18F-FDG PET/CT Did Not Increase the Risk of Cataract Occurrence in Oncology Patients: A Nationwide Population-Based Cohort Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Database
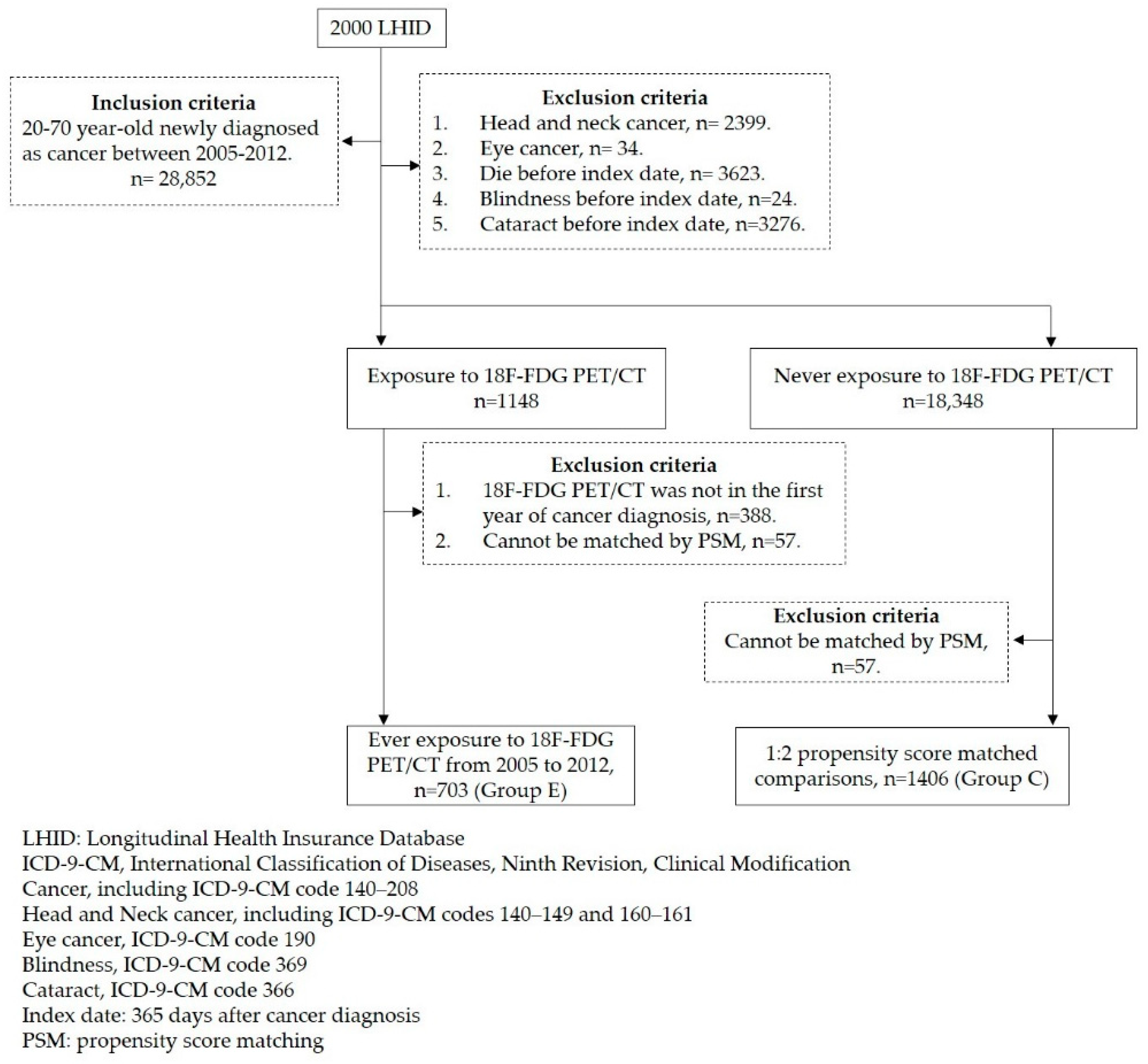
2.2. Study Participants
2.3. Selected Controls
2.4. Identification of Cataract
2.5. Statistical Analysis
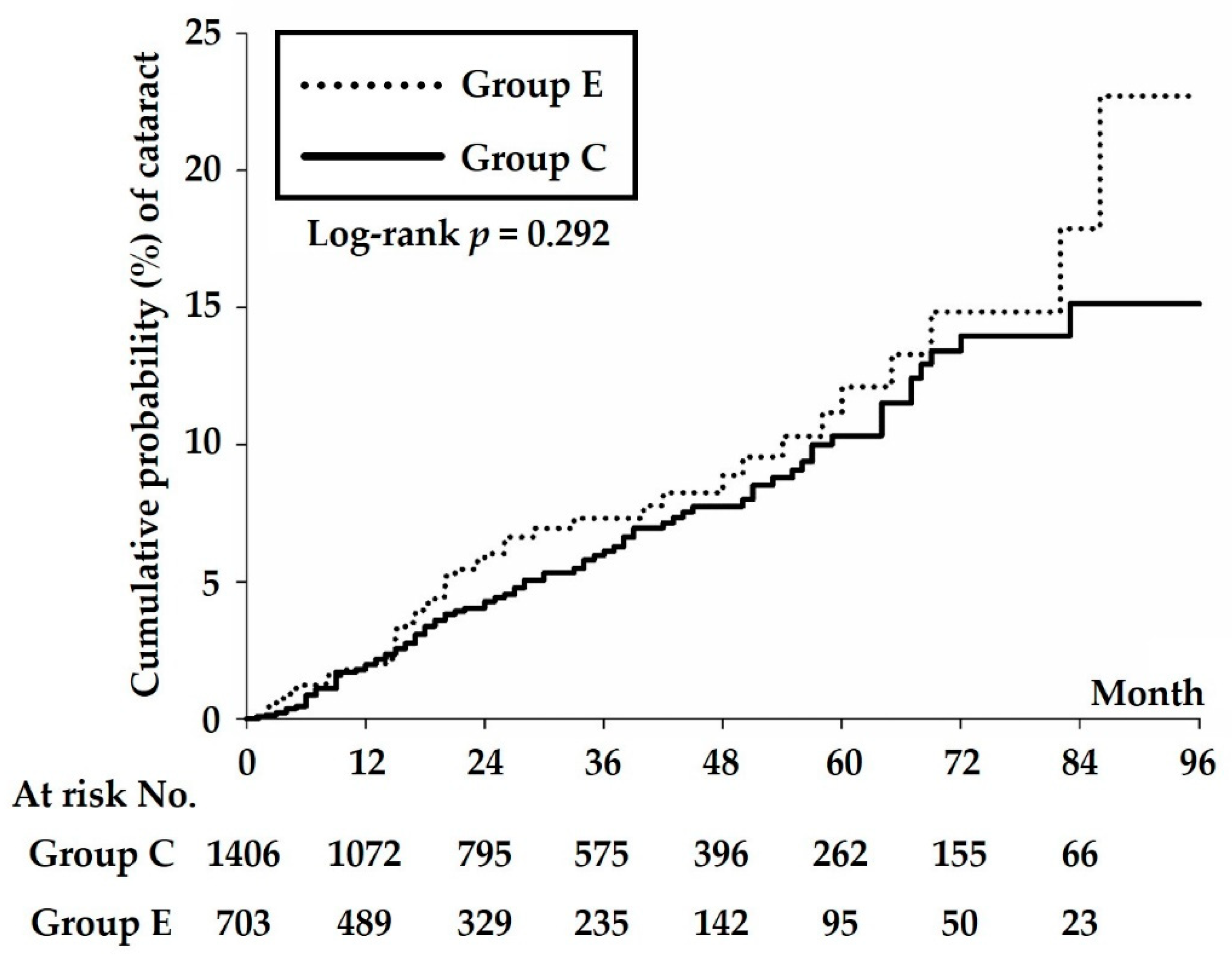
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ainsbury, E.A.; Dalke, C.; Hamada, N.; Benadjaoud, M.A.; Chumak, V.; Ginjaume, M.; Kok, J.L.; Mancuso, M.; Sabatier, L.; Struelens, L.; et al. Radiation-induced lens opacities: Epidemiological, clinical and experimental evidence, methodological issues, research gaps and strategy. Environ. Int. 2021, 146, 106213. [Google Scholar] [CrossRef] [PubMed]
- Cheng, K.L.; Huang, J.Y.; Su, C.L.; Tung, K.C.; Chiou, J.Y. Cataract risk of neuro-interventional procedures: A nationwide population-based matched-cohort study. Clin. Radiol. 2018, 73, 836.e17–836.e22. [Google Scholar] [CrossRef] [PubMed]
- Yuan, M.-K.; Tsai, D.-C.; Chang, S.-C.; Yuan, M.-C.; Chang, S.-J.; Chen, H.-W.; Leu, H.-B. The risk of cataract associated with repeated head and neck CT studies: A nationwide population-based study. Am. J. Roentgenol. 2013, 201, 626–630. [Google Scholar] [CrossRef] [PubMed]
- Chiti, G.; Grazzini, G.; Cozzi, D.; Danti, G.; Matteuzzi, B.; Granata, V.; Pradella, S.; Recchia, L.; Brunese, L.; Miele, V. Imaging of Pancreatic Neuroendocrine Neoplasms. Int. J. Environ. Res. Public Health 2021, 18, 8895. [Google Scholar] [CrossRef]
- Lee, J.W.; Kim, S.Y.; Han, S.W.; Lee, J.E.; Hong, S.H.; Lee, S.M.; Jo, I.Y. Clinical Significance of Peritumoral Adipose Tissue PET/CT Imaging Features for Predicting Axillary Lymph Node Metastasis in Patients with Breast Cancer. J. Pers. Med. 2021, 11, 1029. [Google Scholar] [CrossRef]
