The Psychophysiological Profile and Cardiac Autonomic Reactivity in Long-Term Female Yoga Practitioners: A Comparison with Runners and Sedentary Individuals
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Self-Report Measures
2.3. Physiological Measures
2.4. Stressors
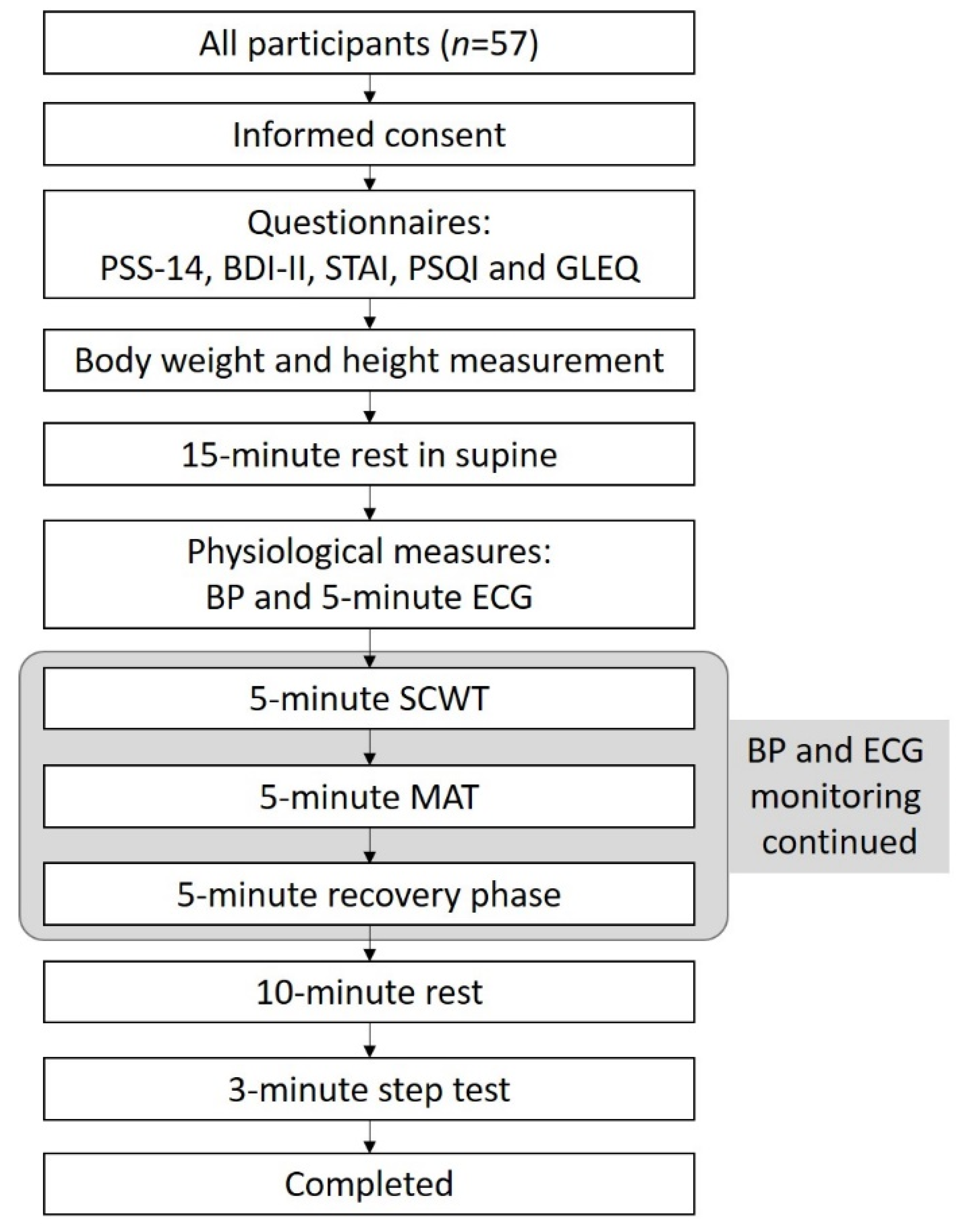
2.5. Procedures
2.6. Statistical Analysis
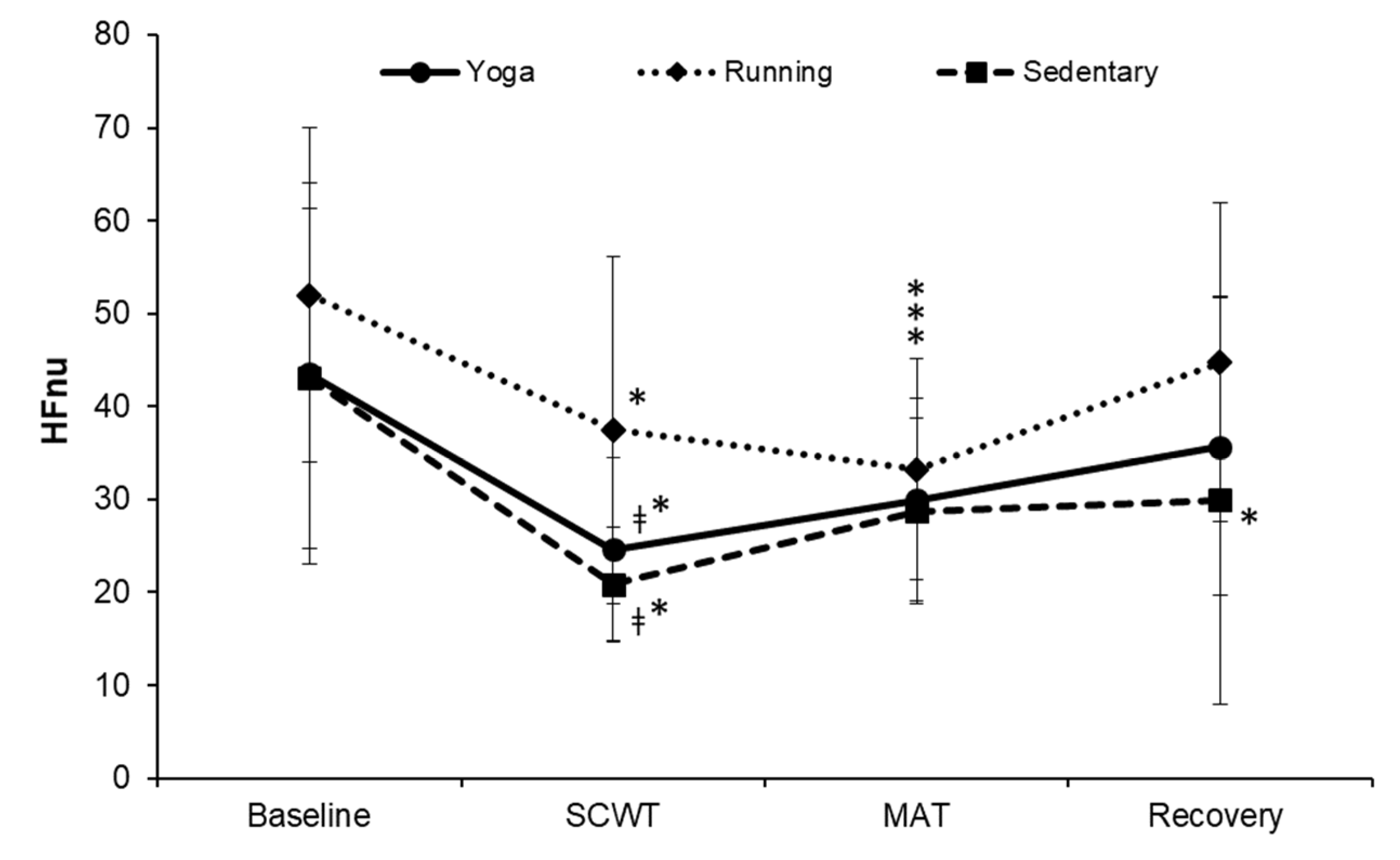
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Innes, K.E.; Bourguignon, C.; Taylor, A.G. Risk indices associated with the insulin resistance syndrome, cardiovascular disease, and possible protection with yoga: A systematic review. J. Am. Board Fam. Pract. 2005, 18, 491–519. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chong, C.S.; Tsunaka, M.; Tsang, H.W.; Chan, E.P.; Cheung, W.M. Effects of yoga on stress management in healthy adults: A systematic review. Altern. Ther. Health Med. 2011, 17, 32–38. [Google Scholar] [PubMed]
- Hartfiel, N.; Burton, C.; Rycroft-Malone, J.; Clarke, G.; Havenhand, J.; Khalsa, S.B.; Edwards, R.T. Yoga for reducing perceived stress and back pain at work. Occup. Med. 2012, 62, 606–612. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Michalsen, A.; Jeitler, M.; Brunnhuber, S.; Ludtke, R.; Bussing, A.; Musial, F.; Dobos, G.; Kessler, C. Iyengar yoga for distressed women: A 3-armed randomized controlled trial. Evid. Based-Complement. Altern. Med. 2012, 2012, 408727. [Google Scholar] [CrossRef]
- Sharma, M. Yoga as an Alternative and Complementary Approach for Stress Management. J. Evid.-Based Complementary Altern. Med. 2013, 19, 59–67. [Google Scholar] [CrossRef]
- Shankarapillai, R.; Nair, M.A.; George, R. The effect of yoga in stress reduction for dental students performing their first periodontal surgery: A randomized controlled study. Int. J. Yoga 2012, 5, 48–51. [Google Scholar] [CrossRef] [Green Version]
- Chu, I.H.; Lin, Y.J.; Wu, W.L.; Chang, Y.K.; Lin, I.M. Effects of Yoga on Heart Rate Variability and Mood in Women: A Randomized Controlled Trial. J. Altern. Complement. Med. 2015, 21, 789–795. [Google Scholar] [CrossRef]
- Chu, I.H.; Wu, W.L.; Lin, I.M.; Chang, Y.K.; Lin, Y.J.; Yang, P.C. Effects of Yoga on Heart Rate Variability and Depressive Symptoms in Women: A Randomized Controlled Trial. J. Altern. Complement. Med. 2017, 23, 310–316. [Google Scholar] [CrossRef]
