A Macro-Level Association of Vaccination Rate with the Number of Confirmed COVID-19 Cases in the United States and Japan
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Materials
2.2. Statistical Analysis
3. Results
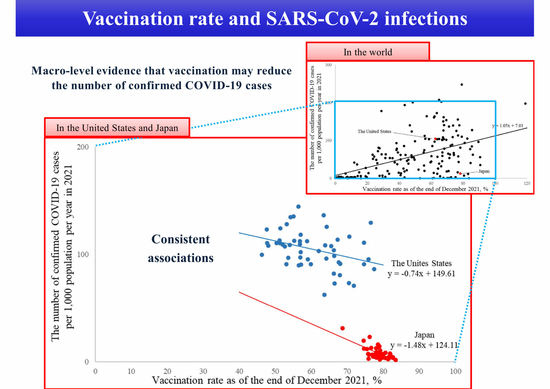
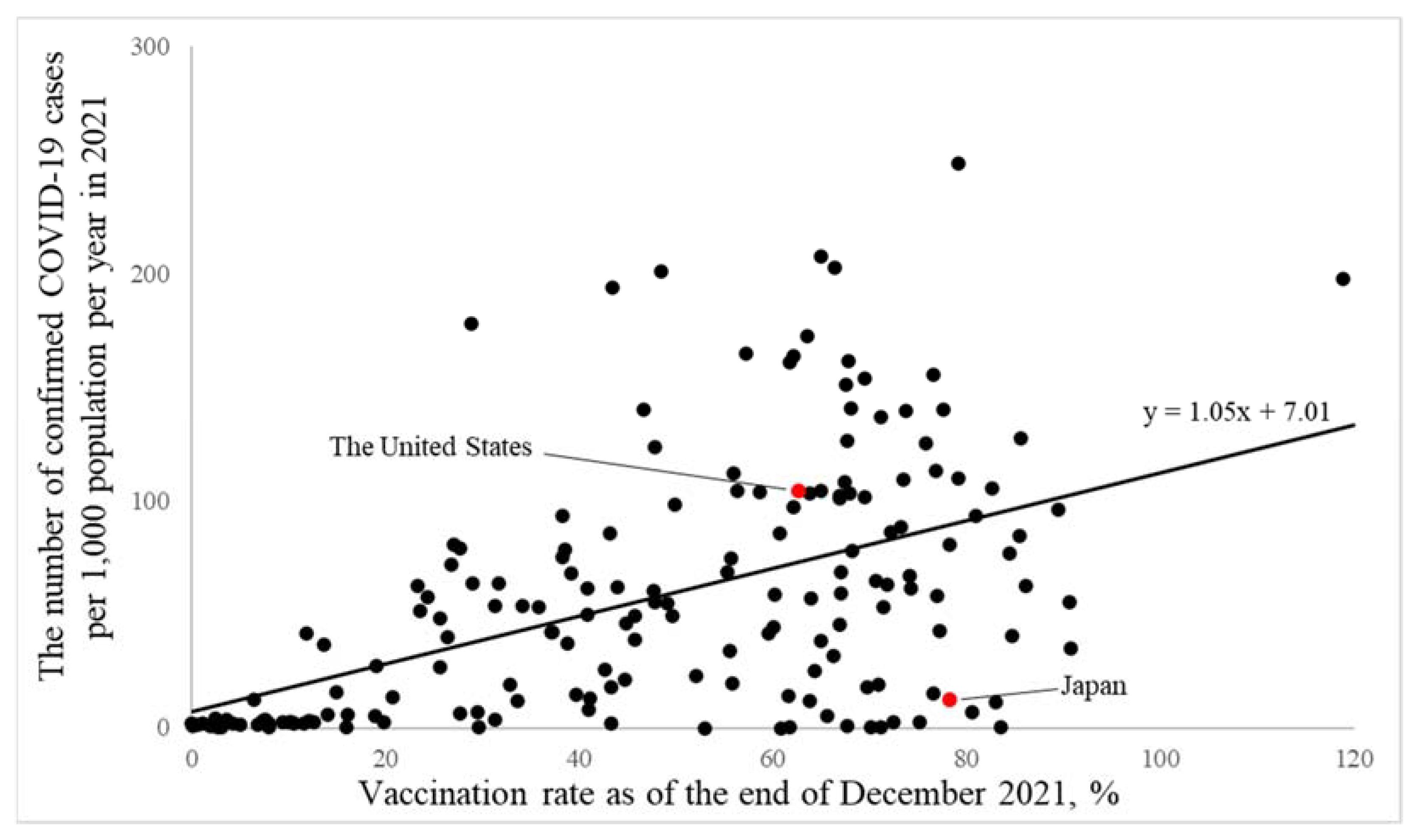
3.1. A Simple International Comparison
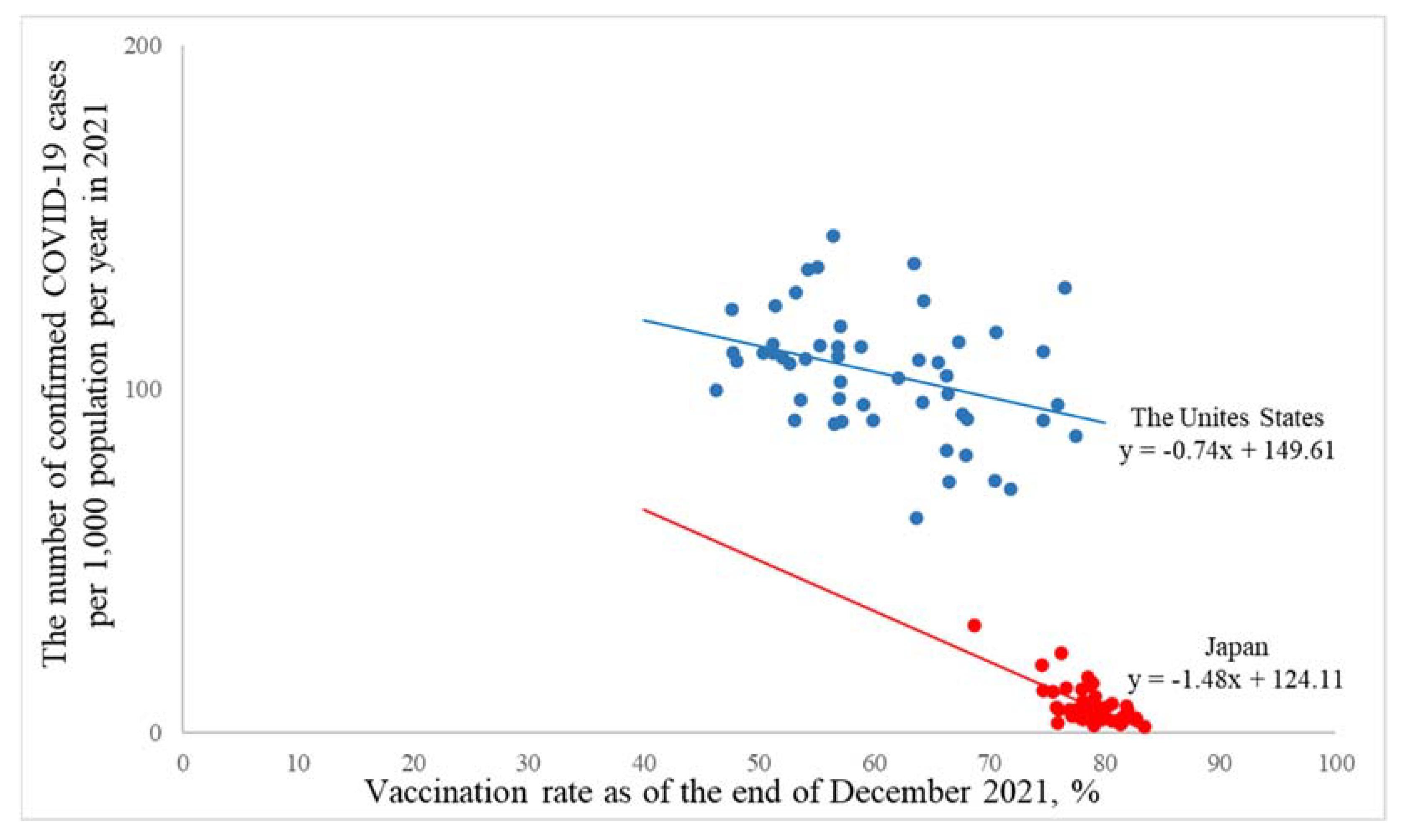
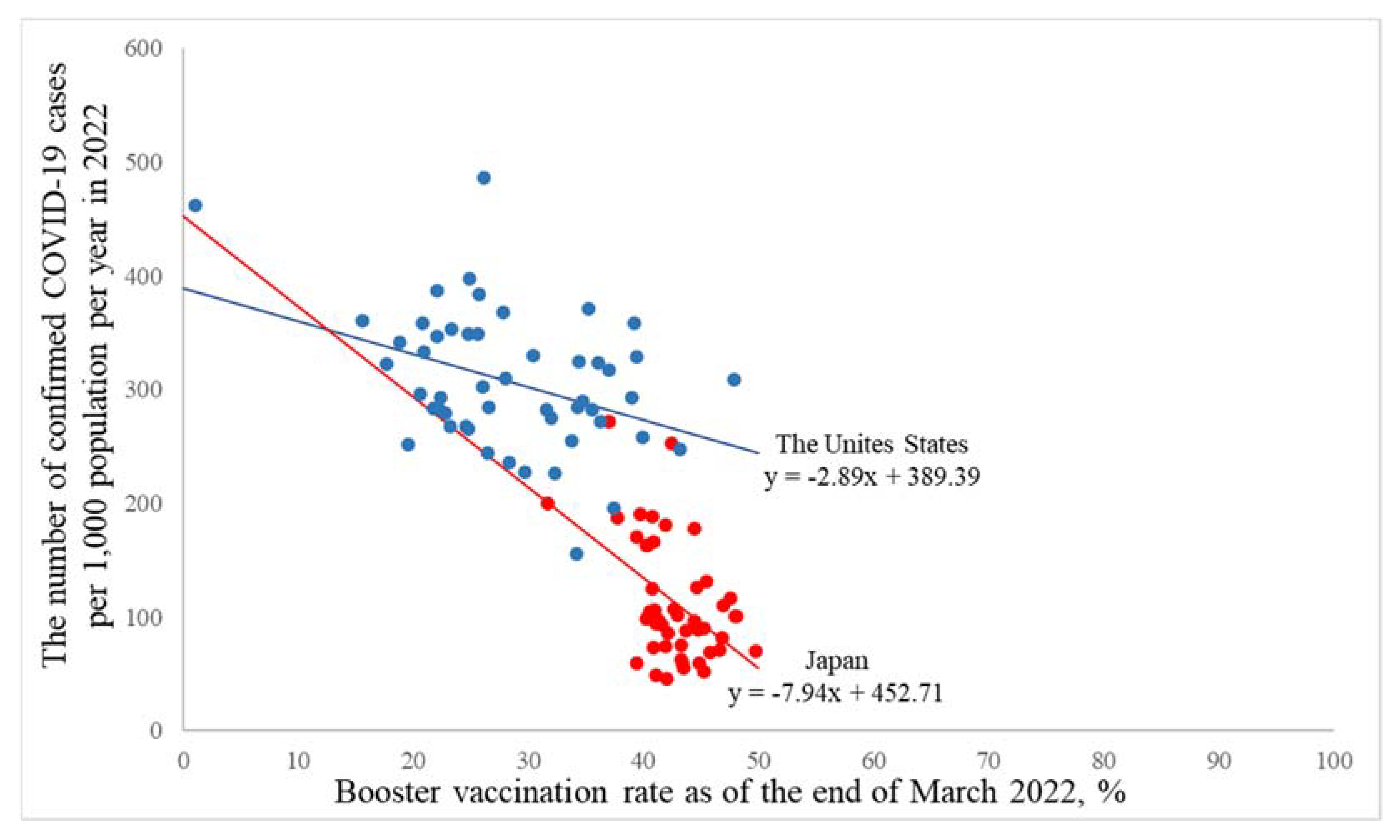
3.2. A Macro-Level Analysis in the United States and Japan
4. Discussion
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dubé, È.; Ward, J.K.; Verger, P.; MacDonald, N.E. Vaccine Hesitancy, Acceptance, and Anti-Vaccination: Trends and Future Prospects for Public Health. Annu. Rev. Public Health 2021, 42, 175–191. [Google Scholar] [CrossRef] [PubMed]
- Pezzoli, L.; Azman, A.S. Moving forward with an imperfect vaccine. Lancet Infect. Dis. 2021, 21, 1339–1341. [Google Scholar] [CrossRef]
- Iboi, E.A.; Ngonghala, C.N.; Gumel, A.B. Will an imperfect vaccine curtail the COVID-19 pandemic in the U.S.? Infect. Dis. Model. 2020, 5, 510–524. [Google Scholar] [CrossRef] [PubMed]
- Greenwood, B.; Dicko, A.; Sagara, I.; Zongo, I.; Tinto, H.; Cairns, M.; Kuepfer, I.; Milligan, P.; Ouedraogo, J.B.; Doumbo, O.; et al. Seasonal vaccination against malaria: A potential use for an imperfect malaria vaccine. Malar J. 2017, 16, 