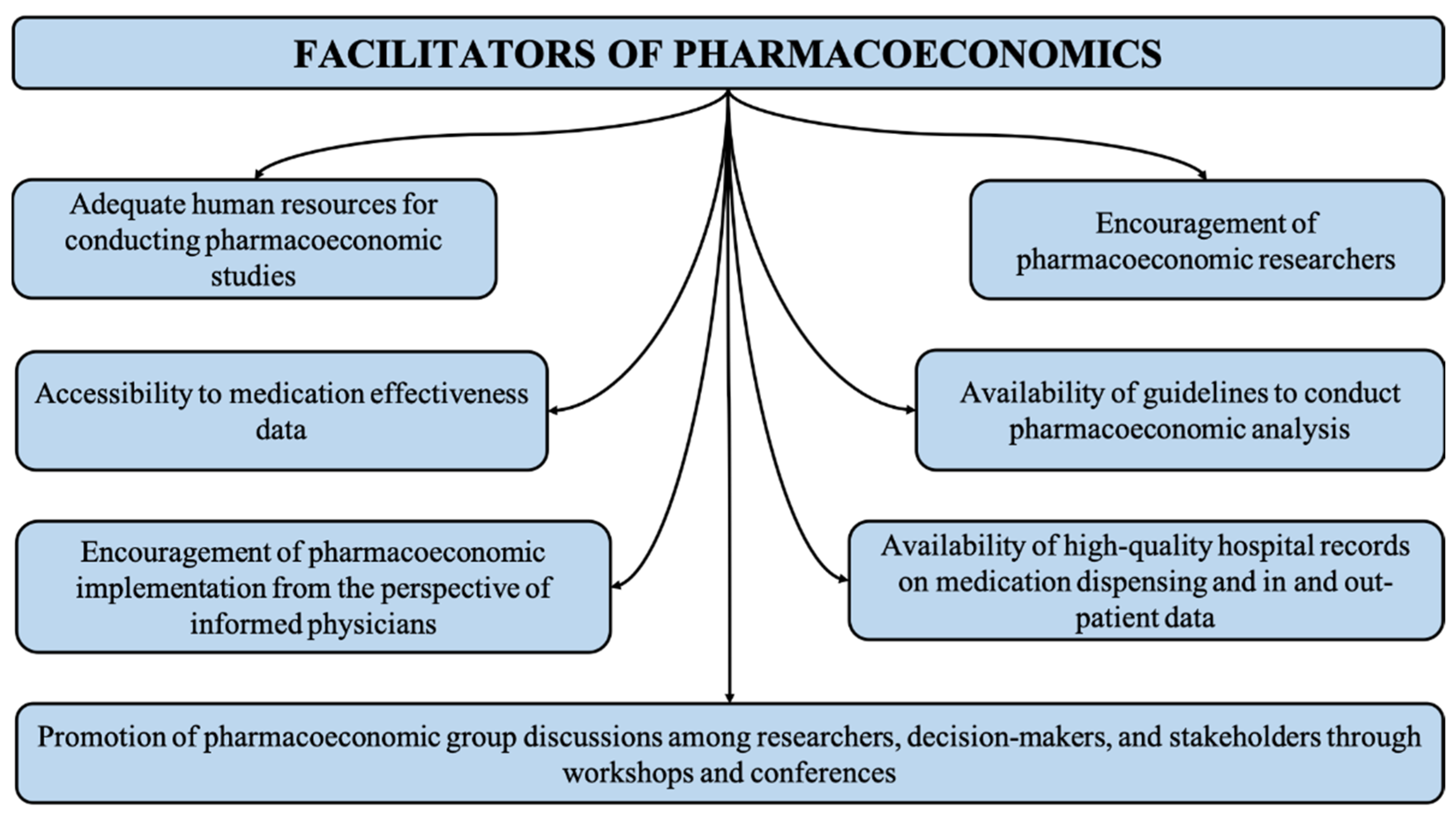
3.3. Facilitators of Pharmacoeconomic Studies
3.3.1. Accessibility to Medication Effectiveness Data
3.3.2. Availability of Guidelines for Conducting Pharmacoeconomic Analysis
3.3.3. Encouragement of Pharmacoeconomic Researchers
3.3.4. Adequate Human Resources for Conducting Pharmacoeconomic Studies
3.3.5. Promotion of Pharmacoeconomic Group Discussions among Researchers, Decision-Makers and Stakeholders through Workshops and Conferences
3.3.6. Availability of High-Quality Hospital Records on Medication Dispensing and In- and Out-Patient Data
3.3.7. Encouragement of Pharmacoeconomic Implementation from the Perspective of Informed Physicians
4. Discussion of Pharmacoeconomic Studies with Middle Eastern Countries
5. Recommendations for Implementation
- The availability of suitable guidelines for formulary management, pharmacoeconomic research and the use of pharmacoeconomic tools must be ensured at the local and national levels. These guidelines should also compare the therapeutic efficiency of drugs and their cost-effectiveness compared to similar drugs [64].
