Abstract
The dramatic lifestyle changes forced by COVID-19-related lockdown promoted weight gain, with a stronger impact on obese subjects, at higher risk of severe infection. The PubMed database was searched to identify original studies assessing: (1) the extent and risk factors of lockdown-induced weight increase; and (2) the impact of obesity on the risk of hospital admission in children and adolescents. A systematic literature review and meta-analyses were performed. Twenty out of 13,986 identified records were included. A significant weight increase was reported in the majority of subjects, with no apparent gender or age differences. It was induced by a higher consumption of hypercaloric/hyperglycemic/junk food and/or the reduction of physical activity, often associated with an altered sleep–wake cycle. On the other hand, obesity increased the risk of hospitalization (OR = 4.38; 95% C.I. 1.46–13.19; p = 0.009; I2 = 96%) as compared to the normal weight population. COVID-19 and obesity represent epidemic conditions with reciprocal detrimental impact. Urgent public health interventions, targeting the various age and social strata, and involving governmental authorities, health care personnel, teachers and families are warranted to increase awareness and actively promote healthy lifestyles to contrast pediatric obesity and its detrimental consequences at a global level.
1. Introduction
COVID-19, caused by SARS-CoV-2 infection and characterized by acute respiratory syndrome with high morbidity and mortality, was first discovered in Wuhan (China) in December 2019 and rapidly spread all over the world, becoming a pandemic [1,2].
To limit its diffusion, most of the countries adopted unprecedented restriction measures to ensure social distancing, including home quarantines and national closure of schools, working sites and other public places [3]. The generalized lockdown forced the entire population to sudden and drastic lifestyle changes and increased the perceived stress and anxiety associated with the pandemic [3].
Overall, caloric intake significantly increased, while physical activity was drastically reduced. A recent meta-analysis showed significant increases in body weight and BMI during closure among school-age children and adolescents, as well as the increase of obesity and overweight prevalence [4]. Moreover, the sleep–wake cycle was altered, with longer day sleep time and night insomnia, the latter contributing to night eating. Their combination promoted weight increase (“obesogenic environment”) in adults and children [5,6].
Since the early 2000s, pediatric obesity has been recognized as an alarming global health issue of increasing proportions, induced by unhealthy eating habits, sedentary lifestyle, stress and psychological disorders [7,8]. To underline the direct detrimental effect of COVID-19 lockdown on obesity, the term ‘covibesity’ has been introduced [9].
Contextually, obesity has been demonstrated as an independent risk factor of more severe COVID-19 disease and worse prognosis in children [10], who otherwise present with mild symptoms [11,12,13,14,15,16,17].
Based on these premises, we aimed to systematically and critically analyze: (1) the incidence and potential predictors of obesity induced by COVID-19 pandemic-lockdown; and (2) the risk of severe COVID-19 disease in obese children and adolescents.
2. Materials and Methods
The PubMed database (https://pubmed.ncbi.nlm.nih.gov, accessed on 31 December 2021) was searched for studies on COVID-19 in children and adolescents using the purposely formulated string: “(SARS-CoV-2 OR COVID-19 OR ncov* or coronavirus) AND (child OR children OR pediatric OR paediatric OR infant OR adolescent)”. Only original studies (i.e., cohort, cross-sectional and case-control) including patients aged 0–18 years of both genders, written in English or in Italian, published from December 2019 to December 2021, for which the abstract was available, were kept.
Reviewer pairs (G.L.F. and M.M.; Z.D.V. and M.G.S; A.S. and G.S.) were created. Each reviewer independently screened articles by title and abstract to identify eligible studies. Any disagreement was solved through discussion among the reviewers and team consensus. The same authors evaluated the full texts of relevant studies. For each full text, the following information was recorded: title, author(s), country, study period (months), study design, sample size, population, age (mean ± SD and range) and gender. To determine the impact of lockdown on weight, we also recorded baseline weight/BMI, weight/BMI increase, modifications of eating habits and/or physical activity. Finally, we recorded information on sleep disorders/modification of sleep–wake habits. On the other hand, to assess the impact of overweight/obesity on hospital admission, we recorded information on the number of obese children/adolescents included in the sample, the number of obese hospitalized and the number of normal weight children/adolescents hospitalized.
We tested statistical heterogeneity to determine if it was appropriate to combine the studies for meta-analysis. We analyzed statistical heterogeneity to test the robustness of matching the studies for meta-analysis, evaluating heterogeneity by the use of graphic forest plots and by calculating the I2 statistic, which represents the percentage of the variance in effect estimates that is caused by heterogeneity rather than by sampling bias (chance). We considered an I2 statistic greater than 40% to be substantially heterogeneous. According to the Cochrane Handbook for Systematic Reviews of Interventions [18], if studies were <5 or substantially heterogeneous, we used a random-effects model, following the method of DerSimonian and Laird, to compute the random-effects estimates for the corresponding statistics [19]. Forest plots were created to display effect estimates with 95% CIs. For all data analyses, the RevMan program was used (Version 5.4.1; The Nordic Cochrane Centre, The Cochrane Collaboration, Copenhagen, Denmark, 2014) [20].
3. Results
The database search retrieved 13,986 articles, including 13,845 from the PubMed search, 28 from a bibliographic search of relevant papers, and 113 from hand search. Sixty-seven were excluded as they were duplicates, while 13,752 were excluded because they were not pertinent with the review aims. Of the remaining 167,147 records were excluded because they were not original studies, or did not focus on the topic of interest, i.e., did not evaluate the impact of lockdown on obesity, or did not report the outcome of COVID-19 specifically in children or adolescents.