- Panjwani, U.; Dudani, S.; Wadhwa, M. Sleep, Cognition, and Yoga. Int. J. Yoga 2021, 14, 100–108. [Google Scholar] [CrossRef]
- Cramer, H.; Lauche, R.; Klose, P.; Lange, S.; Langhorst, J.; Dobos, G.J. Yoga for improving health-related quality of life, mental health and cancer-related symptoms in women diagnosed with breast cancer. Cochrane Database Syst. Rev. 2017, 1, CD010802. [Google Scholar] [CrossRef] [Green Version]
- Pascoe, M.C.; Bauer, I.E. A systematic review of randomised control trials on the effects of yoga on stress measures and mood. J. Psychiatr. Res. 2015, 68, 270–282. [Google Scholar] [CrossRef] [PubMed]
- Cramer, H.; Lauche, R.; Haller, H.; Steckhan, N.; Michalsen, A.; Dobos, G. Effects of yoga on cardiovascular disease risk factors: A systematic review and meta-analysis. Int. J. Cardiol. 2014, 173, 170–183. [Google Scholar] [CrossRef] [PubMed]
- Cohen, D.L.; Boudhar, S.; Bowler, A.; Townsend, R.R. Blood Pressure Effects of Yoga, Alone or in Combination with Lifestyle Measures: Results of the Lifestyle Modification and Blood Pressure Study (LIMBS). J. Clin. Hypertens. 2016, 18, 809–816. [Google Scholar] [CrossRef] [PubMed]
- Haider, T.; Sharma, M.; Branscum, P. Yoga as an Alternative and Complimentary Therapy for Cardiovascular Disease. J. Evid.-Based Complement. Altern. Med. 2016, 22, 310–316. [Google Scholar] [CrossRef]
- Pal, A.; Srivastava, N.; Narain, V.S.; Agrawal, G.G.; Rani, M. Effect of yogic intervention on the autonomic nervous system in the patients with coronary artery disease: A randomized controlled trial. East Mediterr. Health J. 2013, 19, 452–458. [Google Scholar] [CrossRef]
- Krishna, B.H.; Pal, P.; Pal, G.K.; Balachander, J.; Jayasettiaseelon, E.; Sreekanth, Y.; Sridhar, M.G.; Gaur, G.S. Effect of Yoga Therapy on Heart Rate, Blood Pressure and Cardiac Autonomic Function in Heart Failure. J. Clin. Diagn. Res. 2014, 8, 14–16. [Google Scholar]
- Balakrishnan, B.; Metri, K.G.; Day, J.; Ganesan, M. Long-Term Effects of Hatha Yoga on Heart Rate Variability in Healthy Practitioners: Potential Benefits for Cardiovascular Risk Reduction. Altern. Ther. Health Med. 2021. Epub ahead of print. Available online: https://pubmed.ncbi.nlm.nih.gov/34453503/ (accessed on 22 June 2022).