- Effective application of pharmacoeconomic studies must be done in pharmacy schools and other health schools implementing pharmaceutical studies in their curricula. Students should be aware of the importance of pharmacoeconomics for healthcare systems. Workshops and seminars can do this in institutes to develop the interest of the discipline and bring forward more specialists and experts in the field to carry out research and apply pharmacoeconomic tools to healthcare data [65].
- Keeping efficient health data records must be compulsory for all hospitals at the government and private levels. This is highly important because the availability of high-quality medication data is the biggest facilitator of the implementation of pharmacoeconomics. Governments should establish minimum data set requirements and form committees that evaluate medical records and data sets and hold the authorities responsible for poor health records [42].
- Pharmacoeconomic workshops and awareness campaigns should be conducted at the local and national levels, which can also be extended to international collaborations if funds are available. In this way, the importance of pharmacoeconomics can be communicated to young researchers and healthcare professionals.
- There should be effective coordination between health policymakers and researchers so that discrepancies in the system can be conveyed and new and better policies can be devised to overcome barriers to implementing pharmacoeconomics.
- States should establish and develop national organizations and agencies responsible for governing pharmacoeconomic implementation and making all the necessary decisions and actions necessary to make good use of pharmacoeconomic analyses in healthcare systems.
- Recordkeeping of all medical data should be maintained, including the cost of treatment, duration of treatment, lifestyle impacts of a disease and its therapy, epidemiological and demographical medical data and patterns of disease occurrence and clinical practice of various therapies [66].
- National and local pharmacoeconomic organizations should publish the actual costs of medication and healthcare services for specific therapies and medical procedures to set up a standard for pharmacoeconomic studies and aid pharmacoeconomic researchers by providing high-quality cost data.
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ahmad, A.; Patel, I.; Parimilakrishnan, S.; Mohanta, G.P.; Chung, H.; Chang, J. The role of pharmacoeconomics in current Indian healthcare system. J. Res. Pharm. Pract. 2013, 2, 3. [Google Scholar] [CrossRef] [PubMed]
- Drummond, M.F.; Sculpher, M.J.; Claxton, K.; Stoddart, G.L.; Torrance, G.W. Methods for the Economic Evaluation of Health Care Programmes; Oxford University Press: Oxford, UK, 2015. [Google Scholar]
- Ahuja, J.; Gupta, M.; Gupta, A.K.; Kohli, K. Pharmacoeconomics. Natl. Med. J. India 2004, 17, 80–83. [Google Scholar] [PubMed]
- Barnett, M.L.; Mehrotra, A.; Landon, B.E. COVID-19 and the upcoming financial crisis in health care. NEJM Catal. Innov. Care Deliv. 2020, 1. [Google Scholar]
- IMS Institute for Healthcare Informatics. Global Medicines Use 2020; IMS Institute for Healthcare Informatics: Parsippany, NJ, USA, 2020. [Google Scholar]
- Rachmania, I.N.; Basri, M.H. Pharmaceutical inventory management issues in hospital supply chains. Management 2013, 3, 1–5. [Google Scholar]