- Pahk, K.; Ryu, K.-J.; Joung, C.; Kwon, H.W.; Lee, S.; Park, H.; Kim, T.; Song, J.Y.; Kim, S. Metabolic Activity of Visceral Adipose Tissue Is Associated with Metastatic Status of Lymph Nodes in Endometrial Cancer: A 18F-FDG PET/CT Study. Int. J. Environ. Res. Public Health 2022, 19, 92. [Google Scholar] [CrossRef]
- Paiva, F.G.; do Carmo Santana, P.; Mourão, A.P. Evaluation of patient effective dose in a PET/CT test. Appl. Radiat. Isot. 2019, 145, 137–141. [Google Scholar] [CrossRef]
- Authors on behalf of ICRP; Stewart, F.; Akleyev, A.; Hauer-Jensen, M.; Hendry, J.; Kleiman, N.; Macvittie, T.; Aleman, B.; Edgar, A.; Mabuchi, K.; et al. ICRP publication 118: ICRP statement on tissue reactions and early and late effects of radiation in normal tissues and organs–threshold doses for tissue reactions in a radiation protection context. Ann. ICRP 2012, 41, 1–322. [Google Scholar]
- Worgul, B.; Kundiyev, Y.I.; Sergiyenko, N.; Chumak, V.; Vitte, P.; Medvedovsky, C.; Bakhanova, E.; Junk, A.; Kyrychenko, O.; Musijachenko, N.; et al. Cataracts among Chernobyl clean-up workers: Implications regarding permissible eye exposures. Radiat. Res. 2007, 167, 233–243. [Google Scholar] [CrossRef]
- Miller, D.L.; Vañó, E.; Bartal, G.; Balter, S.; Dixon, R.; Padovani, R.; Schueler, B.; Cardella, J.F.; De Baère, T.J.C. Occupational radiation protection in interventional radiology: A joint guideline of the Cardiovascular and Interventional Radiology Society of Europe and the Society of Interventional Radiology. Cardiovasc. Interv. Radiol. 2010, 33, 230–239. [Google Scholar] [CrossRef] [Green Version]
- Hung, M.-C.; Huang, Y.-Y. Three Nuclear Medicine diagnostic procedures and breast cancer mortality in women. A population-analysis in Taiwan based upon National Health Insurance database. Hell. J. Nucl. Med. 2019, 22, 111–115. [Google Scholar] [PubMed]