182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dagan, N.; Barda, N.; Kepten, E.; Miron, O.; Perchik, S.; Katz, M.A.; Hernán, M.A.; Lipsitch, M.; Reis, B.; Balicer, R.D. BNT162b2 mRNA COVID-19 Vaccine in a Nationwide Mass Vaccination Setting. N. Engl. J. Med. 2021, 384, 1412–1423. [Google Scholar] [CrossRef] [PubMed]
- Haas, E.J.; Angulo, F.J.; McLaughlin, J.M.; Anis, E.; Singer, S.R.; Khan, F.; Brooks, N.; Smaja, M.; Mircus, G.; Pan, K.; et al. Impact and effectiveness of mRNA BNT162b2 vaccine against SARS-CoV-2 infections and COVID-19 cases, hospitalisations, and deaths following a nationwide vaccination campaign in Israel: An observational study using national surveillance data. Lancet 2021, 397, 1819–1829. [Google Scholar] [CrossRef]
- Amit, S.; Regev-Yochay, G.; Afek, A.; Kreiss, Y.; Leshem, E. Early rate reductions of SARS-CoV-2 infection and COVID-19 in BNT162b2 vaccine recipients. Lancet 2021, 397, 875–877. [Google Scholar] [CrossRef]
- Angel, Y.; Spitzer, A.; Henig, O.; Saiag, E.; Sprecher, E.; Padova, H.; Ben-Ami, R. Association Between Vaccination with BNT162b2 and Incidence of Symptomatic and Asymptomatic SARS-CoV-2 Infections Among Health Care Workers. JAMA 2021, 325, 2457–2465. [Google Scholar] [CrossRef]
- Thompson, M.G.; Burgess, J.L.; Naleway, A.L.; Tyner, H.L.; Yoon, S.K.; Meece, J.; Olsho, L.E.W.; Caban-Martinez, A.J.; Fowlkes, A.; Lutrick, K.; et al. Interim Estimates of Vaccine Effectiveness of BNT162b2 and mRNA-1273 COVID-19 Vaccines in Preventing SARS-CoV-2 Infection Among Health Care Personnel, First Responders, and Other Essential and Frontline Workers—Eight U.S. Locations, December 2020–March 2021. MMWR Morb. Mortal. Wkly. Rep. 2021, 70, 495–500. [Google Scholar]