Finally, 20 articles were considered eligible for full-text evaluation: 14 evaluated the effect of COVID-19 lockdown on obesity (first review aim), while six focused on obesity as a risk factor for hospital/Intensive Care Unit (ICU) admission (second review aim). The PRISMA flowchart, showing the study selection process, is reported in Figure 1.
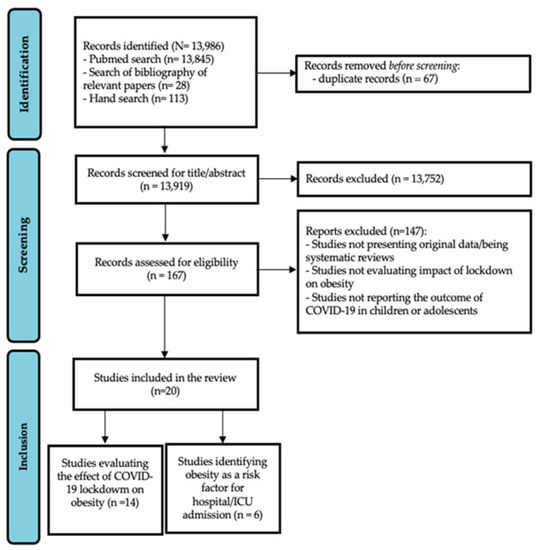
Figure 1.
PRISMA flow chart showing the study selection process.
3.1. Impact of COVID-19 Lockdown on Weight and Lifestyle in Children and Adolescents
Relevant data of the 14 studies focusing on the impact of COVID-19 lockdown on changes in weight and lifestyle in children and adolescents are summarized in Table 1.

Table 1.
Main patient and protocol features of studies assessing the impact of COVID-19 lockdown on weight and lifestyle changes included in the literature review.
Sample size ranged from 40 [31] to 274,456 [32] patients. Males represented 24 (678/2824) [26] to 77.8% (70/90) [28] of the study population. Age ranged from 0 [32] to 18 years [32,33], and mean age ± SD from 7.8 ± 4.1 [21] to 17.5 ± 1.2 [26]. Ten studies were performed in hospitals (71.4%), 2 (14.3%) online/via social-media, and 2 (14.3%) in schools.
Eight (57.1%) studies evaluated changes of patients BMI-SDS/z-score [22,23,25,27,28,29,33,34], six (42.9%) of Body Mass Index (BMI) [24,26,28,29,30,31] and four (28.6%) [21,26,28,29] of patient weight. Vinker-Shuster M et al. reported adjusted weight percentiles [32]; Maltoni et al. reported modifications also in waist circumferences and waist/height ratio [29].
At baseline evaluation, performed before COVID-19 lockdown, BMI ranged from 20.9 (median BMI) [30] to 32.6 ± 4.0 (mean ± SD) [29]; BMI-SDS/z-score ranged from 0.001 (median BMI-SDS) [33] to 2.4 ± 0.5 (mean ± SD) [29], and mean body weight from 32.3 ± 16.9 [21] to 67.2 ± 23.8 [28].
At follow-up evaluation, performed at the end of the lock-down, six studies (42.9%) reported the increase of BMI [25,26,28,29,30,31], 5 (35.7%) of body weight [21,26,28,29,32], and five others of BMI SDS/z-score [23,28,29,33,34]. According to four others (14.3%) weight distribution remained stable [21,22,24,27], although the last two evaluated only a part of the study population.
Three (21.4%) studies reported ‘changes in eating habits’ associated with lockdown that consisted of a significant increase of the intake of fresh fruit, vegetables, dairy products, pasta, sweets (especially at breakfast) and snacks in the study by Androutsos et al. [21], and of pasta, bread and pizza according to Cipolla et al. [24], and in the decrease of vegetable/fruit intake in the study of Maltoni et al. [29]. Valenzise et al. [31] reported an overall increase in BMI during lockdown, although not significant, associated with an overall increase in the number of daily meals, especially in children with parents with elementary school diploma vs. high school diploma (6 ± 0.7 vs. 4.4 ± 1.3; p = 0.019).
Six (42.9%) studies reported a reduction of physical activity [21,24,26,28,29,31], together with an increase in video-gaming [24], while two (14.3%) focused on the impact of COVID-19 lockdown on sleep hygiene and demonstrated an overall increase of sleep hours [21,26]. Azoulay et al. [22] reported the positive effect on body composition associated with engagement in physical activity during the lock-down, although data on lifestyle changes (type and prevalence) were not reported.
According to Brooks et al. [23] and Hu et al. [25], children aged 8–12-years old and 6–11-years old, respectively, had a more marked increase in weight gain than adolescents. Moreover, four studies reported a higher weight increase in boys than in girls in [23,25,29,30], likely related to a more significant increase of sedentary behavior, electronic gaming and screen time, especially in younger children.
Pre-existing weight excess/obesity [23], lower socioeconomic position, a lack of health insurance/Medicaid, Black and Hispanic ethnicity increased the risk of weight increase [22,23]. In the study by Hu et al. [25], no significant differences in BMI increase associated with lockdown were detected in children living in urban and rural areas.