- Worgul, B.V.; Smilenov, L.; Brenner, D.J.; Junk, A.; Zhou, W.; Hall, E.J. Atm heterozygous mice are more sensitive to radiation-induced cataracts than are their wild-type counterparts. Proc. Natl. Acad. Sci. USA 2002, 99, 9836–9839. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neriishi, K.; Nakashima, E.; Akahoshi, M.; Hida, A.; Grant, E.J.; Masunari, N.; Funamoto, S.; Minamoto, A.; Fujiwara, S.; Shore, R.E. Radiation dose and cataract surgery incidence in atomic bomb survivors, 1986–2005. Radiology 2012, 265, 167–174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klein, B.E.; Klein, R.; Linton, K.; Franke, T. Diagnostic X-ray exposure and lens opacities: The Beaver Dam Eye Study. Am. J. Public Health 1993, 83, 588–590. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quinn, B.; Dauer, Z.; Pandit-Taskar, N.; Schoder, H.; Dauer, L.T. Radiation dosimetry of 18F-FDG PET/CT: Incorporating exam-specific parameters in dose estimates. BMC Med. Imaging 2016, 16, 41. [Google Scholar] [CrossRef]
- Huang, B.; Law, M.W.-M.; Khong, P.-L. Whole-Body PET/CT Scanning: Estimation of Radiation Dose and Cancer Risk. Radiology 2009, 251, 166–174. [Google Scholar] [CrossRef]
- Brix, G.; Lechel, U.; Glatting, G.; Ziegler, S.I.; Münzing, W.; Müller, S.P.; Beyer, T. Radiation Exposure of Patients Undergoing Whole-Body Dual-Modality 18F-FDG PET/CT Examinations. J. Nucl. Med. 2005, 46, 608. [Google Scholar]
- Hosono, M.; Takenaka, M.; Monzen, H.; Tamura, M.; Kudo, M.; Nishimura, Y. Cumulative radiation doses from recurrent PET–CT examinations. Br. J. Radiol. 2021, 94, 20210388. [Google Scholar] [CrossRef]
- Hourihan, F.; Mitchell, P.; Cumming, R.G. Possible associations between computed tomography scan and cataract: The Blue Mountains Eye Study. Am. J. Public Health 1999, 89, 1864–1866. [Google Scholar] [CrossRef] [Green Version]
- Klein, R.; Klein, B.E.; Wong, T.Y.; Tomany, S.C.; Cruickshanks, K.J. The association of cataract and cataract surgery with the long-term incidence of age-related maculopathy: The Beaver Dam eye study. Arch. Ophthalmol. 2002, 120, 1551–1558. [Google Scholar] [CrossRef] [Green Version]