- Miles, S.C.; Chun-Chung, C.; Hsin-Fu, L.; Hunter, S.D.; Dhindsa, M.; Nualnim, N.; Tanaka, H. Arterial blood pressure and cardiovascular responses to yoga practice. Altern. Ther. Health Med. 2013, 19, 38–45. [Google Scholar]
- Kleiger, R.E.; Miller, J.P.; Bigger, J.T.; Moss, A.J. Decreased heart rate variability and its association with increased mortality after acute myocardial infarction. Am. J. Cardiol. 1987, 59, 256–262. [Google Scholar] [CrossRef]
- Malik, M.; Farrell, T.; Cripps, T.; Camm, A.J. Heart rate variability in relation to prognosis after myocardial infarction: Selection of optimal processing techniques. Eur. Heart J. 1989, 10, 1060–1074. [Google Scholar] [CrossRef]
- Bigger, J.T.; Fleiss, J.L.; Steinman, R.C.; Rolnitzky, L.M.; Kleiger, R.E.; Rottman, J.N. Frequency domain measures of heart period variability and mortality after myocardial infarction. Circulation 1992, 85, 164–171. [Google Scholar] [CrossRef] [Green Version]
- Malik, M.; Bigger, J.T.; Camm, A.J.; Kleiger, R.E.; Malliani, A.; Moss, A.J. Heart rate variability:Standards of measurement, physiological interpretation, and clinical use. Eur. Heart J. 1996, 17, 354–381. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.G.; Cheon, E.J.; Bai, D.S.; Lee, Y.H.; Koo, B.H. Stress and Heart Rate Variability: A Meta-Analysis and Review of the Literature. Psychiatry Investig. 2018, 15, 235–245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakao, M. Heart Rate Variability and Perceived Stress as Measurements of Relaxation Response. J. Clin. Med. 2019, 8, 1704. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jensen-Urstad, K.; Saltin, B.; Ericson, M.; Storck, N.; Jensen-Urstad, M. Pronounced resting bradycardia in male elite runners is associated with high heart rate variability. Scand. J. Med. Sci. Sports 1997, 7, 274–278. [Google Scholar] [CrossRef]
- Peter, R.; Sood, S.; Dhawan, A. Spectral Parameters of HRV In Yoga Practitioners, Athletes and Sedentary Males. Indian J. Physiol. Pharmacol. 2015, 59, 380–387. [Google Scholar]
- Hoshikawa, Y.; Yamamoto, Y. Effects of Stroop color-word conflict test on the autonomic nervous system responses. Am. J. Physiol. 1997, 272, H1113–H1121. [Google Scholar] [CrossRef]
- Ruediger, H.; Seibt, R.; Scheuch, K.; Krause, M.; Alam, S. Sympathetic and parasympathetic activation in heart rate variability in male hypertensive patients under mental stress. J. Hum. Hypertens 2004, 18, 307–315. [Google Scholar] [CrossRef] [Green Version]
- Boutcher, S.H.; Nugent, F.W.; McLaren, P.F.; Weltman, A.L. Heart period variability of trained and untrained men at rest and during mental challenge. Psychophysiology 1998, 35, 16–22. [Google Scholar] [CrossRef]
- Tyagi, A.; Cohen, M.; Reece, J.; Telles, S.; Jones, L. Heart Rate Variability, Flow, Mood and Mental Stress During Yoga Practices in Yoga Practitioners, Non-yoga Practitioners and People with Metabolic Syndrome. Appl. Psychophysiol. Biofeedback 2016, 41, 381–393. [Google Scholar] [CrossRef]
- Satin, J.R.; Linden, W.; Millman, R.D. Yoga and psychophysiological determinants of cardiovascular health: Comparing yoga practitioners, runners, and sedentary individuals. Ann. Behav. Med. 2014, 47, 231–241. [Google Scholar] [CrossRef]
- Voss, A.; Heitmann, A.; Schroeder, R.; Peters, A.; Perz, S. Short-term heart rate variability--age dependence in healthy subjects. Physiol. Meas. 2012, 33, 1289–1311. [Google Scholar] [CrossRef] [PubMed]
- Katzmarzyk, P.T.; Reeder, B.A.; Elliott, S.; Joffres, M.R.; Pahwa, P.; Raine, K.; Kirkland, S.A.; Paradis, G. Body mass index and risk of cardiovascular disease, cancer and all-cause mortality. Can. J. Public Health 2012, 103, 147–151. [Google Scholar] [CrossRef] [PubMed]
- Voss, A.; Schroeder, R.; Heitmann, A.; Peters, A.; Perz, S. Short-term heart rate variability--influence of gender and age in healthy subjects. PLoS ONE 2015, 10, e0118308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Faul, F.; Erdfelder, E.; Lang, A.-G.; Buchner, A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef]
- Buysse, D.J.; Reynolds, C.F.; Monk, T.H.; Berman, S.R.; Kupfer, D.J. The Pittsburgh sleep quality index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989, 28, 193–213. [Google Scholar] [CrossRef]
- Amireault, S.; Godin, G. The Godin-Shephard leisure-time physical activity questionnaire: Validity evidence supporting its use for classifying healthy adults into active and insufficiently active categories. Percept. Mot. Ski. 2015, 120, 604–622. [Google Scholar] [CrossRef]
- Godin, G.; Shephard, R.J. A simple method to assess exercise behavior in the community. Can. J. Appl. Sport Sci. 1985, 10, 141–146. [Google Scholar]
- Sports Administration, Ministry of Education, Taiwan. Three-Minute Step Test. Available online: https://www.fitness.org.tw/measure06.php (accessed on 1 April 2022).