3.2. Impact of Overweight/Obesity on the Risk of Hospital/ICU Admission in Children/Adolescents with COVID-19
The main data of patients enrolled in the six studies [35,36,37,38,39,40] assessing the impact of overweight/obesity on hospital/ICU admission in children/adolescents with COVID-19 are shown in Table 2.

Table 2.
Main patient and study features of articles assessing the risk of hospital admission/ICU in obese children and adolescents included in literature review.
Sample size ranged from 48 [40] to 30,527 [39] patients. All studies included both children and adolescents. Males represented 45.6 [37] to 63% [40] of the sample. Prevalence of obesity varied from 2.8 [39] to 39.6% [40]; all obese patients presented with COVID-19 symptoms.
The rate of hospital admission among obese patients ranged from 31.3 [37] to 60.3% [36], and from 9.3% [38] to 24.8% [37] in normal weight ones. In three studies, all patients recruited were admitted to hospital/ICU [35,39,40].
Obesity was found to be an independent risk factor for severe/critical illness in two (33.3%) studies [35,37] and for hospital admission in three (50%) studies [36,38,40].
Other risk factors associated with more severe COVID-19 with hospital/ICU admission were adolescence [37,39], age < 1 year [39], Type 1 diabetes [38] and black ethnicity [39].
Due to the wide heterogeneity of outcomes, investigation tools and statistical analysis, only three studies could be included in the meta-analysis [36,37,38], showing a significantly increased risk of hospitalization for obese children/adolescents as compared to normal weight peers (OR = 4.38; 95% C.I. = 1.46–13.19; p = 0.009; I2 = 96%) (Figure 2).
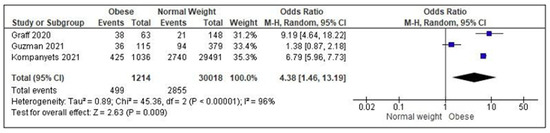
Figure 2.
Meta-analysis assessing the risk of hospitalization/admission to Intensive Care Units in obese children/adolescents as compared to normal weight peers. Studies are listed in alphabetical order [36,37,38].
4. Discussion
The aims of the present systematic literature review and meta-analysis were to assess in children and adolescents the impact of COVID-19 lockdown in promoting weight gain and obesity, on one hand, and the risk of developing severe COVID-19 disease, with consequent admission to hospital/ICU, associated with overweight/obesity on the other hand.
COVID-19 and pediatric obesity can be approached as “syndemic conditions”. This notion, conceived by the medical anthropologist Merrill Singer in the 1990s, goes beyond comorbidities as it identifies conditions characterized by biological and social interactions that increase a person’s susceptibility to harm or worsen health outcomes, so are important for prognosis, treatment and health policy. As the same author explained, a “syndemic approach” provides a very different orientation to clinical medicine and public health as an integrated approach to understanding and treating diseases can be significantly more successful than simply controlling epidemic disease or treating individual patients [41]. In 2020, Horton suggested applying the term ‘syndemic’ to COVID-19 for its clustering and interactions with pre-existing conditions, and the influence of larger political, economic and social factors [42].
4.1. Impact of COVID-19 Lockdown on Weight and Lifestyle Changes in Children and Adolescents
Data analysis clearly demonstrated the detrimental impact of COVID-19 lockdown on children and adolescents’ body weight and BMI, children with pre-existing overweight/obesity being more at risk of gaining weight.
Obesity is a chronic disease resulting from the interaction of genetic, environmental, and psychosocial factors leading to the predominance of caloric intake over expenditure. Based on the standardized growth charts of the Centers for Disease Control and Prevention [43], youths are defined as ‘overweight’ for body weight in the 85th–94th centile, ‘obese’ in the 95th–98th centile, and ‘severely obese’ if >99th centile. Even before the COVID-19 pandemic, pediatric weight excess was considered a worrisome epidemic condition affecting over 337 million children globally, with more than 124 million cases of obesity and severe obesity, worsening over the time, with rates varying with age, ethnicity, location, and social determinants [7]. The associated health and social burden depend on the several physical and psychological co-morbidities, characterized by early onset and lifetime duration, with an overall poor patient quality of life and significant social costs [44,45,46].
Weight gain seems to be explained primarily by the increase of sedentary life, secondary to the longer time spent at home sitting and screen time for homeschooling and recreational activities (i.e., video games, computers/tablets, and television), in the absence of a structured environment on weekdays, to the detriment of compulsory physical activity at school and extracurricular physical activity in dedicated recreational spaces and outdoors [21,26,27,29,32,39]. This phenomenon appeared to be more prevalent among younger children with respect to adolescents, and in boys as compared to females [23,25,29,30]. As demonstrated by previous studies, the abrupt cessation of exercise and prolonged inactivity promote several other adverse health changes, including insulin resistance, muscle atrophy and bone loss [47].
Change in dietary habits towards unhealthy patterns and lifestyles characterized by an overall increase of ingested calories, secondary to a higher number of meals per day [31], a more abundant breakfast [21], and the consumption of hypercaloric food at the various meals (i.e., sweets, snacks, carbohydrates, junk food [21,24,29]), significantly contributed to weight increase during COVID-19 lockdown.
Some studies also reported the alteration of sleep-wake cycles during the lockdown period—predisposing to night eating [26,27,32] and altering the hormone circadian rhythms—[48,49] and the overall increase of sleep time, reducing hours dedicated to physical activity and, thus, further contributing to the sedentary lifestyle [21,26].