- Tande, A.J.; Pollock, B.D.; Shah, N.D.; Farrugia, G.; Virk, A.; Swift, M.; Breeher, L.; Binnicker, M.; Berbari, E.F. Impact of the Coronavirus Disease 2019 (COVID-19) Vaccine on Asymptomatic Infection Among Patients Undergoing Preprocedural COVID-19 Molecular Screening. Clin. Infect. Dis. 2022, 74, 59–65. [Google Scholar] [CrossRef]
- Tang, L.; Hijano, D.R.; Gaur, A.H.; Geiger, T.L.; Neufeld, E.J.; Hoffman, J.M.; Hayden, R.T. Asymptomatic and Symptomatic SARS-CoV-2 Infections After BNT162b2 Vaccination in a Routinely Screened Workforce. JAMA 2021, 325, 2500–2502. [Google Scholar] [CrossRef] [PubMed]
- Jones, N.K.; Rivett, L.; Seaman, S.; Samworth, R.J.; Warne, B.; Workman, C.; Ferris, M.; Wright, J.; Quinnell, N.; Shaw, A.; et al. Single-dose BNT162b2 vaccine protects against asymptomatic SARS-CoV-2 infection. eLife 2021, 10, e68808. [Google Scholar] [CrossRef] [PubMed]
- Fabiani, M.; Ramigni, M.; Gobbetto, V.; Mateo-Urdiales, A.; Pezzotti, P.; Piovesan, C. Effectiveness of the Comirnaty (BNT162b2, BioNTech/Pfizer) vaccine in preventing SARS-CoV-2 infection among healthcare workers, Treviso province, Veneto region, Italy, 27 December 2020 to 24 March 2021. Euro Surveill. 2021, 26, 2100420. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Baz, I.; Trobajo-Sanmartín, C.; Miqueleiz, A.; Guevara, M.; Fernández-Huerta, M.; Burgui, C.; Casado, I.; Portillo, M.E.; Navascués, A.; Ezpeleta, C.; et al. Working Group for the Study of COVID-19 in Navarre. Product-specific COVID-19 vaccine effectiveness against secondary infection in close contacts, Navarre, Spain, April to August 2021. Euro Surveill. 2021, 26, 2100894. [Google Scholar]
- Goldberg, Y.; Mandel, M.; Bar-On, Y.M.; Bodenheimer, O.; Freedman, L.; Haas, E.J.; Milo, R.; Alroy-Preis, S.; Ash, N.; Huppert, A. Waning Immunity after the BNT162b2 Vaccine in Israel. N. Engl. J. Med. 2021, 385, e85. [Google Scholar] [CrossRef]
- Chemaitelly, H.; Tang, P.; Hasan, M.R.; AlMukdad, S.; Yassine, H.M.; Benslimane, F.M.; Al Khatib, H.A.; Coyle, P.; Ayoub, H.H.; Al Kanaani, Z.; et al. Waning of BNT162b2 Vaccine Protection against SARS-CoV-2 Infection in Qatar. N. Engl. J. Med. 2021, 385, e83. [Google Scholar] [CrossRef]
- Tartof, S.Y.; Slezak, J.M.; Fischer, H.; Hong, V.; Ackerson, B.K.; Ranasinghe, O.N.; Frankland, T.B.; Ogun, O.A.; Zamparo, J.M.; Gray, S.; et al. Effectiveness of mRNA BNT162b2 COVID-19 vaccine up to 6 months in a large integrated health system in the USA: A retrospective cohort study. Lancet 2021, 398, 1407–1416. [Google Scholar] [CrossRef]
