Factors Predicting Tongue Pressure Decline among Community-Dwelling Older Adults: The Takashimadaira Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
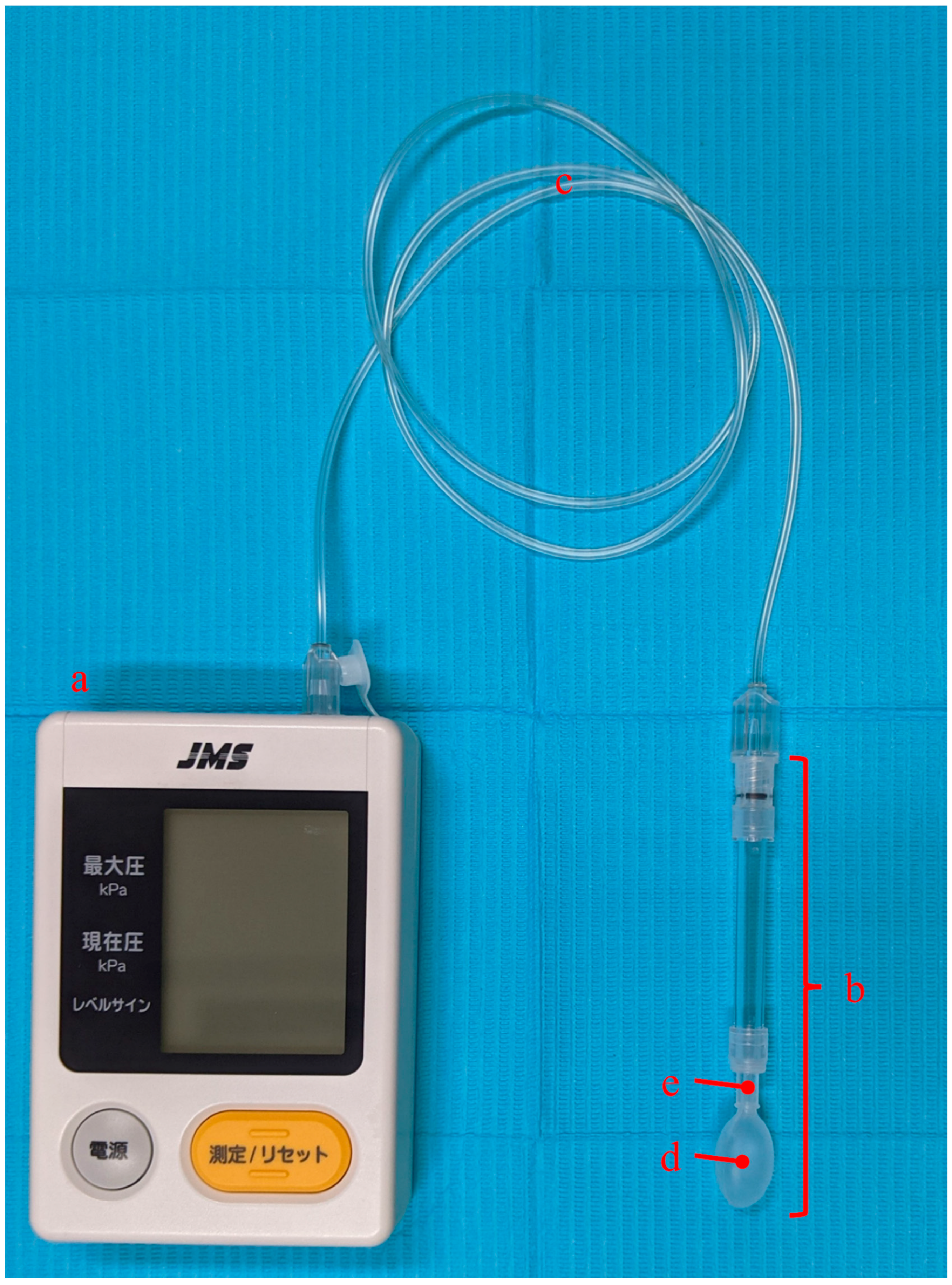
2.2. TP Measurement
2.3. Assessment of Dental Status
2.4. Anthropometric and Body Composition Measurements
2.5. Assessments of Physical Function
2.6. Cognitive Assessment
2.7. Questionnaire Survey
2.8. Medical Interviews
2.9. Statistical Analyses
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tsuga, K.; Yoshikawa, M.; Oue, H.; Okazaki, Y.; Tsuchioka, H.; Maruyama, M.; Yoshida, M.; Akagawa, Y. Maximal voluntary tongue pressure is decreased in Japanese frail elderly persons. Gerodontology 2012, 29, e1078–e1085. [Google Scholar] [CrossRef] [PubMed]
- Ogino, Y.; Suzuki, H.; Ayukawa, Y.; Jinnouchi, A.; Koyano, K. Analyses of Swallowing Function and Its Related Factors in Community-Dwelling Elderly Patients: A Case-Control Study. J. Clin. Med. 2021, 10, 3437. [Google Scholar] [CrossRef] [PubMed]
- Nishida, T.; Yamabe, K.; Honda, S. The Influence of Dysphagia on Nutritional and Frailty Status among Community-Dwelling Older Adults. Nutrients 2021, 13, 512. [Google Scholar] [CrossRef]
- Tanaka, T.; Takahashi, K.; Hirano, H.; Kikutani, T.; Watanabe, Y.; Ohara, Y.; Furuya, H.; Tetsuo, T.; Akishita, M.; Iijima, K. Oral Frailty as a Risk Factor for Physical Frailty and Mortality in Community-Dwelling Elderly. J. Gerontol. A Biol. Sci. Med. Sci. 2018, 73, 1661–1667. [Google Scholar] [CrossRef]
- Machida, N.; Tohara, H.; Hara, K.; Kumakura, A.; Wakasugi, Y.; Nakane, A.; Minakuchi, S. Effects of aging and sarcopenia on tongue pressure and jaw-opening force. Geriatr. Gerontol. Int. 2017, 17, 295–301. [Google Scholar] [CrossRef]
- Nagayoshi, M.; Higashi, M.; Takamura, N.; Tamai, M.; Koyamatsu, J.; Yamanashi, H.; Kadota, K.; Sato, S.; Kawashiri, S.Y.; Koyama, Z.; et al. Social networks, leisure activities and maximum tongue pressure: Cross-sectional associations in the Nagasaki Islands Study. BMJ Open 2017, 7, e014878. [Google Scholar] [CrossRef] [PubMed]
- Kaji, A.; Hashimoto, Y.; Kobayashi, Y.; Sakai, R.; Okamura, T.; Miki, A.; Hamaguchi, M.; Kuwahata, M.; Yamazaki, M.; Fukui, M. Sarcopenia is associated with tongue pressure in older patients with type 2 diabetes: A cross-sectional study of the KAMOGAWA-DM cohort study. Geriatr. Gerontol. Int. 2019, 19, 153–158. [Google Scholar] [CrossRef]
- Yoshimi, K.; Nakagawa, K.; Hara, K.; Yamaguchi, K.; Nakane, A.; Kubota, K.; Furuya, J.; Tohara, H. Relationship between tongue pressure and back muscle strength in healthy elderly individuals. Aging Clin. Exp. Res. 2020, 32, 2549–2555. [Google Scholar] [CrossRef]