- Leahy, J.; Hickey, C.; McConnell, D.; Cassidy, O.; Trela-Larsen, L.; Barry, M.; Tilson, L.; McCullagh, L.; Group, N.R. Coronavirus Disease 2019: Considerations for Health Technology Assessment from the National Centre for Pharmacoeconomics Review Group; Elsevier: Amsterdam, The Netherlands, 2020; pp. 1423–1426. [Google Scholar]
- Zaidi, S.T.R.; Babar, Z.-U.-D. Applying pharmacoeconomics in community and hospital pharmacy research. In Pharmacy Practice Research Methods; Springer: Cham, Switzerland, 2015; pp. 157–173. [Google Scholar]
- Iram, M.; Hiremath, S.R.R. Pharmacoeconomics: Need for the day. Indian J. Pharm. Pract. 2009, 2, 16–18. [Google Scholar]
- Farid, S.; Baines, D. Pharmacoeconomics Education in the Middle East and North Africa Region: A Web-Based Research Project. Value Health Reg. Issues 2021, 25, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Oberoi, S.; Oberoi, A. Pharmacoeconomics guidelines: The need of hour for India. Int. J. Pharm. Investig. 2014, 4, 109–111. [Google Scholar] [CrossRef]
- DeSanVicente-Célis, Z.; Salazar, J.C.; Pineda-Tamayo, R.; Anaya, J.-M. On the need of pharmacoeconomics. Beginning by the principles. Rev. Colomb. De Reumatol. 2011, 18, 187–202. [Google Scholar]
- Shete, R.V.; Pattan, S.R.; Dhikale, R.S.; Shetkar, U.B.; Gaikwad, P.M. Pharmacoeco Omics. Pharmacologyonline 2010, 1, 877–884. [Google Scholar]
- Kumar, S.; Baldi, A. Pharmacoeconomics: Principles, methods and economic evaluation of drug therapies. Pharm. Tech. Med. 2013, 2, 362–369. [Google Scholar]
- Hasamnis, A.A.; Patil, S.S.; Shaik, I.; Narendiran, K. A Review of Pharmacoeconomics: The key to “Healthcare for All”. Syst. Rev. Pharm. 2019, 10, s40–s42. [Google Scholar]
- Babar, Z.-U.-D.; Scahill, S. Is there a role for pharmacoeconomics in developing countries? Pharmacoeconomics 2010, 28, 1069–1074. [Google Scholar] [PubMed]
- Williams, I.; Bryan, S. Understanding the limited impact of economic evaluation in health care resource allocation: A conceptual framework. Health Policy 2007, 80, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, N.H.H.; Becerra, L.M.S.; Rojas, J.A.D. The assessment of pharmacoeconomic studies quality of Levodopa’s use for the management of Parkinson’s diseases, 2010–2015. Rev. Colomb. Cienc. Químico-Farm. 2017, 46, 256–277. [Google Scholar]
- Walkom, E.; Robertson, J.; Newby, D.; Pillay, T. The role of pharmacoeconomics in formulary decision-making. Formulary 2006, 41, 374–386. [Google Scholar]
- Tarn, Y.-H.; Hu, S.; Kamae, I.; Yang, B.-M.; Li, S.-C.; Tangcharoensathien, V.; Teerawattananon, Y.; Limwattananon, S.; Hameed, A.; Aljunid, S.M.; et al. Health-Care Systems and Pharmacoeconomic Research in Asia-Pacific Region. Value Health 2008, 11, S137–S155. [Google Scholar] [CrossRef][Green Version]
- Mori, A.; Gavaza, P.; Robberstad, B. Role of pharmacoeconomics in developing countries. Farmeconomia. Health Econ. Ther. Pathways 2013, 14, 3–5. [Google Scholar] [CrossRef][Green Version]
- Lakdawalla, D.N.; Doshi, J.A.; Garrison, L.P.; Phelps, C.E.; Basu, A.; Danzon, P.M. Defining Elements of Value in Health Care—A Health Economics Approach: An ISPOR Special Task Force Report [3]. Value Health 2018, 21, 131–139. [Google Scholar] [CrossRef]