- Spitzer, A.; Angel, Y.; Marudi, O.; Zeltser, D.; Saiag, E.; Goldshmidt, H.; Goldiner, I.; Stark, M.; Halutz, O.; Gamzu, R.; et al. Association of a Third Dose of BNT162b2 Vaccine With Incidence of SARS-CoV-2 Infection Among Health Care Workers in Israel. JAMA 2022, 327, 341–349. [Google Scholar] [CrossRef]
- Bar-On, Y.M.; Goldberg, Y.; Mandel, M.; Bodenheimer, O.; Freedman, L.; Kalkstein, N.; Mizrahi, B.; Alroy-Preis, S.; Ash, N.; Milo, R.; et al. Protection of BNT162b2 Vaccine Booster against COVID-19 in Israel. N. Engl. J. Med. 2021, 385, 1393–1400. [Google Scholar] [CrossRef]
- Tseng, H.F.; Ackerson, B.K.; Luo, Y.; Sy, L.S.; Talarico, C.A.; Tian, Y.; Bruxvoort, K.J.; Tubert, J.E.; Florea, A.; Ku, J.H.; et al. Effectiveness of mRNA-1273 against SARS-CoV-2 Omicron and Delta variants. Nat. Med. 2022, in press. [CrossRef]
- Abu-Raddad, L.J.; Chemaitelly, H.; Ayoub, H.H.; AlMukdad, S.; Yassine, H.M.; Al-Khatib, H.A.; Smatti, M.K.; Tang, P.; Hasan, M.R.; Coyle, P.; et al. Effect of mRNA Vaccine Boosters against SARS-CoV-2 Omicron Infection in Qatar. N. Engl. J. Med. 2022, in press. [CrossRef] [PubMed]
- Feikin, D.R.; Higdon, M.M.; Abu-Raddad, L.J.; Andrews, N.; Araos, R.; Goldberg, Y.; Groome, M.J.; Huppert, A.; O’Brien, K.L.; Smith, P.G.; et al. Duration of effectiveness of vaccines against SARS-CoV-2 infection and COVID-19 disease: Results of a systematic review and meta-regression. Lancet 2022, 399, 924–944. [Google Scholar] [CrossRef]
- Leung, K.; Wu, J.T. Managing waning vaccine protection against SARS-CoV-2 variants. Lancet 2022, 399, 2–3. [Google Scholar] [CrossRef]