- Kanthan, G.L.; Wang, J.J.; Rochtchina, E.; Tan, A.G.; Lee, A.; Chia, E.-M.; Mitchell, P. Ten-year incidence of age-related cataract and cataract surgery in an older Australian population: The Blue Mountains Eye Study. Ophthalmology 2008, 115, 808–814.e1. [Google Scholar] [CrossRef] [PubMed]
- Mukesh, B.N.; Le, A.; Dimitrov, P.N.; Ahmed, S.; Taylor, H.R.; McCarty, C.A. Development of Cataract and Associated Risk Factors: The Visual Impairment Project. Arch. Ophthalmol. 2006, 124, 79–85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Non-PET | PET | p Value | |
|---|---|---|---|
| Sex | 1.000 | ||
| Female | 776 (55.19%) | 388 (55.19%) | |
| Male | 630 (44.81%) | 315 (44.81%) | |
| Age at index date | 0.496 | ||
| 20–49 | 481 (34.21%) | 245 (34.85%) | |
| 50–59 | 557 (39.62%) | 261 (37.13%) | |
| ≥60 | 368 (26.17%) | 197 (28.02%) | |
| Cancer type | 1.000 | ||
| Lung cancer | 400 (28.45%) | 200 (28.45%) | |
| Colorectal cancer | 314 (22.33%) | 157 (22.33%) | |
| Breast cancer | 210 (14.94%) | 105 (14.94%) | |
| Esophagus cancer | 66 (4.69%) | 33 (4.69%) | |
| Lymphoma | 134 (9.53%) | 67 (9.53%) | |
| Others * | 282 (20.06%) | 141 (20.06%) | |
| Co-morbidities | |||
| Hypertension | 381 (27.10%) | 191 (27.17%) | 0.972 |
| Diabetes mellitus | 206 (14.65%) | 94 (13.37%) | 0.428 |
| COPD | 178 (12.66%) | 85 (12.09%) | 0.709 |
| Coronary artery disease | 119 (8.46%) | 34 (4.84%) | 0.003 |
| Chronic renal disease | 76 (5.41%) | 27 (3.84%) | 0.116 |
| Gout | 90 (6.40%) | 24 (3.41%) | 0.004 |
| Rheumatoid arthritis | 20 (1.42%) | 3 (0.43%) | 0.038 |
| Dementia | 10 (0.71%) | 8 (1.14%) | 0.315 |
| Alcohol-related diseases | 24 (1.71%) | 8 (1.14%) | 0.314 |
| Depression | 205 (14.58%) | 107 (15.22%) | 0.696 |
| Sleep disorders | 254 (18.07%) | 136 (19.35%) | 0.475 |
| Obesity | 9 (0.64%) | 2 (0.28%) | 0.285 |
| Hyperlipidemia: | 263 (18.71%) | 73 (10.38%) | <0.0001 |
| Atopic dermatitis | 20 (1.42%) | 11 (1.56%) | 0.798 |
| No. of CT in the first year | <0.0001 | ||
| 0 | 690 (49.08%) | 114 (16.22%) | |