- Jazieh, A.R.; Pizzo, E.; Gulacsi, L.; Eldahiyat, F.; Abu-Helalah, M.; Ibrahim, N.; AlAbdulkareem, H.; Maraiki, F.; AlSaggabi, A.; Cornes, P. Implementation of country-wide pharmacoeconomic principles in cancer care in developing countries: Expert-based recommendations. Glob. J. Qual. Saf. Healthc. 2019, 2, 109–114. [Google Scholar] [CrossRef]
- Serritella, A.V.; Strohbehn, G.W.; Goldstein, D.; Lichter, A.S.; Ratain, M.J. Interventional Pharmacoeconomics: A Novel Mechanism for Unlocking Value. Clin. Pharmacol. Ther. 2020, 108, 487–493. [Google Scholar] [CrossRef]
- Nepal, G.; Yadav, J.K.; Bhandari, S.; Gautam, J.; Gajurel, B.P. Low-cost alternatives for the management of acute ischemic stroke in low and middle-income countries. Ann. Med. Surg. 2021, 72, 102969. [Google Scholar] [CrossRef]
- White, A.; Srinivasan, M.; Wingate, L.M.; Peasah, S.; Fleming, M. Development of a pharmacoeconomic registry: An example using hormonal contraceptives. Health Econ. Rev. 2021, 11, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Fasseeh, A.; Karam, R.; Jameleddine, M.; George, M.; Kristensen, F.B.; Al-Rabayah, A.A.; AlSaggabi, A.H.; El Rabbat, M.; Alowayesh, M.S.; Chamova, J.; et al. Implementation of Health Technology Assessment in the Middle East and North Africa: Comparison Between the Current and Preferred Status. Front. Pharmacol. 2020, 11, 15. [Google Scholar] [CrossRef] [PubMed]
- Eljilany, I.; El-Dahiyat, F.; Curley, L.E.; Babar, Z.-U. Evaluating quantity and quality of literature focusing on health economics and pharmacoeconomics in Gulf Cooperation Council countries. Expert Rev. Pharmacoecon. Outcomes Res. 2018, 18, 403–414. [Google Scholar] [CrossRef] [PubMed]
- Alefan, Q.; Hamdouni, E.; Alhamad, H.; Mukattash, T.; Rascati, K. Barriers to implementing pharmacoeconomics: Interview study. Expert Rev. Pharmacoecon. Outcomes Res. 2020, 21, 93–104. [Google Scholar] [CrossRef] [PubMed]
- Farid, S.; Elmahdawy, M.; Baines, D. A Systematic Review on the Extent and Quality of Pharmacoeconomic Publications in Egypt. Clin. Drug Investig. 2018, 39, 157–168. [Google Scholar] [CrossRef]
- Alshakka, M.; Alshammari, T.; Ansari, M.; Aldhubhani, A.; Ali, H.; Ibrahim, M.I.M. State of Pharmacoeconomic in Low Income Countries: A Case Study of Yemen. J. Middle East N. Afr. Sci. 2017, 3, 43–49. [Google Scholar] [CrossRef]
- Heerey, A.; McGowan, B.; Ryan, M.; Barry, M. Microcosting versus DRGs in the provision of cost estimates for use in pharmacoeconomic evaluation. Expert Rev. Pharm. Outcomes Res. 2002, 2, 29–33. [Google Scholar] [CrossRef]
- Alsultan, M.S. The role of pharmacoeconomics in formulary decision making in different hospitals in Riyadh, Saudi Arabia. Saudi Pharm. J. 2010, 19, 51–56. [Google Scholar] [CrossRef]
- Ibrahim, N.; Altwoijri, A.; Alabdulkarim, H.; Alnajjar, F.; AlSaqa’aby, M. Challenges in applying pharmacoeconomics at the hospital level: Experts based approach. Glob. J. Med Ther. 2019, 1, 1–4. [Google Scholar] [CrossRef]
- Haslé-Pham, E.; Arnould, B.; Späth, H.-M.; Follet, A.; Duru, G.; Marquis, P. Role of clinical, patient-reported outcome and medico-economic studies in the public hospital drug formulary decision-making process: Results of a European survey. Health Policy 2005, 71, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Drummond, M.; Brown, R.; Fendrick, A.M.; Fullerton, P.; Neumann, P.; Taylor, R.; Barbieri, M. Use of Pharmacoeconomics Information—Report of the ISPOR Task Force on Use of Pharmacoeconomic/Health Economic Information in Health-Care Decision Making. Value Health 2003, 6, 407–416. [Google Scholar] [CrossRef] [PubMed]