- Tashiro, K.; Soutome, S.; Funahara, M.; Kawashita, Y.; Kitamura, M.; Fukuda, H.; Furugen, R.; Iwasaki, T.; Hayashida, H.; Kawasaki, K.; et al. The Relationship between Dental Findings and Tongue Pressure: A Survey of 745 Community-Dwelling Adults and Elderly Persons in Japan. Gerontology 2021, 67, 517–524. [Google Scholar] [CrossRef]
- Taniguchi, Y.; Watanabe, Y.; Osuka, Y.; Kitamura, A.; Seino, S.; Kim, H.; Kawai, H.; Sakurai, R.; Inagaki, H.; Awata, S.; et al. Characteristics for gait parameters of community-dwelling elderly Japanese with lower cognitive function. PLoS ONE 2019, 14, e0212646. [Google Scholar] [CrossRef]
- Minakuchi, S.; Tsuga, K.; Ikebe, K.; Ueda, T.; Tamura, F.; Nagao, K.; Furuya, J.; Matsuo, K.; Yamamoto, K.; Kanazawa, M.; et al. Oral hypofunction in the older population: Position paper of the Japanese Society of Gerodontology in 2016. Gerodontology 2018, 35, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, H.; Ayukawa, Y.; Ueno, Y.; Atsuta, I.; Jinnouchi, A.; Koyano, K. Relationship between Maximum Tongue Pressure Value and Age, Occlusal Status, or Body Mass Index among the Community-Dwelling Elderly. Medicina 2020, 56, 623. [Google Scholar] [CrossRef] [PubMed]
- Murakami, M.; Iijima, K.; Watanabe, Y.; Tanaka, T.; Iwasa, Y.; Edahiro, A.; Ohara, Y.; Motokawa, K.; Shirobe, M.; Hirano, H. Development of a simple method to measure masseter muscle mass. Gerodontology 2020, 37, 383–388. [Google Scholar] [CrossRef]
- Chen, L.K.; Woo, J.; Assantachai, P.; Auyeung, T.W.; Chou, M.Y.; Iijima, K.; Jang, H.C.; Kang, L.; Kim, M.; Kim, S.; et al. Asian Working Group for Sarcopenia: 2019 Consensus Update on Sarcopenia Diagnosis and Treatment. J. Am. Med. Dir. Assoc. 2020, 21, 300–307.e2. [Google Scholar] [CrossRef] [PubMed]
- Sugishita, M.; Hemmi, I.; Takeuchi, T. Reexamination of the validity and reliability of the Japanese version of the mini-mental state examination MMSE-J. Jpn. J. Cogn. Neurosci. 2016, 18, 168–183. [Google Scholar] [CrossRef]
- Satake, S.; Arai, H. The revised Japanese version of the Cardiovascular Health Study criteria (revised J-CHS criteria). Geriatr. Gerontol. Int. 2020, 20, 992–993. [Google Scholar] [CrossRef]
- Wilson, M.M.; Thomas, D.R.; Rubenstein, L.Z.; Chibnall, J.T.; Anderson, S.; Baxi, A.; Diebold, M.R.; Morley, J.E. Appetite assessment: Simple appetite questionnaire predicts weight loss in community-dwelling adults and nursing home residents. Am. J. Clin. Nutr. 2005, 82, 1074–1081. [Google Scholar] [CrossRef]
- Kobayashi, E.; Fujiwara, Y.; Fukaya, T.; Nishi, M.; Saito, M.; Shinkai, S. Social support availability and psychological well-being among the socially isolated elderly. Differences by living arrangement and gender. Nihon Koshu Eisei Zasshi 2011, 58, 446–456. (In Japanese) [Google Scholar]