| 1 to 4 | 647 (46.02%) | 486 (69.13%) | |
| ≥5 | 69 (4.91%) | 103 (14.65%) |
| Group C | Group E | p Value | |
|---|---|---|---|
| N | 1406 | 703 | |
| Follow up person months | 47,373 | 20,079 | |
| Event of cataract | 86 | 44 | |
| Incidence density § | 18.15 (14.70–22.42) | 21.91 (16.31–29.45) | 0.310 |
| Crude hazard ratio | Reference | 1.276 (0.885–1.841) | 0.192 |
| Adjusted hazard ratio † | Reference | 1.264 (0.845–1.891) | 0.255 |
| aHR (95% CI) | p Value | |
|---|---|---|
| PET exposure | ||
| No | Reference | |
| Yes | 1.281 (0.854–1.921) | 0.231 |
| Sex | ||
| Female | Reference | |
| Male | 0.947 (0.639–1.401) | 0.784 |
| Age at index date | ||
| 20–49 | Reference | |
| 50–59 | 6.330 (2.835–14.135) | <0.0001 |
| ≥60 | 14.151 (6.312–31.728) | <0.0001 |
| Cancer type | ||
| Lung cancer, | Reference | |
| Colorectal cancer | 1.000 (0.620–1.614) | 0.998 |
| Breast cancer | 0.580 (0.276–1.218) | 0.150 |
| Esophagus cancer | 0.787 (0.299–2.071) | 0.627 |
| Lymphoma | 0.582 (0.254–1.333) | 0.201 |
| Others | 1.001 (0.579–1.728) | 0.999 |
| Co-morbidities | ||
| Hypertension | 1.384 (0.936–2.048) | 0.104 |
| Diabetes mellitus | 1.078 (0.679–1.712) | 0.750 |
| COPD | 1.222 (0.733–2.038) | 0.442 |
| Coronary artery disease | 1.212 (0.726–2.025) | 0.462 |
| Chronic renal disease | 0.939 (0.481–1.832) | 0.853 |
| Gout | 0.732 (0.352–1.520) | 0.402 |
| Rheumatoid arthritis | 2.182 (0.662–7.184) | 0.200 |
| Dementia | 1.091 (0.146–8.135) | 0.932 |
| Alcohol-related diseases | 0.710 (0.096–5.240) | 0.737 |
| Depression | 1.202 (0.751–1.926) | 0.443 |
| Sleep disorders | 1.232 (0.785–1.935) | 0.364 |
| Obesity | 2.005 (0.271–14.807) | 0.495 |
| Hyperlipidemia: | 1.400 (0.905–2.166) | 0.131 |
| Atopic dermatitis | 0.366 (0.050–2.661) | 0.321 |
| No. of CT in the first year | ||
| 0 | Reference | |
| 1 to 4 | 0.840 (0.549–1.285) | 0.421 |
| ≥5 | 1.608 (0.830–3.114) | 0.159 |
| Person-Months | Event of Cataract | Incidence Density § | Adjusted HR † | |