- Sports Administration, Ministry of Education, Taiwan. Audio File for the Three-Minute Step Test. Available online: https://www.fitness.org.tw/download.php (accessed on 10 June 2022).
- Stroop, J.R. Studies of interference in serial verbal reactions. J. Exp. Psychol. 1935, 18, 643–662. [Google Scholar] [CrossRef]
- Sports Administration, Ministry of Education, Taiwan. National Norms for the Aerobic Fitness Index. Available online: https://www.fitness.org.tw/model07.php (accessed on 1 April 2022).
- Jayasinghe, S.R. Yoga in cardiac health (A Review). Eur. J. Cardiovasc. Prev. Rehabil. 2004, 11, 369–375. [Google Scholar] [CrossRef]
- Bernardi, L.; Sleight, P.; Bandinelli, G.; Cencetti, S.; Fattorini, L.; Wdowczyc-Szulc, J.; Lagi, A. Effect of rosary prayer and yoga mantras on autonomic cardiovascular rhythms: Comparative study. BMJ 2001, 323, 1446–1449. [Google Scholar] [CrossRef] [Green Version]
- Prakash, S.; Meshram, S.; Ramtekkar, U. Athletes, yogis and individuals with sedentary lifestyles; do their lung functions differ? Indian J. Physiol. Pharmacol. 2007, 1, 76–80. [Google Scholar]
- Rennie, K.L.; Hemingway, H.; Kumari, M.; Brunner, E.; Malik, M.; Marmot, M. Effects of moderate and vigorous physical activity on heart rate variability in a British study of civil servants. Am. J. Epidemiol. 2003, 158, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.A.; Oh, D.J. The effects of long-term aerobic exercise on cardiac structure, stroke volume of the left ventricle, and cardiac output. J. Exerc. Rehabil. 2016, 12, 37–41. [Google Scholar] [CrossRef] [PubMed]