- Schreiner, A.S.; Hayakawa, H.; Morimoto, T.; Kakuma, T. Screening for late life depression: Cut-off scores for the Geriatric Depression Scale and the Cornell Scale for Depression in Dementia among Japanese subjects. Int J. Geriatr. Psychiatry 2003, 18, 498–505. [Google Scholar] [CrossRef]
- Masnoon, N.; Shakib, S.; Kalisch-Ellett, L.; Caughey, G.E. What is polypharmacy? A systematic review of definitions. BMC Geriatr. 2017, 17, 230. [Google Scholar] [CrossRef] [Green Version]
- Hernan, M.A.; Hernandez-Diaz, S.; Robins, J.M. A structural approach to selection bias. Epidemiology 2004, 15, 615–625. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Utanohara, Y.; Hayashi, R.; Yoshikawa, M.; Yoshida, M.; Tsuga, K.; Akagawa, Y. Standard values of maximum tongue pressure taken using newly developed disposable tongue pressure measurement device. Dysphagia 2008, 23, 286–290. [Google Scholar] [CrossRef] [PubMed]
- Arakawa, I.; Igarashi, K.; Imamura, Y.; Müller, F.; Abou-Ayash, S.; Schimmel, M. Variability in tongue pressure among elderly and young healthy cohorts: A systematic review and meta-analysis. J. Oral Rehabil. 2021, 48, 430–448. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, M.; Ohara, Y.; Motokawa, K.; Hayakawa, M.; Shirobe, M.; Edahiro, A.; Watanabe, Y.; Awata, S.; Okamura, T.; Inagaki, H.; et al. Population-based reference values for tongue pressure in Japanese older adults: A pooled analysis of over 5000 participants. J. Prosthodont. Res. 2022, in press. [Google Scholar] [CrossRef]
- Wakabayashi, H. Presbyphagia and Sarcopenic Dysphagia: Association between Aging, Sarcopenia, and Deglutition Disorders. J. Frailty Aging 2014, 3, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Wakabayashi, H.; Sakuma, K. Rehabilitation nutrition for sarcopenia with disability: A combination of both rehabilitation and nutrition care management. J. Cachexia Sarcopenia Muscle 2014, 5, 269–277. [Google Scholar] [CrossRef]
- Yoshimura, Y.; Wakabayashi, H.; Bise, T.; Nagano, F.; Shimazu, S.; Shiraishi, A.; Yamaga, M.; Koga, H. Sarcopenia is associated with worse recovery of physical function and dysphagia and a lower rate of home discharge in Japanese hospitalized adults undergoing convalescent rehabilitation. Nutrition 2019, 61, 111–118. [Google Scholar] [CrossRef]
- Murakami, M.; Hirano, H.; Watanabe, Y.; Sakai, K.; Kim, H.; Katakura, A. Relationship between chewing ability and sarcopenia in Japanese community-dwelling older adults. Geriatr. Gerontol. Int. 2015, 15, 1007–1012. [Google Scholar] [CrossRef] [Green Version]
- Takagi, D.; Watanabe, Y.; Edahiro, A.; Ohara, Y.; Murakami, M.; Murakami, K.; Hironaka, S.; Taniguchi, Y.; Kitamura, A.; Shinkai, S.; et al. Factors affecting masticatory function of community-dwelling older people: Investigation of the differences in the relevant factors for subjective and objective assessment. Gerodontology 2017, 34, 357–364. [Google Scholar] [CrossRef]