- Cavanaugh, T.M.; Buring, S.; Cluxton, R. A Pharmacoeconomics and Formulary Management Collaborative Project to Teach Decision Analysis Principles. Am. J. Pharm. Educ. 2012, 76, 115. [Google Scholar] [CrossRef] [PubMed]
- Jayasree, D.; Uppu, B.; Devi, B. A study on awareness of pharmacoeconomics among post graduates in a tertiary care teaching hospital. Int. J. Res. Med. Sci. 2016, 4, 1597–1603. [Google Scholar] [CrossRef]
- Cheung, K.; Evers, S.; De Vries, H.; Levy, P.; Pokhrel, S.; Jones, T.; Danner, M.; Wentlandt, J.; Knufinke, L.; Mayer, S.; et al. Most important barriers and facilitators of HTA usage in decision-making in Europe. Expert Rev. Pharmacoecon. Outcomes Res. 2018, 18, 297–304. [Google Scholar] [CrossRef]
- Zhao, Y.; Feng, H.-M.; Qu, J.; Luo, X.; Ma, W.-J.; Tian, J.-H. A systematic review of pharmacoeconomic guidelines. J. Med. Econ. 2017, 21, 85–96. [Google Scholar] [CrossRef]
- Arnold, R.J. Pharmacoeconomics: From Theory to Practice; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Almazrou, S.H.; Alaujan, S.S.; Al-Aqeel, S.A. Barriers and facilitators to conducting economic evaluation studies of Gulf Cooperation Council (GCC) countries: A survey of researchers. Health Res. Policy Syst. 2021, 19, 1–11. [Google Scholar] [CrossRef]
- Godfrey, K.M. Availability of Pharmacoeconomic Data and Its Use in the Development of Drug Formularies in South Africa; SEALS: Port Elizabeth, South, Africa, 2008. [Google Scholar]
- Fattore, G.; Torbica, A. Economic Evaluation in Health Care: The Point of View of Informed Physicians. Value Health 2006, 9, 157–167. [Google Scholar] [CrossRef]
- Mate, K.; Bryan, C.; Deen, N.; McCall, J. Review of Health Systems of the Middle East and North Africa Region. In International Encyclopedia of Public Health, 2nd ed.; Academic Press: Cambridge, MA, USA, 2017; pp. 347–356. [Google Scholar]
- Hamadeh, N.; van Pompaey, C.; Metreaum, E. New World Bank Country Classifications by Income Level: 2021–2022; Data Blog; The World Bank: Washington, DC, USA, 2021. [Google Scholar]
- World Health Organization. Global Health Expenditure Database. 2022. Available online: https://apps.who.int/nha/database (accessed on 19 April 2022).
- Al-Jazairi, A.S.; Al-Qadheeb, N.S.; Ajlan, A. Pharmacoeconomic Analysis in Saudi Arabia: An Overdue Agenda Item for Action. Ann. Saudi Med. 2011, 31, 335–341. [Google Scholar] [CrossRef]
- El-Jardali, F.; Lavis, J.N.; Ataya, N.; Jamal, D. Use of health systems and policy research evidence in the health policymaking in eastern Mediterranean countries: Views and practices of researchers. Implement. Sci. 2012, 7, 1–16. [Google Scholar] [CrossRef]
- Luz, A.; Santatiwongchai, B.; Pattanaphesaj, J.; Teerawattananon, Y. Identifying priority technical and context-specific issues in improving the conduct, reporting and use of health economic evaluation in low- and middle-income countries. Health Res. Policy Syst. 2018, 16, 1–12. [Google Scholar] [CrossRef] [PubMed]
- The World Bank. World Development Indicators. 2021. Available online: https://databank.worldbank.org/source/world-development-indicators (accessed on 20 April 2022).