Finally, the high levels of stress, fear and anxiety experienced by children and adolescents during COVID-19 pandemic could have contributed to weight increase during the lockdown [26,28]. Previous studies had demonstrated the complex relationship between stress, mental health, and obesity [50]. First, stress can cause weight gain by stimulating chronic cortisol secretion [51,52]. Second, prolonged stress periods can lead to depression with consequent isolation, home stay, sedentary behavior, and unhealthy nutrition, overall contributing to weight increase and obesity, that are, in turn, responsible for social stigma, stress and isolation [50]. Factors promoting stress and anxiety in children and adolescents were self-experienced but also parent-transmitted and included poor knowledge (especially at the beginning of the pandemic) of SARS-CoV-2 mechanisms of transmission, potentially severe outcomes of infection, and absence of efficacious treatments, social isolation, drastic changes in family dynamics and exacerbation of dysfunctional aspects, economic difficulties secondary to job loss/unemployment and difficulties in the use of technology necessary for home schooling and working [53,54].
Interestingly, the significant increase of body weight and BMI reported by the various studies occurred in a very short period, confirming previous observations on higher weight gain during summer vacations than during the school year, thus reinforcing the importance of school in obesity prevention through structured routine with meals, physical activity and a routine that promotes an adequate sleep schedule [55,56]. Forced school closure during the COVID-19 pandemic had a greater impact because of the previously mentioned associated psychological aspects impairing mental health and well-being, and the detrimental effect on academic progression [50].
The highest weight/BMI increase was observed in children with pre-existing overweight/obesity, of Hispanic and African American ethnicity, and in those living unfavorable socioeconomic conditions, therefore, most vulnerable to unhealthy lifestyle, food insecurity, family and social stress (i.e., lower parental psychological and educational support, and higher financial concerns/limitations), and with difficult access to academic resources and healthcare services [22,23,50].
4.2. Impact of Overweight/Obesity on the Severity and Outcome of COVID-19 Disease
Our study clearly pointed out a significant increase in the risk of severe COVID-19 disease and, consequently, of hospital/ICU admission in youths with overweight/obesity as compared with normoweight pairs [35,36,40,57].
The prevalence and severity of COVID-19 disease in children is significantly lower than in adults. It seems that, because of their immune systems’ immaturity, younger individuals are less likely to develop severe disease than adults and are more often asymptomatic. Most of the cases occurred in children aged 5–10 years old, and the prevalence in males was slightly higher than in females [58,59].
Around 3% developed critical conditions and few died. Racial/ethnic minorities and low-income populations, as well as children with comorbidities (i.e., chronic cardiac, respiratory, kidney, oncological, immunological, and hematological diseases, especially if associated with immunosuppression; diabetes and obesity) presented a significantly higher risk of infection and adverse disease outcome [60,61].
It should be underlined that obesity appeared to be the most significant independent risk factor, even in the mildest cases, in agreement with data from the adult population [12,14,16,61]. Several altered mechanisms, related to obesity itself and to its comorbidities, are involved. These include: (1) altered respiratory physiology, secondary to the pressure exerted by abdominal adiposity on the lungs, and the defective lung mesenchymal stem cells, responsible for ineffective tissue repair processes and immune response, with an overall increased risk of pulmonary infections, asthma, and obstructive apneas; (2) insulin resistance and hyperinsulinemia, because of which, in situations of intense metabolic activity, like during the response to SARS-CoV-2 infection, beta cells, already working at their limit, were not able to increase insulin secretion and can also be damaged by the virus [62,63]. Moreover, they contribute to the onset of other metabolic and cardiovascular alterations; including (3) dyslipidemia (low HDL- and increased LDL-cholesterol levels contribute to endothelial dysfunction and atherosclerosis); (4) hypertension with consequent left ventricular hypertrophy; (5) non-alcoholic steatohepatitis; and (6) hyperuricemia [62,63]. Finally, insulin resistance increases the (7) oxidative stress and, together with visceral adiposity, endothelium damage and micronutrients deficiencies (i.e., vitamin D, C, A, E and B12, iron and folate, with anti-oxidative action) lead to (8) chronic inflammation, excessive and dysregulated inflammatory response, and increased coagulation activity [62,63].
4.3. Study Strengths and Limitations
The main strengths of this work are: (1) the ‘syndemic’ approach to pediatric obesity and COVID-19 disease, according to which their reciprocal influence has been evaluated by dedicated analyses in the same study; (2) the accurate study search—through the initial application of a purposely-created highly sensitive string to identify as many pertinent articles as possible, so reducing the risk of missing important data—and selection; (3) the inclusion of studies focusing only on children and adolescents, thus discarding those including adults together with youths, thus increasing the specificity of the study.
On the other hand, the study presents some important limitations. First, the limited number and heterogeneity of the studies, in terms of sample size, gender distribution, ethnicity, study design and parameters used for the assessment of study variables, outcomes and confounders could be only partially limited by the accurate selection made. Most of the studies were cross sectional and some were based on parent interviews, thus limiting the validity of collected data because of intrinsic study limitations. Moreover, some population samples may be overlapping. Finally, the majority of the studies considered absolute weight increase and/or BMI, while only few reported weight and BMI percentiles, which are fundamental to assessing the presence and degree of weight excess in children and adolescents.