- Umeki, K.; Watanabe, Y.; Hirano, H.; Edahiro, A.; Ohara, Y.; Yoshida, H.; Obuchi, S.; Kawai, H.; Murakami, M.; Takagi, D.; et al. The relationship between masseter muscle thickness and appendicular skeletal muscle mass in Japanese community-dwelling elders: A cross-sectional study. Arch. Gerontol. Geriatr. 2018, 78, 18–22. [Google Scholar] [CrossRef]
- Yamaguchi, K.; Tohara, H.; Hara, K.; Nakane, A.; Kajisa, E.; Yoshimi, K.; Minakuchi, S. Relationship of aging, skeletal muscle mass, and tooth loss with masseter muscle thickness. BMC Geriatr. 2018, 18, 67. [Google Scholar] [CrossRef]
- Donini, L.M.; Poggiogalle, E.; Piredda, M.; Pinto, A.; Barbagallo, M.; Cucinotta, D.; Sergi, G. Anorexia and eating patterns in the elderly. PLoS ONE 2013, 8, e63539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van der Meij, B.S.; Wijnhoven, H.A.H.; Lee, J.S.; Houston, D.K.; Hue, T.; Harris, T.B.; Kritchevsky, S.B.; Newman, A.B.; Visser, M. Poor Appetite and Dietary Intake in Community-Dwelling Older Adults. J. Am. Geriatr. Soc. 2017, 65, 2190–2197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, K.S.; Hong, C.H.; Cheong, H.K.; Oh, B.H. Difference in nutritional risk between mild cognitive impairment group and normal cognitive function elderly group. Arch. Gerontol. Geriatr. 2009, 49, 49–53. [Google Scholar] [CrossRef] [PubMed]
- Kai, K.; Hashimoto, M.; Amano, K.; Tanaka, H.; Fukuhara, R.; Ikeda, M. Relationship between eating disturbance and dementia severity in patients with Alzheimer’s disease. PLoS ONE 2015, 10, e0133666. [Google Scholar] [CrossRef]
- Kimura, A.; Sugimoto, T.; Niida, S.; Toba, K.; Sakurai, T. Association Between Appetite and Sarcopenia in Patients With Mild Cognitive Impairment and Early-Stage Alzheimer’s Disease: A Case-Control Study. Front. Nutr. 2018, 5, 128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suma, S.; Watanabe, Y.; Hirano, H.; Kimura, A.; Edahiro, A.; Awata, S.; Yamashita, Y.; Matsushita, K.; Arai, H.; Sakurai, T. Factors affecting the appetites of persons with Alzheimer’s disease and mild cognitive impairment. Geriatr. Gerontol. Int. 2018, 18, 1236–1243. [Google Scholar] [CrossRef]
- Zekry, D.; Herrmann, F.R.; Grandjean, R.; Meynet, M.P.; Michel, J.P.; Gold, G.; Krause, K.H. Demented versus non-demented very old inpatients: The same comorbidities but poorer functional and nutritional status. Age Ageing 2008, 37, 83–89. [Google Scholar] [CrossRef] [Green Version]
- Khater, M.S.; Abouelezz, N.F. Nutritional status in older adults with mild cognitive impairment living in elderly homes in Cairo, Egypt. J. Nutr. Health Aging 2011, 15, 104–108. [Google Scholar] [CrossRef]