- Murugesan, R.; Govindarajulu, N.; Bera, T.K. Effect of selected yogic practices on the management of hypertension. Indian J. Physiol. Pharmacol. 2000, 44, 207–210. [Google Scholar] [PubMed]
- Posadzki, P.; Cramer, H.; Kuzdzal, A.; Lee, M.S.; Ernst, E. Yoga for hypertension: A systematic review of randomized clinical trials. Complementary Ther. Med. 2014, 22, 511–522. [Google Scholar] [CrossRef]
- Chobanian, A.V.; Bakris, G.L.; Black, H.R.; Cushman, W.C.; Green, L.A.; Izzo, J.L., Jr.; Jones, D.W.; Materson, B.J.; Oparil, S.; Wright, J.T., Jr.; et al. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension 2003, 42, 1206–1252. [Google Scholar] [CrossRef] [Green Version]
- Grässler, B.; Thielmann, B.; Böckelmann, I.; Hökelmann, A. Effects of different exercise interventions on heart rate variability and cardiovascular health factors in older adults: A systematic review. Eur. Rev. Aging Phys. Act. 2021, 18, 24. [Google Scholar] [CrossRef]
- Sandercock, G.R.; Bromley, P.D.; Brodie, D.A. Effects of exercise on heart rate variability: Inferences from meta-analysis. Med. Sci. Sports Exerc. 2005, 37, 433–439. [Google Scholar] [CrossRef]
- Chida, Y.; Steptoe, A. Greater Cardiovascular Responses to Laboratory Mental Stress Are Associated with Poor Subsequent Cardiovascular Risk Status. Meta-Anal. Prospect. Evid. 2010, 55, 1026–1032. [Google Scholar] [CrossRef] [Green Version]