- Shillcutt, S.D.; Walker, D.; Goodman, C.A.; Mills, A. Cost Effectiveness in Low- and Middle-Income Countries. PharmacoEconomics 2009, 27, 903–917. [Google Scholar] [CrossRef] [PubMed]
- Al-Harakeh, L.; Abbas, H.; Hassan, H.; Hallal, Z.; Hamadeh, G.; Kurdi, M.; Selwan, C.A.; Karam, R. Survey investigating the knowledge and awareness of payers and patient advocacy groups about the health technology assessment process in Lebanon. Int. J. Technol. Assess. Health Care 2021, 37, e72. [Google Scholar] [CrossRef] [PubMed]
- Cheraghali, A.M. Implications of pharmacoeconomics for Iran national health system. Iran. J. Pharm. Sci. 2013, 9, 81–85. [Google Scholar]
- Soliman, A.M.; Hussein, M.; Abdulhalim, A.M. Pharmacoeconomic Education in Egyptian Schools of Pharmacy. Am. J. Pharm. Educ. 2013, 77, 57. [Google Scholar] [CrossRef]
- Alefan, Q.; AlImam, S.; Mukattash, T.; Mhaidat, N.; Alabbadi, I.; Rascati, K. Pharmacoeconomics education in WHO Eastern Mediterranean region. Curr. Pharm. Teach. Learn. 2015, 7, 819–825. [Google Scholar] [CrossRef]
- Hammad, E.A.; Mousa, R.; Hammad, A.A.; Al-Qudah, M. Awareness, knowledge, and attitudes of health professions students toward health economics and pharmacoeconomics education in Jordan. Curr. Pharm. Teach. Learn. 2020, 12, 1072–1080. [Google Scholar] [CrossRef]
- Nwokeji, E.D.; Rascati, K.L. Pharmacoeconomic Education in Colleges of Pharmacy Outside of the United States. Am. J. Pharm. Educ. 2005, 69, 348. [Google Scholar] [CrossRef][Green Version]
- Alsuwaidan, A.; Almedlej, N.; Alsabti, S.; Daftardar, O.; Al Deaji, F.; Al Amri, A.; Alsuwaidan, S. A Comprehensive Overview of Polypharmacy in Elderly Patients in Saudi Arabia. Geriatrics 2019, 4, 36. [Google Scholar] [CrossRef]
- Balkhi, B.; AlQahtani, N.; Alwhaibi, M.; Alshammari, T.M.; Alhawassi, T.M.; Mahmoud, M.A.; Almetwazi, M.; Ata, S.; Basyoni, M.; Aljadhey, H. Prevalence and Factors Associated with Polypharmacy Use Among Adult Patients in Saudi Arabia. J. Patient Saf. 2017, 17, e1119–e1124. [Google Scholar] [CrossRef]
- Abu Farha, R.K.; Mukattash, T.L.; Al-Sakran, L.; Abu Hammour, K.; Zawiah, M. Prevalence and predictors of polypharmacy in Jordanian hospitalised patients: A cross-sectional Study. Int. J. Clin. Pract. 2020, 75, e13742. [Google Scholar] [CrossRef] [PubMed]
- Bagher, A.M.; Neamatallah, T.; Balto, G.; Almikhy, L.; Almutairi, S.S.; Abushal, M.O.; Baghlaf, K.; Bagher, S.M. Knowledge, perception, and confidence of hospital pharmacists toward pharmacogenetics in Jeddah, Kingdom of Saudi Arabia. Saudi Pharm. J. 2020, 29, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Al-Omar, H.A. Cost-conscious medications-prescribing behavior among physicians working in Saudi Arabia. Arch. Pharm. Pract. 2020, 1, 143. [Google Scholar]
- Bentkover, J.D.; Corey, R. Effective Utilization of Pharmacoeconomics for Decision Makers. Dis. Manag. Health Outcomes 2002, 10, 75–80. [Google Scholar] [CrossRef]
- Choi, S.-E. Current State and Challenges of Pharmacoeconomic Evaluation in Korea. J. Prev. Med. Public Health 2008, 41, 74–79. [Google Scholar] [CrossRef]
- Arenas-Guzman, R.; Tosti, A.; Hay, R.; Haneke, E. Pharmacoeconomics–an aid to better decision-making. J. Eur. Acad. Dermatol. Venereol. 2005, 19, 34–39. [Google Scholar] [CrossRef]
| Barrier | Strategies to Overcome | Reference |
|---|---|---|
| Absence of a national body to govern pharmacoeconomics | Development of a national regulatory committee on pharmacoeconomics | [23] |