- Bischoff-Ferrari, H.A.; Dietrich, T.; Orav, E.J.; Hu, F.B.; Zhang, Y.; Karlson, E.W.; Dawson-Hughes, B. Higher 25-hydroxyvitamin D concentrations are associated with better lower-extremity function in both active and inactive persons aged > or =60 y. Am. J. Clin. Nutr. 2004, 80, 752–758. [Google Scholar] [CrossRef]
- Chaput, J.P.; Lord, C.; Cloutier, M.; Aubertin Leheudre, M.; Goulet, E.D.; Rousseau, S.; Khalil, A.; Dionne, I.J. Relationship between antioxidant intakes and class I sarcopenia in elderly men and women. J. Nutr. Health Aging 2007, 11, 363–369. [Google Scholar]
- Thomas, D.R. Loss of skeletal muscle mass in aging: Examining the relationship of starvation, sarcopenia and cachexia. Clin. Nutr. 2007, 26, 389–399. [Google Scholar] [CrossRef] [PubMed]
- Nieuwenhuizen, W.F.; Weenen, H.; Rigby, P.; Hetherington, M.M. Older adults and patients in need of nutritional support: Review of current treatment options and factors influencing nutritional intake. Clin. Nutr. 2010, 29, 160–169. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.K.; Manaf, Z.A.; Yusoff, N.A.; Muhammad, N.A.; Phan, M.F.; Shahar, S. Correlation between nutritional status and comprehensive physical performance measures among older adults with undernourishment in residential institutions. Clin. Interv. Aging 2014, 9, 1415–1423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iwasaki, M.; Motokawa, K.; Watanabe, Y.; Shirobe, M.; Ohara, Y.; Edahiro, A.; Kawai, H.; Fujiwara, Y.; Kim, H.; Ihara, K.; et al. Oral hypofunction and malnutrition among community—Dwelling older adults: Evidence from the Otassha study. Gerodontology 2022, 39, 17–25. [Google Scholar] [CrossRef]
- Shimazaki, Y.; Nonoyama, T.; Tsushita, K.; Arai, H.; Matsushita, K.; Uchibori, N. Oral hypofunction and its association with frailty in community-dwelling older people. Geriatr. Gerontol. Int. 2020, 20, 917–926. [Google Scholar] [CrossRef]
- Kugimiya, Y.; Iwasaki, M.; Ohara, Y.; Motokawa, K.; Edahiro, A.; Shirobe, M.; Watanabe, Y.; Obuchi, S.; Kawai, H.; Fujiwara, Y.; et al. Relationship between Oral Hypofunction and Sarcopenia in Community-Dwelling Older Adults: The Otassha Study. Int. J. Environ. Res. Public Health 2021, 18, 6666. [Google Scholar] [CrossRef]

| Total | Women | Men | ||
|---|---|---|---|---|
| N = 357 | N = 198 | N = 159 | p Value | |
| Oral health status | ||||
| Tongue pressure (kPa) * | 36.8 (5.5) | 36.2 (5.1) | 37.5 (5.9) | 0.03 |
| n of natural teeth † | 23 (13–27) | 24 (16–27) | 22 (12–27) | 0.09 |
| Posterior occlusal support | 0.07 | |||
| Eichner group C | 96 (26.9%) | 48 (24.2%) | 48 (30.2%) | |
| Eichner group B | 102 (28.6%) | 51 (25.8%) | 51 (32.1%) | |