- Kiecolt-Glaser, J.K.; Christian, L.; Preston, H.; Houts, C.R.; Malarkey, W.B.; Emery, C.F.; Glaser, R. Stress, inflammation, and yoga practice. Psychosom. Med. 2010, 72, 113–121. [Google Scholar] [CrossRef] [Green Version]
- Anderson, E.; Shivakumar, G. Effects of exercise and physical activity on anxiety. Front. Psychiatry 2013, 4, 27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wipfli, B.M.; Rethorst, C.D.; Landers, D.M. The anxiolytic effects of exercise: A meta-analysis of randomized trials and dose-response analysis. J. Sport Exerc. Psychol. 2008, 30, 392–410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mirko, W.; Ingo, H.; Sergio, M.; Antonio, E.N.; Oscar, A.-C.; Henning, B. Effects of Exercise on Anxiety and Depression Disorders: Review of Meta- Analyses and Neurobiological Mechanisms. CNS Neurol. Disord. Drug Targets 2014, 13, 1002–1014. [Google Scholar]
- Mikkelsen, K.; Stojanovska, L.; Polenakovic, M.; Bosevski, M.; Apostolopoulos, V. Exercise and mental health. Maturitas 2017, 106, 48–56. [Google Scholar] [CrossRef] [PubMed]
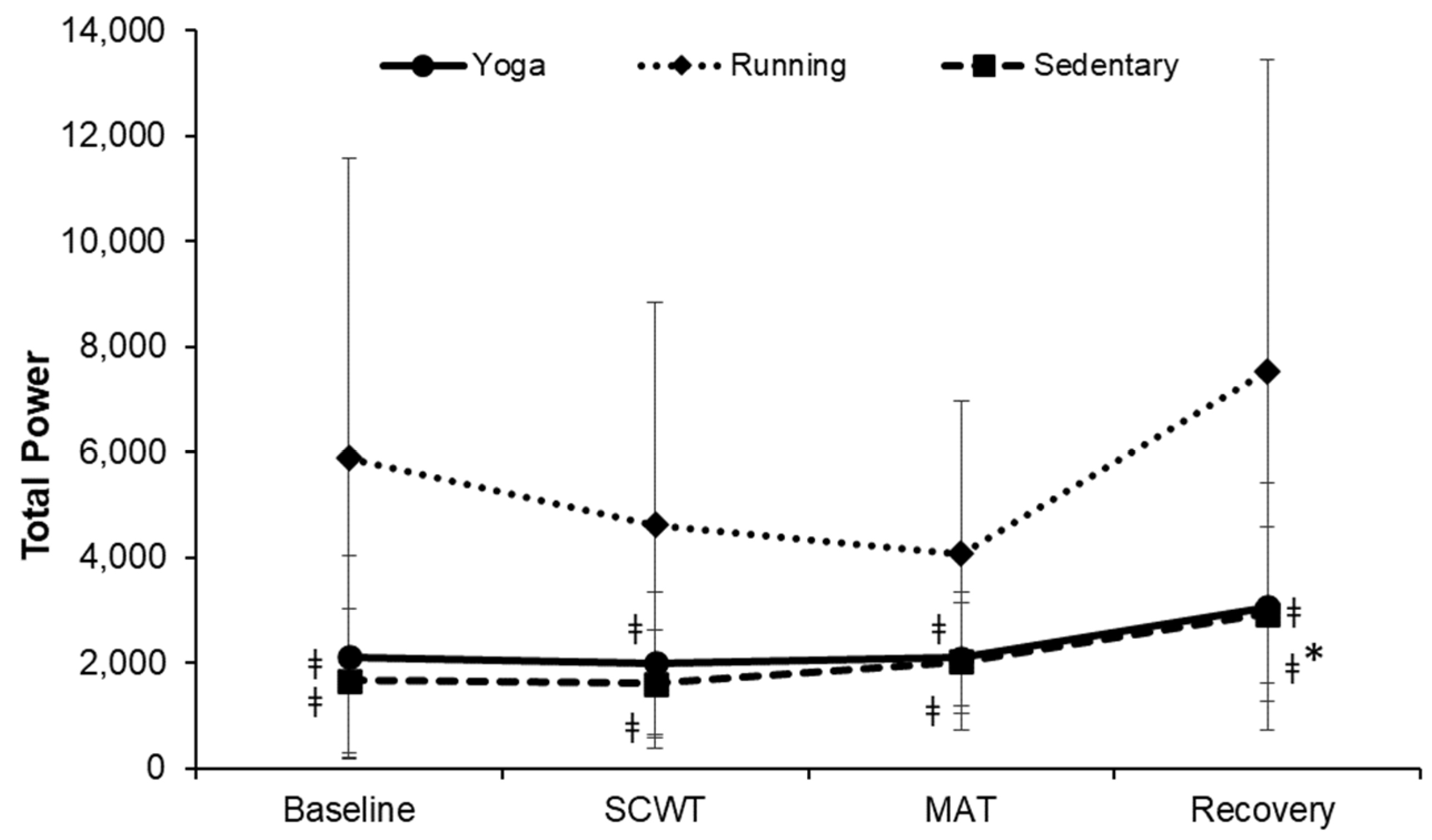
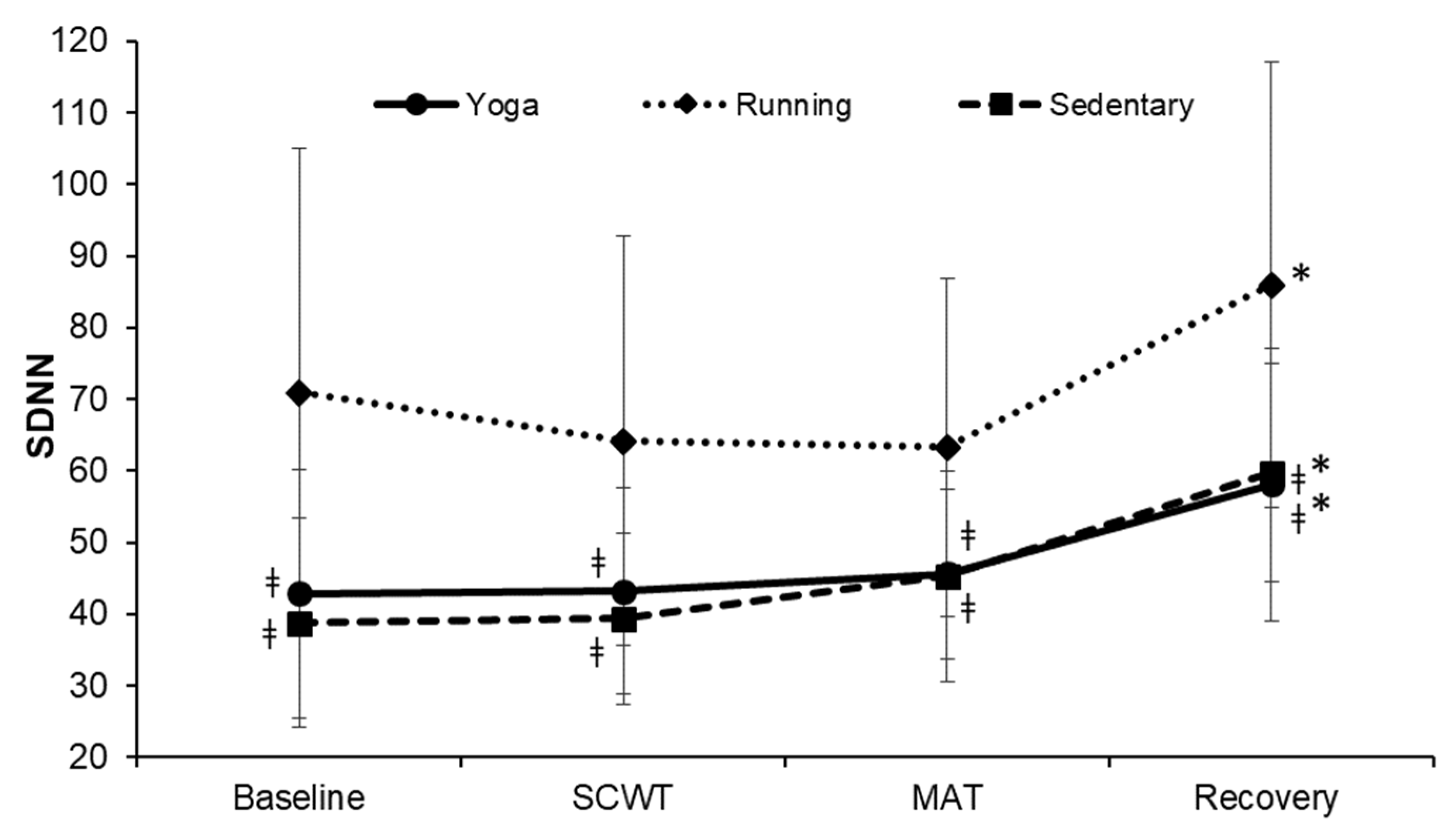
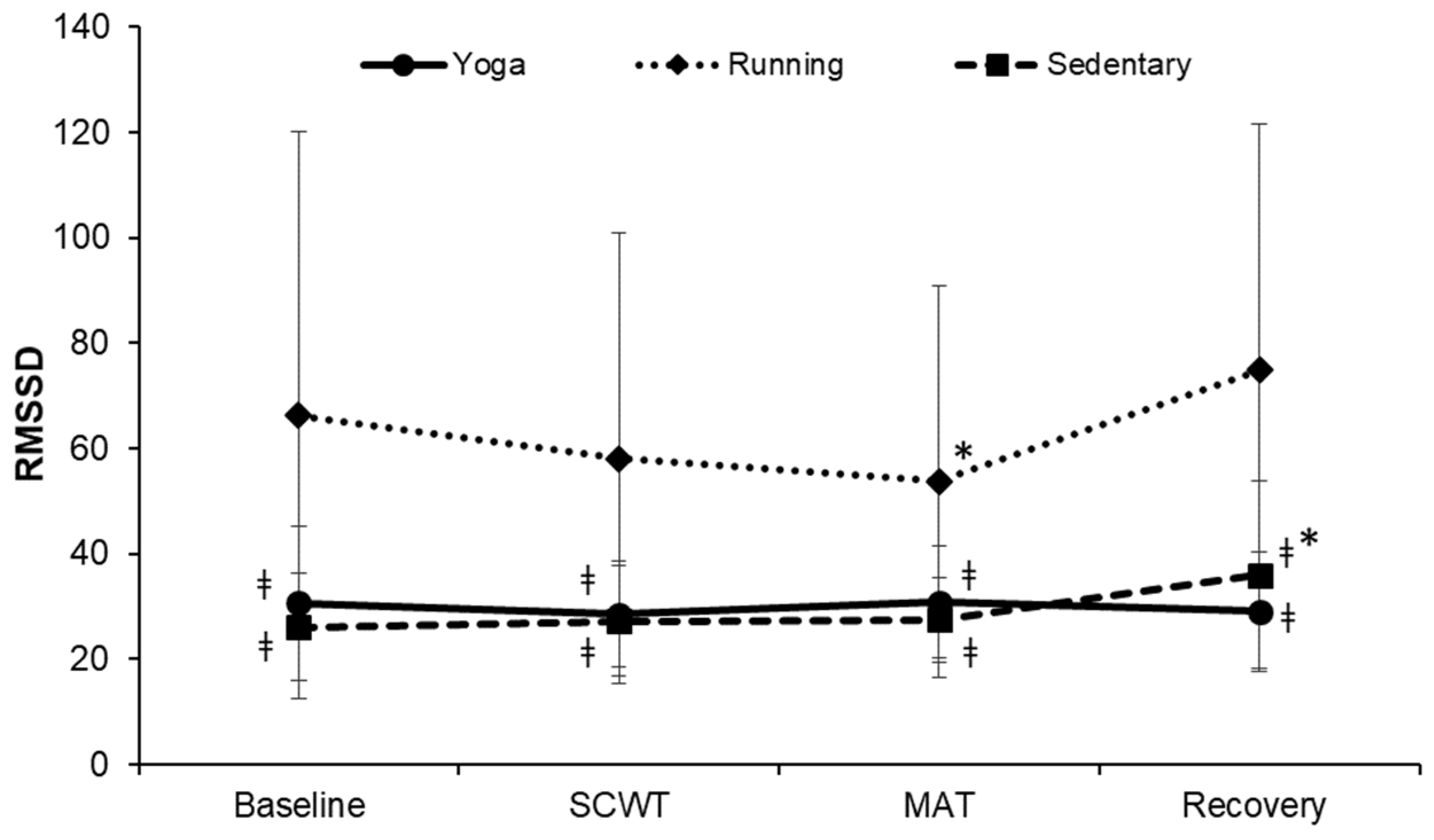
| Characteristics | Yoga (n = 20) | Running (n = 19) | Sedentary (n = 18) | F | p-Value |
|---|---|---|---|---|---|
| Age (years) | 37.95 ± 7.65 | 37.53 ± 6.58 | 37.33 ± 5.58 | 0.04 | 0.958 |
| Height (cm) | 161.48 ± 4.87 | 160.18 ± 2.56 | 158.17 ± 3.84 # | 3.45 | 0.039 |
| Weight (kg) | 54.35 ± 5.02 | 56.71 ± 5.10 | 58.59 ± 7.49 | 2.45 | 0.096 |