|---|---|---|---|---|
| CT < 5 and PET = 0 (n = 1337) | 45,999 | 81 | 17.61 (14.16–21.89) | Reference |
| CT < 5 and PET = 1 (n = 482) | 13,957 | 26 | 18.63 (12.68–27.36) | 1.116 (0.693–1.795) |
| CT < 5 and PET ≥ 2 (n = 118) | 3448 | 9 | 26.10 (13.58–50.16) | 2.029 (0.976–4.219) |
| CT ≥ 5 and PET = 0 (n = 69) | 1374 | 5 | 36.39 (15.15–87.43) | 1.460 (0.567–3.760) |
| CT ≥ 5 and PET = 1 (n = 82) | 1646 | 8 | 48.60 (24.30–97.19) | 2.380 (1.097–5.161) |
| CT ≥ 5 and PET ≥ 2 (n = 21) | 1028 | 1 | 9.73 (1.37–69.06) | 0.727 (0.098–5.403) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, K.-L.; Huang, J.-Y.; Weng, J.-H.; Chiou, J.-Y.; Lan, C.-T.; Tung, K.-C. 18F-FDG PET/CT Did Not Increase the Risk of Cataract Occurrence in Oncology Patients: A Nationwide Population-Based Cohort Study. Int. J. Environ. Res. Public Health 2022, 19, 7651. https://doi.org/10.3390/ijerph19137651
Cheng K-L, Huang J-Y, Weng J-H, Chiou J-Y, Lan C-T, Tung K-C. 18F-FDG PET/CT Did Not Increase the Risk of Cataract Occurrence in Oncology Patients: A Nationwide Population-Based Cohort Study. International Journal of Environmental Research and Public Health. 2022; 19(13):7651. https://doi.org/10.3390/ijerph19137651
Chicago/Turabian StyleCheng, Kai-Lun, Jing-Yang Huang, Jui-Hung Weng, Jeng-Yuan Chiou, Chyn-Tair Lan, and Kwong-Chung Tung. 2022. "18F-FDG PET/CT Did Not Increase the Risk of Cataract Occurrence in Oncology Patients: A Nationwide Population-Based Cohort Study" International Journal of Environmental Research and Public Health 19, no. 13: 7651. https://doi.org/10.3390/ijerph19137651
APA StyleCheng, K.-L., Huang, J.-Y., Weng, J.-H., Chiou, J.-Y., Lan, C.-T., & Tung, K.-C. (2022). 18F-FDG PET/CT Did Not Increase the Risk of Cataract Occurrence in Oncology Patients: A Nationwide Population-Based Cohort Study. International Journal of Environmental Research and Public Health, 19(13), 7651. https://doi.org/10.3390/ijerph19137651