| Lack of balance between effectiveness and cost effectiveness on the part of doctors and pharmacists | Proper monitoring and comparison of the cost–benefit ratio of drugs and patient follow-up procedures | [25] |
| Absence of local and national registries containing patient data and pharmacoeconomic records | Allocation of specialized personnel for maintaining local and national registries and conducting proper accountability | [27] |
| Lack of funding to conduct a pharmacoeconomic evaluation | Allocation of a specific health budget by governments to conduct pharmacoeconomic research and evaluation | [28] |
| Lack of good quality pharmacoeconomic data | Maintenance of high-quality patient records in hospitals | [31] |
| Inadequate Pharmacoeconomic Workshops | Organization of pharmacoeconomic workshops at a national and international level, encouragement of experts to participate in workshops. | [33] |
| Lack of belief in the importance of pharmacoeconomics | Awareness campaigns for young students and researchers to disseminate information about the importance of pharmacoeconomics | [10] |
| Inability to make conscious decisions | Education of decision makers and experts on new approaches arising in the field and encouragement of group discussions with researchers | [19] |
| Lack of pharmacoeconomic evaluation experts | Encouragement of young researchers to participate in pharmacoeconomic studies, allocation of funds for experts | [34] |
| Lack of efficient formulary management | Establishment of appropriate guidelines for formularies that frequently investigate the quality of hospital formularies by national governing bodies | [35] |
| Lack of patient participation in the decision-making process | Encouragement of patients to participate in decision making through interviews and opinion sharing | [2] |
| Lack of public awareness regarding the importance of pharmacoeconomics | Workshops, training programs and seminars to spread knowledge of pharmacoeconomics among decision makers, students and researchers | [38] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alzarea, A.I.; Khan, Y.H.; Alanazi, A.S.; Butt, M.H.; Almalki, Z.S.; AlAhmari, A.K.; Alsahali, S.; Mallhi, T.H. Barriers and Facilitators of Pharmacoeconomic Studies: A Review of Evidence from the Middle Eastern Countries. Int. J. Environ. Res. Public Health 2022, 19, 7862. https://doi.org/10.3390/ijerph19137862
Alzarea AI, Khan YH, Alanazi AS, Butt MH, Almalki ZS, AlAhmari AK, Alsahali S, Mallhi TH. Barriers and Facilitators of Pharmacoeconomic Studies: A Review of Evidence from the Middle Eastern Countries. International Journal of Environmental Research and Public Health. 2022; 19(13):7862. https://doi.org/10.3390/ijerph19137862
Chicago/Turabian StyleAlzarea, Abdulaziz Ibrahim, Yusra Habib Khan, Abdullah Salah Alanazi, Muhammad Hammad Butt, Ziyad Saeed Almalki, Abdullah K. AlAhmari, Saud Alsahali, and Tauqeer Hussain Mallhi. 2022. "Barriers and Facilitators of Pharmacoeconomic Studies: A Review of Evidence from the Middle Eastern Countries" International Journal of Environmental Research and Public Health 19, no. 13: 7862. https://doi.org/10.3390/ijerph19137862
APA StyleAlzarea, A. I., Khan, Y. H., Alanazi, A. S., Butt, M. H., Almalki, Z. S., AlAhmari, A. K., Alsahali, S., & Mallhi, T. H. (2022). Barriers and Facilitators of Pharmacoeconomic Studies: A Review of Evidence from the Middle Eastern Countries. International Journal of Environmental Research and Public Health, 19(13), 7862. https://doi.org/10.3390/ijerph19137862