| BMI (kg/m2) | 20.87 ± 2.02 | 22.09 ± 1.81 | 23.43 ± 2.93 # | 5.95 | 0.005 |
| Resting RR (times/min) | 10.35 ± 2.13 | 13.47 ± 2.84 # | 14.17 ± 4.19 # | 8.15 | 0.001 |
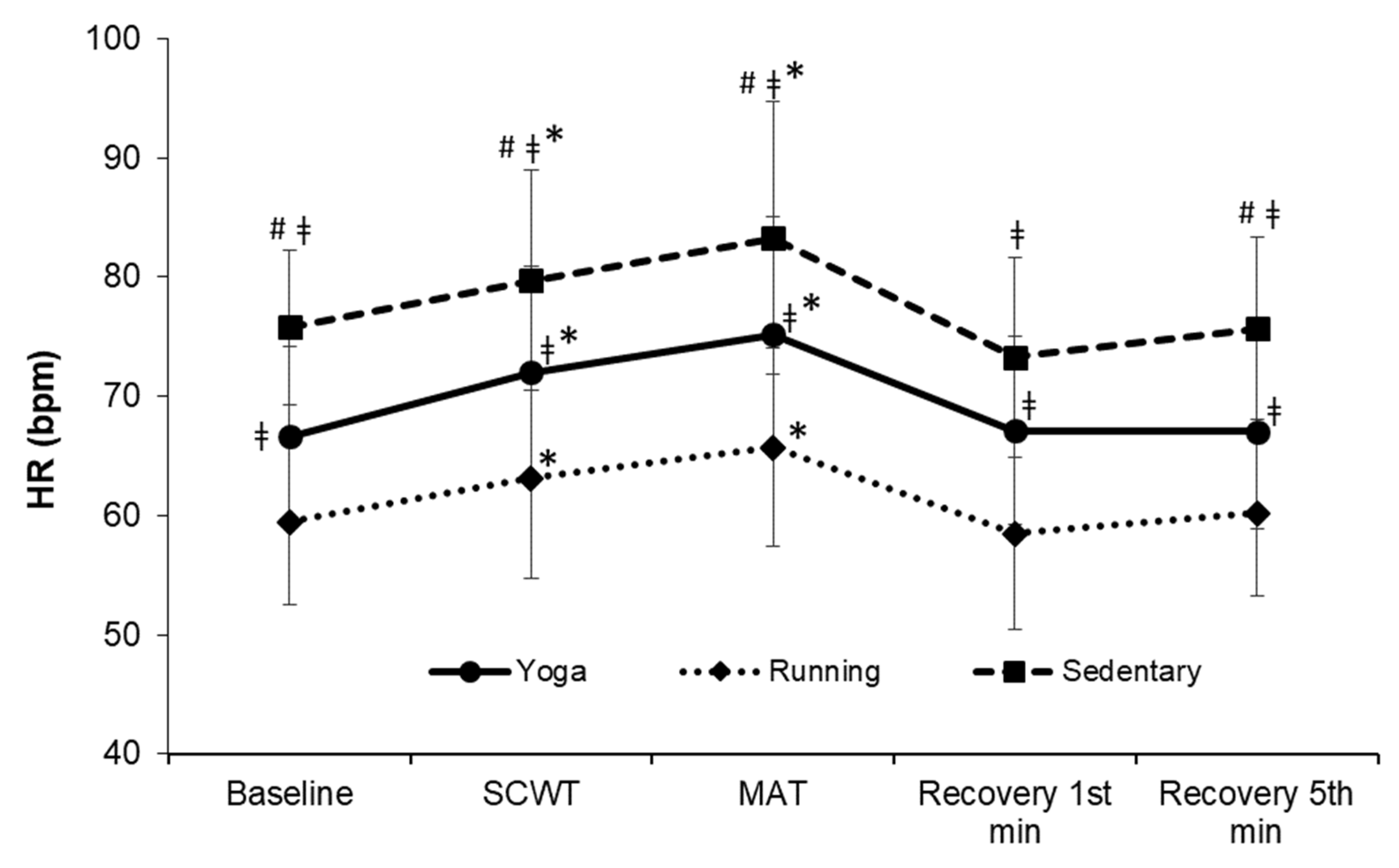
| Resting HR (bpm) | 66.60 ± 7.55 ǂ | 59.47 ± 6.92 | 75.78 ± 6.52 #ǂ | 24.96 | <0.001 |
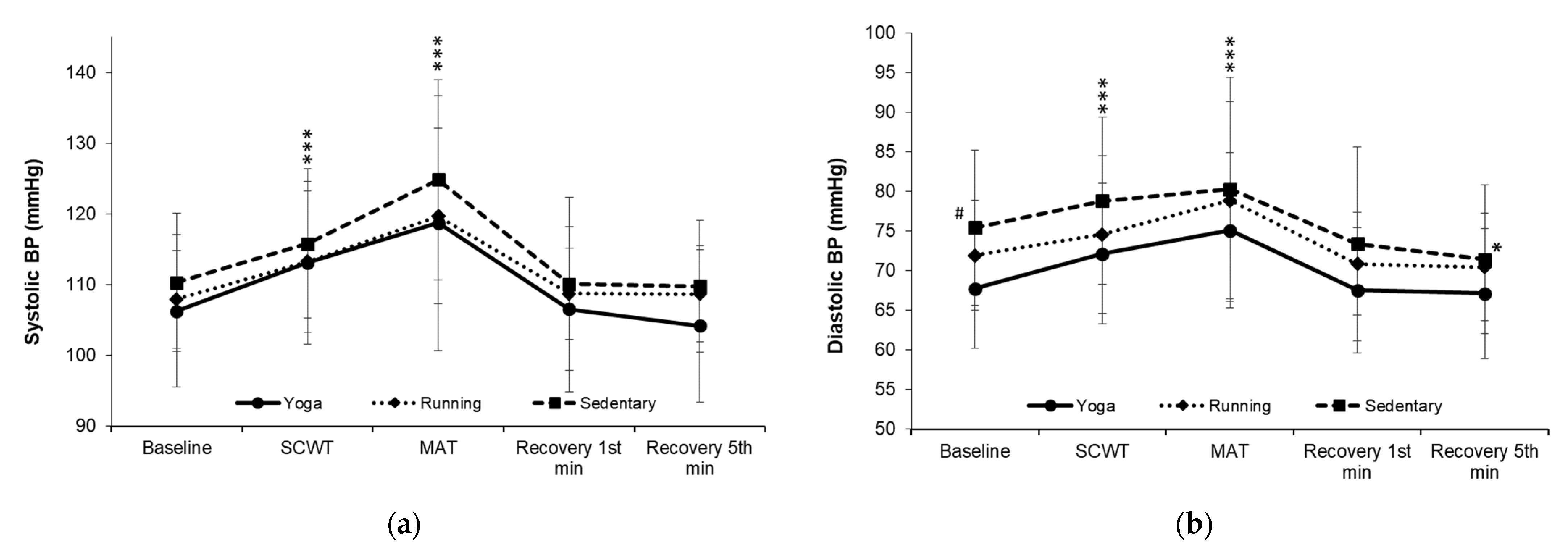
| Resting systolic BP (mmHg) | 106.30 ± 10.79 | 107.95 ± 6.89 | 110.33 ± 9.80 | 0.89 | 0.416 |
| Resting diastolic BP (mmHg) | 67.75 ± 8.38 | 71.95 ± 6.58 | 75.44 ± 7.03 # | 5.16 | 0.009 |
| GLEQ score | 17.45 ± 9.57 ǂ | 36.79 ± 9.41 | 2.22 ± 4.65 #,ǂ | 81.17 | <0.001 |
| Years of practice (years) | 4.60 ± 2.84 | 4.47 ± 3.32 | -- | 0.02 | 0.899 |
| Frequency of practice (times/week) | 4.15 ± 2.70 | 3.53 ± 1.07 | -- | 0.88 | 0.354 |
| Duration of practice (min/session) | 70.00 ± 25.13 | 61.58 ± 33.50 | -- | 0.79 | 0.379 |
| Weekly duration of practice (min/week) | 273.50 ± 158.39 | 213.95 ± 140.98 | -- | 1.53 | 0.224 |
| Aerobic fitness index | 65.59 ± 5.41 ǂ | 83.34 ± 15.66 | 52.73 ± 6.09 #ǂ | 42.26 | <0.001 |
| Variables | Yoga | Running | Sedentary | F | p-Value |
|---|---|---|---|---|---|
| (n = 20) | (n = 19) | (n = 18) | |||
| PSS-14 | 17.40 ± 6.12 | 19.11 ± 5.75 | 21.89 ± 7.10 | 2.41 | 0.099 |
| State-anxiety | 34.35 ± 7.88 | 33.00 ± 6.75 | 39.83 ± 6.87 ǂ | 4.66 | 0.014 |
| Trait-anxiety | 37.05 ± 7.73 | 36.11 ± 7.48 | 42.44 ± 8.05 ǂ | 3.59 | 0.034 |
| BDI-II | 3.40 ± 3.02 | 2.89 ± 3.67 | 5.56 ± 3.79 | 3.01 | 0.058 |