- Pogue, K.; Jensen, J.L.; Stancil, C.K.; Ferguson, D.G.; Hughes, S.J.; Mello, E.J.; Burgess, R.; Berges, B.K.; Quaye, A.; Poole, B.D. Influences on Attitudes Regarding Potential COVID-19 Vaccination in the United States. Vaccines 2020, 8, 582. [Google Scholar] [CrossRef] [PubMed]
- Takeya, Y.; Popper, J.S.; Shimizu, Y.; Kato, H.; Rhoads, G.G.; Kagan, A. Epidemiologic studies of coronary heart disease and stroke in Japanese men living in Japan, Hawaii and California: Incidence of stroke in Japan and Hawaii. Stroke 1984, 15, 15–23. [Google Scholar] [CrossRef] [Green Version]
- Iso, H.; Sato, S.; Kitamura, A.; Naito, Y.; Shimamoto, T.; Komachi, Y. Fat and protein intakes and risk of intraparenchymal hemorrhage among middle-aged Japanese. Am. J. Epidemiol. 2003, 157, 32–39. [Google Scholar] [CrossRef] [Green Version]
- COVID-19 Vaccinations in the United States, Jurisdiction. Available online: https://www.cdc.gov/coronavirus/2019-ncov/vaccines/distributing/about-vaccine-data.html (accessed on 10 January 2022).
- Total Number of Vaccinations So Far (by Prefecture). Available online: https://www.kantei.go.jp/jp/headline/kansensho/vaccine.html (accessed on 4 January 2022).
- United States COVID-19 Cases and Deaths by State over Time. Available online: https://data.cdc.gov/Case-Surveillance/United-States-COVID-19-Cases-and-Deaths-by-State-o/9mfq-cb36 (accessed on 3 April 2022).