5. Conclusions
The COVID-19 pandemic had a huge impact on the health and well-being of children and adolescents. Lockdown measures affected healthy lifestyle behaviors through the modification of dietary habits, the reduction of physical activity and the alteration of sleep patterns, and also increased the levels of stress and anxiety, overall promoting weight gain and obesity. Moreover, pediatric overweight/obesity—already representing an alarming epidemic of increasing proportions, with detrimental effects on physical and mental health before COVID-19, has been demonstrated to be the most important independent risk factor for the development of severe SARS-CoV-2 infections in youths, requiring admission to hospital/ICUs.
Urgent measures aimed at supporting children and their families through counseling and active implementation of services for the promotion of healthy lifestyle in the different settings as well as medical care at all population levels are mandatory to limit the outburst of pediatric obesity. Finally, active population surveillance, as well as structured prospective studies focusing on physical and psychological aspects, are warranted to assess the real impact of COVID-19 disease and related containment measures on youth health and wellbeing in the long-term, and, consequently, promote targeted, more efficacious Public Health interventions.
Author Contributions
Conceptualization and methodology, all authors contributed equally; formal analysis and data curation G.L.F., M.M., Z.D.V., D.G., M.G.S., A.S. and G.S.; writing—original draft preparation, G.L.F., M.M. and F.G.; writing—review and editing and supervision D.G. and F.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare to have no conflict of interest.
References
- Cucinotta, D.; Vanelli, M. WHO Declares COVID-19 a Pandemic. Acta Biomed. 2020, 91, 157–160. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.-C.; Shih, T.-P.; Ko, W.-C.; Tang, H.-J.; Hsueh, P.-R. Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) and Coronavirus Disease-2019 (COVID-19): The Epidemic and the Challenges. Int. J. Antimicrob. Agents 2020, 55, 105924. [Google Scholar] [CrossRef] [PubMed]
- Han, E.; Tan, M.M.J.; Turk, E.; Sridhar, D.; Leung, G.M.; Shibuya, K.; Asgari, N.; Oh, J.; García-Basteiro, A.L.; Hanefeld, J.; et al. Lessons Learnt from Easing COVID-19 Restrictions: An Analysis of Countries and Regions in Asia Pacific and Europe. Lancet 2020, 396, 1525–1534. [Google Scholar] [CrossRef]
- Chang, T.-H.; Chen, Y.-C.; Chen, W.-Y.; Chen, C.-Y.; Hsu, W.-Y.; Chou, Y.; Chang, Y.-H. Weight Gain Associated with COVID-19 Lockdown in Children and Adolescents: A Systematic Review and Meta-Analysis. Nutrients 2021, 13, 3668. [Google Scholar] [CrossRef]
- Bremner, J.D.; Moazzami, K.; Wittbrodt, M.T.; Nye, J.A.; Lima, B.B.; Gillespie, C.F.; Rapaport, M.H.; Pearce, B.D.; Shah, A.J.; Vaccarino, V. Diet, Stress and Mental Health. Nutrients 2020, 12, 2428. [Google Scholar] [CrossRef] [PubMed]
- Tester, J.M.; Rosas, L.G.; Leung, C.W. Food Insecurity and Pediatric Obesity: A Double Whammy in the Era of COVID-19. Curr. Obes. Rep. 2020, 9, 442–450. [Google Scholar] [CrossRef]
- Deal, B.J.; Huffman, M.D.; Binns, H.; Stone, N.J. Perspective: Childhood Obesity Requires New Strategies for Prevention. Adv. Nutr. 2020, 11, 1071–1078. [Google Scholar] [CrossRef]
- Statistics on Obesity, Physical Activity and Diet. Available online: https://digital.nhs.uk/data-and-information/publications/statistical/statistics-on-obesity-physical-activity-and-diet (accessed on 22 March 2022).
- Khan, M.A.; Moverley Smith, J.E. “Covibesity”, a New Pandemic. Obes. Med. 2020, 19, 100282. [Google Scholar] [CrossRef]
- Frenkel, L.; Gomez, F.; Bellanti, J.A. COVID-19 in Children: Pathogenesis and Current Status. Allergy Asthma Proc. 2020, 42, 8–15. [Google Scholar] [CrossRef]
- Simonnet, A.; Chetboun, M.; Poissy, J.; Raverdy, V.; Noulette, J.; Duhamel, A.; Labreuche, J.; Mathieu, D.; Pattou, F.; Jourdain, M.; et al. High Prevalence of Obesity in Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2) Requiring Invasive Mechanical Ventilation. Obesity 2020, 28, 1195–1199. [Google Scholar] [CrossRef]
- Zhou, Y.; Chi, J.; Lv, W.; Wang, Y. Obesity and Diabetes as High-Risk Factors for Severe Coronavirus Disease 2019 (COVID-19). Diabetes Metab. Res. Rev. 2021, 37, e3377. [Google Scholar] [CrossRef] [PubMed]
- Caussy, C.; Wallet, F.; Laville, M.; Disse, E. Obesity Is Associated with Severe Forms of COVID-19. Obesity 2020, 28, 1175. [Google Scholar] [CrossRef] [PubMed]
- Richardson, S.; Hirsch, J.S.; Narasimhan, M.; Crawford, J.M.; McGinn, T.; Davidson, K.W.; the Northwell COVID-19 Research Consortium; Barnaby, D.P.; Becker, L.B.; Chelico, J.D.; et al. Presenting Characteristics, Comorbidities, and Outcomes Among 5700 Patients Hospitalized With COVID-19 in the New York City Area. JAMA 2020, 323, 2052–2059. [Google Scholar] [CrossRef] [PubMed]
- Lockhart, S.M.; O’Rahilly, S. When Two Pandemics Meet: Why Is Obesity Associated with Increased COVID-19 Mortality? Med 2020, 1, 33–42. [Google Scholar] [CrossRef]
- Chen, Q.; Zheng, Z.; Zhang, C.; Zhang, X.; Wu, H.; Wang, J.; Wang, S.; Zheng, C. Clinical Characteristics of 145 Patients with Corona Virus Disease 2019 (COVID-19) in Taizhou, Zhejiang, China. Infection 2020, 48, 543–551. [Google Scholar] [CrossRef]
- Petrilli, C.M.; Jones, S.A.; Yang, J.; Rajagopalan, H.; O’Donnell, L.; Chernyak, Y.; Tobin, K.A.; Cerfolio, R.J.; Francois, F.; Horwitz, L.I. Factors Associated with Hospital Admission and Critical Illness among 5279 People with Coronavirus Disease 2019 in New York City: Prospective Cohort Study. BMJ 2020, 369, m1966. [Google Scholar] [CrossRef]
- Cochrane Handbook for Systematic Reviews of Interventions. Available online: https://training.cochrane.org/handbook (accessed on 22 March 2022).