| Eichner group A | 159 (44.5%) | 99 (50.0%) | 60 (37.7%) | |
| Denture use ‡ | 178 (49.9%) | 84 (42.4%) | 94 (59.1%) | <0.01 |
| n of functional teeth † | 28 (27–28) | 28 (27–28) | 28 (27–28) | 0.51 |
| Other characteristics | ||||
| Age * | 75.9 (4.1) | 75.9 (4.1) | 75.9 (4.0) | 0.89 |
| Educational status (years of schooling) † | 12 (12–16) | 12 (12–14) | 13 (12–16) | <0.01 |
| Annual income < 3 million JPY ‡ | 209 (58.5%) | 135 (68.2%) | 74 (46.5%) | <0.01 |
| Daily drinker ‡ | 53 (14.8%) | 10 (5.1%) | 43 (27.0%) | <0.01 |
| Current smoker ‡ | 28 (7.8%) | 4 (2.0%) | 24 (15.1%) | <0.01 |
| Social isolation ‡ | 134 (37.5%) | 45 (22.7%) | 89 (56.0%) | <0.01 |
| Living alone ‡ | 128 (35.9%) | 97 (49.0%) | 31 (19.5%) | <0.01 |
| Poor appetite ‡ | 106 (29.7%) | 58 (29.3%) | 48 (30.2%) | 0.85 |
| Underweight ‡ | 11 (3.1%) | 9 (4.5%) | 2 (1.3%) | 0.07 |
| Low SMI ‡ | 103 (28.9%) | 66 (33.3%) | 37 (23.3%) | 0.04 |
| Low grip strength ‡ | 16 (4.5%) | 5 (2.5%) | 11 (6.9%) | 0.05 |
| Low usual gait speed ‡ | 23 (6.4%) | 9 (4.5%) | 14 (8.8%) | 0.10 |
| Low physical activity level ‡ | 40 (11.2%) | 16 (8.1%) | 24 (15.1%) | 0.04 |
| Comorbidity status ‡ | ||||
| Hypertension | 176 (49.3%) | 95 (48.0%) | 81 (50.9%) | 0.58 |
| Heart disease | 67 (18.8%) | 37 (18.7%) | 30 (18.9%) | 0.97 |
| Stroke | 24 (6.7%) | 11 (5.6%) | 13 (8.2%) | 0.33 |
| Diabetes | 50 (14.0%) | 21 (10.6%) | 29 (18.2%) | 0.04 |
| Depressive symptoms ‡ | 89 (24.9%) | 51 (25.8%) | 38 (23.9%) | 0.69 |
| Cognitive impairment ‡ | 17 (4.8%) | 9 (4.5%) | 8 (5.0%) | 0.83 |
| Polypharmacy ‡ | 109 (30.5%) | 55 (27.8%) | 54 (34.0%) | 0.21 |
| Variables * | IRRs †,‡ | 95% CIs | p Value |
|---|---|---|---|
| Oral health status | |||
| n of natural teeth (per one tooth increase) | 0.99 | (0.96–1.01) | 0.36 |
| Posterior occlusal support | |||
| Eichner group C | Ref. | ||
| Eichner group B | 0.94 | (0.52–1.70) | 0.84 |
| Eichner group A | 0.81 | (0.46–1.43) | 0.47 |
| Denture use | 1.23 | (0.79–1.91) | 0.35 |
| n of functional teeth (per one tooth increase) | 1.01 | (0.92–1.10) | 0.86 |
| Other characteristics | |||
| Age (per one year increase) | 1.01 | (0.96–1.07) | 0.67 |
| Men (vs. women) | 1.03 | (0.65–1.62) | 0.91 |
| Educational Status (per one year of schooling increase) | 0.95 | (0.87–1.04) | 0.28 |
| Annual income < 3 million JPY | 0.86 | (0.55–1.35) | 0.51 |
| Daily drinker | 0.71 | (0.33–1.50) | 0.36 |
| Current smoker | 0.67 | (0.24–1.86) | 0.44 |
| Social isolation | 0.83 | (0.51–1.35) | 0.45 |
| Living alone | 1.23 | (0.78–1.95) | 0.37 |
| Poor appetite | 1.57 | (1.001–2.46) | 0.049 |
| Underweight | 1.16 | (0.33–4.16) | 0.82 |
| Low SMI | 1.67 | (1.06–2.63) | 0.03 |
| Low grip strength | 1.26 | (0.45–3.51) | 0.66 |