| PSQI | 5.25 ± 2.65 | 4.58 ± 2.93 | 6.00 ± 1.94 | 1.43 | 0.248 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, J.-R.; Wu, P.-T.; Wu, W.-L.; Chang, Y.-K.; Chu, I.-H. The Psychophysiological Profile and Cardiac Autonomic Reactivity in Long-Term Female Yoga Practitioners: A Comparison with Runners and Sedentary Individuals. Int. J. Environ. Res. Public Health 2022, 19, 7671. https://doi.org/10.3390/ijerph19137671
Lin J-R, Wu P-T, Wu W-L, Chang Y-K, Chu I-H. The Psychophysiological Profile and Cardiac Autonomic Reactivity in Long-Term Female Yoga Practitioners: A Comparison with Runners and Sedentary Individuals. International Journal of Environmental Research and Public Health. 2022; 19(13):7671. https://doi.org/10.3390/ijerph19137671
Chicago/Turabian StyleLin, Jia-Ru, Pei-Tzu Wu, Wen-Lan Wu, Yu-Kai Chang, and I-Hua Chu. 2022. "The Psychophysiological Profile and Cardiac Autonomic Reactivity in Long-Term Female Yoga Practitioners: A Comparison with Runners and Sedentary Individuals" International Journal of Environmental Research and Public Health 19, no. 13: 7671. https://doi.org/10.3390/ijerph19137671
APA StyleLin, J.-R., Wu, P.-T., Wu, W.-L., Chang, Y.-K., & Chu, I.-H. (2022). The Psychophysiological Profile and Cardiac Autonomic Reactivity in Long-Term Female Yoga Practitioners: A Comparison with Runners and Sedentary Individuals. International Journal of Environmental Research and Public Health, 19(13), 7671. https://doi.org/10.3390/ijerph19137671