- DerSimonian, R.; Laird, N. Meta-Analysis in Clinical Trials. Control. Clin. Trials 1986, 7, 177–188. [Google Scholar] [CrossRef]
- RevMan 5 Download. Available online: https://training.cochrane.org/online-learning/core-software-cochrane-reviews/revman/revman-5-download (accessed on 22 March 2022).
- Androutsos, O.; Perperidi, M.; Georgiou, C.; Chouliaras, G. Lifestyle Changes and Determinants of Children’s and Adolescents’ Body Weight Increase during the First COVID-19 Lockdown in Greece: The COV-EAT Study. Nutrients 2021, 13, 930. [Google Scholar] [CrossRef]
- Azoulay, E.; Yackobovitch-Gavan, M.; Yaacov, H.; Gilboa, I.; Lopez, A.; Sheppes, T.; Waksman, Y.; Lebenthal, Y.; Brener, A. Weight Status and Body Composition Dynamics in Children and Adolescents During the COVID-19 Pandemic. Front. Pediatr. 2021, 9, 707773. [Google Scholar] [CrossRef]
- Brooks, C.G.; Spencer, J.R.; Sprafka, J.M.; Roehl, K.A.; Ma, J.; Londhe, A.A.; He, F.; Cheng, A.; Brown, C.A.; Page, J. Pediatric BMI Changes during COVID-19 Pandemic: An Electronic Health Record-Based Retrospective Cohort Study. EClinicalMedicine 2021, 38, 101026. [Google Scholar] [CrossRef]
- Cipolla, C.; Curatola, A.; Ferretti, S.; Giugno, G.; Condemi, C.; Delogu, A.B.; Birritella, L.; Lazzareschi, I. Eating Habits and Lifestyle in Children with Obesity during the COVID19 Lockdown: A Survey in an Italian Center. Acta Biomed. 2021, 92, e2021196. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Liu, J.; Wang, J.; Shen, M.; Ge, W.; Shen, H.; Zhang, T.; Yang, H.; Yin, J. Unfavorable Progression of Obesity in Children and Adolescents Due to COVID-19 Pandemic: A School-Based Survey in China. Obesity 2021, 29, 1907–1915. [Google Scholar] [CrossRef] [PubMed]
- Jia, P.; Zhang, L.; Yu, W.; Yu, B.; Liu, M.; Zhang, D.; Yang, S. Impact of COVID-19 Lockdown on Activity Patterns and Weight Status among Youths in China: The COVID-19 Impact on Lifestyle Change Survey (COINLICS). Int. J. Obes. 2021, 45, 695–699. [Google Scholar] [CrossRef]
- Kang, H.M.; Jeong, D.C.; Suh, B.K.; Ahn, M.B. The Impact of the Coronavirus Disease-2019 Pandemic on Childhood Obesity and Vitamin D Status. J. Korean Med. Sci. 2021, 36, e21. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.S.; Kwon, Y.; Choe, Y.H.; Kim, M.J. COVID-19-Related School Closing Aggravate Obesity and Glucose Intolerance in Pediatric Patients with Obesity. Sci. Rep. 2021, 11, 5494. [Google Scholar] [CrossRef] [PubMed]
- Maltoni, G.; Zioutas, M.; Deiana, G.; Biserni, G.B.; Pession, A.; Zucchini, S. Gender Differences in Weight Gain during Lockdown Due to COVID-19 Pandemic in Adolescents with Obesity. Nutr. Metab. Cardiovasc. Dis. 2021, 31, 2181–2185. [Google Scholar] [CrossRef]
- Qiu, N.; He, H.; Qiao, L.; Ding, Y.; Ji, S.; Guo, X.; Luo, J.; Luo, Z.; Li, Y.; Pang, H.; et al. Sex Differences in Changes in BMI and Blood Pressure in Chinese School-Aged Children during the COVID-19 Quarantine. Int. J. Obes. 2021, 45, 2132–2136. [Google Scholar] [CrossRef]