| Low usual gait speed | 1.37 | (0.66–2.85) | 0.40 |
| Low physical activity level | 0.96 | (0.48–1.95) | 0.92 |
| Comorbidity status | |||
| Hypertension | 1.06 | (0.68–1.66) | 0.80 |
| Heart disease | 1.11 | (0.63–1.96) | 0.71 |
| Stroke | 1.06 | (0.45–2.46) | 0.90 |
| Diabetes | 1.16 | (0.56–2.41) | 0.69 |
| Depressive symptoms | 0.95 | (0.56–1.62) | 0.85 |
| Cognitive impairment | 2.22 | (1.38–3.57) | <0.01 |
| Polypharmacy | 1.26 | (0.78–2.05) | 0.34 |
| Outcome = Having Tongue Pressure of <30 kPa at 2-Year Follow-Up Assessment | ||||||
|---|---|---|---|---|---|---|
| Model 1 (Baseline Variables That Yielded p Values < 0.05 in the Models Adjusted Only for Baseline Tongue Pressure *) | Model 2 (Model 1 + Age and Sex) | |||||
| Variables | IRRs †,‡ | 95% CIs | p Value | IRRs †,‡ | 95% CIs | p Value |
| Poor appetite | 1.58 | (1.01–2.45) | 0.04 | 1.58 | (1.01–2.48) | 0.046 |
| Low SMI | 1.62 | (1.02–2.59) | 0.04 | 1.66 | (1.02–2.70) | 0.04 |
| Cognitive impairment | 1.88 | (1.16–3.04) | 0.01 | 1.93 | (1.12–3.33) | 0.02 |
| Age (per one-year increase) | 0.99 | (0.94–1.04) | 0.67 | |||
| Men (vs. women) | 1.02 | (0.64–1.63) | 0.94 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takahashi, C.; Iwasaki, M.; Motokawa, K.; Watanabe, Y.; Hayakawa, M.; Mikami, Y.; Shirobe, M.; Inagaki, H.; Edahiro, A.; Ohara, Y.; et al. Factors Predicting Tongue Pressure Decline among Community-Dwelling Older Adults: The Takashimadaira Study. Int. J. Environ. Res. Public Health 2022, 19, 7850. https://doi.org/10.3390/ijerph19137850
Takahashi C, Iwasaki M, Motokawa K, Watanabe Y, Hayakawa M, Mikami Y, Shirobe M, Inagaki H, Edahiro A, Ohara Y, et al. Factors Predicting Tongue Pressure Decline among Community-Dwelling Older Adults: The Takashimadaira Study. International Journal of Environmental Research and Public Health. 2022; 19(13):7850. https://doi.org/10.3390/ijerph19137850
Chicago/Turabian StyleTakahashi, Chika, Masanori Iwasaki, Keiko Motokawa, Yutaka Watanabe, Misato Hayakawa, Yurie Mikami, Maki Shirobe, Hiroki Inagaki, Ayako Edahiro, Yuki Ohara, and et al. 2022. "Factors Predicting Tongue Pressure Decline among Community-Dwelling Older Adults: The Takashimadaira Study" International Journal of Environmental Research and Public Health 19, no. 13: 7850. https://doi.org/10.3390/ijerph19137850
APA StyleTakahashi, C., Iwasaki, M., Motokawa, K., Watanabe, Y., Hayakawa, M., Mikami, Y., Shirobe, M., Inagaki, H., Edahiro, A., Ohara, Y., Hirano, H., Shinkai, S., & Awata, S. (2022). Factors Predicting Tongue Pressure Decline among Community-Dwelling Older Adults: The Takashimadaira Study. International Journal of Environmental Research and Public Health, 19(13), 7850. https://doi.org/10.3390/ijerph19137850