- Valenzise, M.; D’Amico, F.; Cucinotta, U.; Lugarà, C.; Zirilli, G.; Zema, A.; Wasniewska, M.; Pajno, G.B. The Lockdown Effects on a Pediatric Obese Population in the COVID-19 Era. Ital. J. Pediatr. 2021, 47, 209. [Google Scholar] [CrossRef]
- Vinker-Shuster, M.; Grossman, E.S.; Yeshayahu, Y. Increased Weight Gain of Children during the COVID-19 Lockdown. Isr. Med. Assoc. J. 2021, 23, 219–222. [Google Scholar]
- Vogel, M.; Geserick, M.; Gausche, R.; Beger, C.; Poulain, T.; Meigen, C.; Körner, A.; Keller, E.; Kiess, W.; Pfäffle, R. Age- and Weight Group-Specific Weight Gain Patterns in Children and Adolescents during the 15 Years before and during the COVID-19 Pandemic. Int. J. Obes. 2022, 46, 144–152. [Google Scholar] [CrossRef]
- Woolford, S.J.; Sidell, M.; Li, X.; Else, V.; Young, D.R.; Resnicow, K.; Koebnick, C. Changes in Body Mass Index Among Children and Adolescents During the COVID-19 Pandemic. JAMA 2021, 326, 1434–1436. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, D.M.; Oliveira, C.R.; Guerguis, S.; Eisenberg, R.; Choi, J.; Kim, M.; Abdelhemid, A.; Agha, R.; Agarwal, S.; Aschner, J.L.; et al. Severe Acute Respiratory Syndrome Coronavirus 2 Clinical Syndromes and Predictors of Disease Severity in Hospitalized Children and Youth. J. Pediatr. 2021, 230, 23–31.e10. [Google Scholar] [CrossRef] [PubMed]
- Graff, K.; Smith, C.; Silveira, L.; Jung, S.; Curran-Hays, S.; Jarjour, J.; Carpenter, L.; Pickard, K.; Mattiucci, M.; Fresia, J.; et al. Risk Factors for Severe COVID-19 in Children. Pediatr. Infect. Dis. J. 2021, 40, e137–e145. [Google Scholar] [CrossRef] [PubMed]
- Guzman, B.V.; Elbel, B.; Jay, M.; Messito, M.J.; Curado, S. Age-Dependent Association of Obesity with COVID-19 Severity in Paediatric Patients. Pediatr. Obes. 2022, 17, e12856. [Google Scholar] [CrossRef]
- Kompaniyets, L.; Agathis, N.T.; Nelson, J.M.; Preston, L.E.; Ko, J.Y.; Belay, B.; Pennington, A.F.; Danielson, M.L.; DeSisto, C.L.; Chevinsky, J.R.; et al. Underlying Medical Conditions Associated With Severe COVID-19 Illness Among Children. JAMA Netw. Open 2021, 4, e2111182. [Google Scholar] [CrossRef]
- Swann, O.V.; Holden, K.A.; Turtle, L.; Pollock, L.; Fairfield, C.J.; Drake, T.M.; Seth, S.; Egan, C.; Hardwick, H.E.; Halpin, S.; et al. Clinical Characteristics of Children and Young People Admitted to Hospital with COVID-19 in United Kingdom: Prospective Multicentre Observational Cohort Study. BMJ 2020, 370, m3249. [Google Scholar] [CrossRef]
- Verma, S.; Lumba, R.; Dapul, H.M.; Gold-von Simson, G.; Phoon, C.K.; Lighter, J.L.; Farkas, J.S.; Vinci, A.; Noor, A.; Raabe, V.N.; et al. Characteristics of Hospitalized Children With SARS-CoV-2 in the New York City Metropolitan Area. Hosp. Pediatr. 2021, 11, 71–78. [Google Scholar] [CrossRef]
- Singer, M.; Bulled, N.; Ostrach, B.; Mendenhall, E. Syndemics and the Biosocial Conception of Health. Lancet 2017, 389, 941–950. [Google Scholar] [CrossRef]
- Horton, R. Offline: COVID-19 Is Not a Pandemic. Lancet 2020, 396, 874. [Google Scholar] [CrossRef]
- CDC. BMI for Children and Teens. Available online: https://www.cdc.gov/obesity/basics/childhood-defining.html (accessed on 11 May 2022).
- Djalalinia, S.; Qorbani, M.; Peykari, N.; Kelishadi, R. Health Impacts of Obesity. Pak. J. Med. Sci. 2015, 31, 239–242. [Google Scholar] [CrossRef]
- Scott, K.M.; Bruffaerts, R.; Simon, G.E.; Alonso, J.; Angermeyer, M.; de Girolamo, G.; Demyttenaere, K.; Gasquet, I.; Haro, J.M.; Karam, E.; et al. Obesity and Mental Disorders in the General Population: Results from the World Mental Health Surveys. Int. J. Obes. 2008, 32, 192–200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rey-López, J.P.; Vicente-Rodríguez, G.; Biosca, M.; Moreno, L.A. Sedentary Behaviour and Obesity Development in Children and Adolescents. Nutr. Metab. Cardiovasc. Dis. 2008, 18, 242–251. [Google Scholar] [CrossRef] [PubMed]
- Kelishadi, R. Childhood Overweight, Obesity, and the Metabolic Syndrome in Developing Countries. Epidemiol. Rev. 2007, 29, 62–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leproult, R.; Van Cauter, E. Role of Sleep and Sleep Loss in Hormonal Release and Metabolism. Endocr. Dev. 2010, 17, 11–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mancuso, P. Obesity and Respiratory Infections: Does Excess Adiposity Weigh down Host Defense? Pulm. Pharm. 2013, 26, 412–419. [Google Scholar] [CrossRef] [Green Version]
- Browne, N.T.; Snethen, J.A.; Greenberg, C.S.; Frenn, M.; Kilanowski, J.F.; Gance-Cleveland, B.; Burke, P.J.; Lewandowski, L. When Pandemics Collide: The Impact of COVID-19 on Childhood Obesity. J. Pediatr. Nurs. 2021, 56, 90–98. [Google Scholar] [CrossRef]
- Doom, J.R.; Lumeng, J.C.; Sturza, J.; Kaciroti, N.; Vazquez, D.M.; Miller, A.L. Longitudinal Associations between Overweight/Obesity and Stress Biology in Low-Income Children. Int. J. Obes. 2020, 44, 646–655. [Google Scholar] [CrossRef]
- de Figueiredo, C.S.; Sandre, P.C.; Portugal, L.C.L.; Mázala-de-Oliveira, T.; da Silva Chagas, L.; Raony, Í.; Ferreira, E.S.; Giestal-de-Araujo, E.; Dos Santos, A.A.; Bomfim, P.O.-S. COVID-19 Pandemic Impact on Children and Adolescents’ Mental Health: Biological, Environmental, and Social Factors. Prog. Neuropsychopharmacol. Biol. Psychiatry 2021, 106, 110171. [Google Scholar] [CrossRef]
- Singh, S.; Roy, D.; Sinha, K.; Parveen, S.; Sharma, G.; Joshi, G. Impact of COVID-19 and Lockdown on Mental Health of Children and Adolescents: A Narrative Review with Recommendations. Psychiatry Res. 2020, 293, 113429. [Google Scholar] [CrossRef]
- Muscogiuri, G.; Barrea, L.; Annunziata, G.; Di Somma, C.; Laudisio, D.; Colao, A.; Savastano, S. Obesity and Sleep Disturbance: The Chicken or the Egg? Crit. Rev. Food Sci. Nutr. 2019, 59, 2158–2165. [Google Scholar] [CrossRef]
- Moreno, J.P.; Johnston, C.A.; Chen, T.-A.; O’Connor, T.A.; Hughes, S.O.; Baranowski, J.; Woehler, D.; Baranowski, T. Seasonal Variability in Weight Change during Elementary School. Obesity 2015, 23, 422–428. [Google Scholar] [CrossRef] [PubMed]
- Pietrobelli, A.; Pecoraro, L.; Ferruzzi, A.; Heo, M.; Faith, M.; Zoller, T.; Antoniazzi, F.; Piacentini, G.; Fearnbach, S.N.; Heymsfield, S.B. Effects of COVID-19 Lockdown on Lifestyle Behaviors in Children with Obesity Living in Verona, Italy: A Longitudinal Study. Obesity 2020, 28, 1382–1385. [Google Scholar] [CrossRef] [PubMed]
- Rundle, A.G.; Park, Y.; Herbstman, J.B.; Kinsey, E.W.; Wang, Y.C. COVID-19-Related School Closings and Risk of Weight Gain Among Children. Obesity 2020, 28, 1008–1009. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Viner, R.M.; Mytton, O.T.; Bonell, C.; Melendez-Torres, G.J.; Ward, J.; Hudson, L.; Waddington, C.; Thomas, J.; Russell, S.; van der Klis, F.; et al. Susceptibility to SARS-CoV-2 Infection Among Children and Adolescents Compared With Adults: A Systematic Review and Meta-Analysis. JAMA Pediatr. 2021, 175, 143–156. [Google Scholar] [CrossRef]
- Irfan, O.; Muttalib, F.; Tang, K.; Jiang, L.; Lassi, Z.S.; Bhutta, Z. Clinical Characteristics, Treatment and Outcomes of Paediatric COVID-19: A Systematic Review and Meta-Analysis. Arch. Dis. Child. 2021, 106, 440–448. [Google Scholar] [CrossRef]
- Harman, K.; Verma, A.; Cook, J.; Radia, T.; Zuckerman, M.; Deep, A.; Dhawan, A.; Gupta, A. Ethnicity and COVID-19 in Children with Comorbidities. Lancet Child. Adolesc. Health 2020, 4, e24–e25. [Google Scholar] [CrossRef]
- Tsankov, B.K.; Allaire, J.M.; Irvine, M.A.; Lopez, A.A.; Sauvé, L.J.; Vallance, B.A.; Jacobson, K. Severe COVID-19 Infection and Pediatric Comorbidities: A Systematic Review and Meta-Analysis. Int. J. Infect. Dis. 2021, 103, 246–256. [Google Scholar] [CrossRef]
- Nogueira-de-Almeida, C.A.; Del Ciampo, L.A.; Ferraz, I.S.; Del Ciampo, I.R.L.; Contini, A.A.; Ued, F.D.V. COVID-19 and Obesity in Childhood and Adolescence: A Clinical Review. J. Pediatr. 2020, 96, 546–558. [Google Scholar] [CrossRef]
- Cena, H.; Fiechtner, L.; Vincenti, A.; Magenes, V.C.; De Giuseppe, R.; Manuelli, M.; Zuccotti, G.V.; Calcaterra, V. COVID-19 Pandemic as Risk Factors for Excessive Weight Gain in Pediatrics: The Role of Changes in Nutrition Behavior. A Narrative Review. Nutrients 2021, 13, 4255. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).