- Visualizing the Data: Information on COVID-19 Infections. Available online: https://covid19.mhlw.go.jp/ (accessed on 3 April 2022).
- Vaccination Record System (VRS). Available online: https://info.vrs.digital.go.jp/dashboard (accessed on 9 April 2022).
- Coronavirus Pandemic (COVID-19). Available online: https://ourworldindata.org/coronavirus (accessed on 5 April 2022).
- Gujarati, D.N. Basic Econometrics, 4th ed.; McGraw-Hill: New York, NY, USA, 2009. [Google Scholar]
- O’Brien, R.M. A Caution Regarding Rules of Thumb for Variance Inflation Factors. Qual. Quant. Int. J. Methodol. 2007, 41, 673–690. [Google Scholar]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. C4591001 Clinical Trial Group. Safety and Efficacy of the BNT162b2 mRNA COVID-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef]
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B.; et al. COVE Study Group. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2021, 384, 403–416. [Google Scholar] [CrossRef]
- Thomas, S.J.; Moreira, E.D., Jr.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Polack, F.P.; Zerbini, C.; et al. C4591001 Clinical Trial Group. Safety and Efficacy of the BNT162b2 mRNA COVID-19 Vaccine through 6 Months. N. Engl. J. Med. 2021, 385, 1761–1773. [Google Scholar] [CrossRef]
- El Sahly, H.M.; Baden, L.R.; Essink, B.; Doblecki-Lewis, S.; Martin, J.M.; Anderson, E.J.; Campbell, T.B.; Clark, J.; Jackson, L.A.; Fichtenbaum, C.J.; et al. COVE Study Group. Efficacy of the mRNA-1273 SARS-CoV-2 Vaccine at Completion of Blinded Phase. N. Engl. J. Med. 2021, 385, 1774–1785. [Google Scholar] [CrossRef] [PubMed]
- Levine-Tiefenbrun, M.; Yelin, I.; Alapi, H.; Katz, R.; Herzel, E.; Kuint, J.; Chodick, G.; Gazit, S.; Patalon, T.; Kishony, R. Viral loads of Delta-variant SARS-CoV-2 breakthrough infections after vaccination and booster with BNT162b2. Nat. Med. 2021, 27, 2108–2110. [Google Scholar] [CrossRef] [PubMed]
- Levine-Tiefenbrun, M.; Yelin, I.; Katz, R.; Herzel, E.; Golan, Z.; Schreiber, L.; Wolf, T.; Nadler, V.; Ben-Tov, A.; Kuint, J.; et al. Initial report of decreased SARS-CoV-2 viral load after inoculation with the BNT162b2 vaccine. Nat. Med. 2021, 27, 790–792. [Google Scholar] [CrossRef] [PubMed]
- Chia, P.Y.; Ong, S.W.X.; Chiew, C.J.; Ang, L.W.; Chavatte, J.M.; Mak, T.M.; Cui, L.; Kalimuddin, S.; Chia, W.N.; Tan, C.W.; et al. Virological and serological kinetics of SARS-CoV-2 Delta variant vaccine breakthrough infections: A multicentre cohort study. Clin. Microbiol Infect. 2022, 28, 612.e1–612.e7. [Google Scholar] [CrossRef] [PubMed]
- Wald, A. Booster Vaccination to Reduce SARS-CoV-2 Transmission and Infection. JAMA 2022, 327, 327–328. [Google Scholar] [CrossRef]
- Diez-Roux, A.V. Bringing context back into epidemiology: Variables and fallacies in multilevel analysis. Am. J. Public Health 1998, 88, 216–222. [Google Scholar] [CrossRef] [Green Version]
- Diez-Roux, A.V. Multilevel analysis in public health research. Annu. Rev. Public Health 2000, 21, 171–192. [Google Scholar] [CrossRef] [Green Version]
- Dong, M.; He, F.; Deng, Y. How to Understand Herd Immunity in the Context of COVID-19. Viral Immunol. 2021, 34, 174–181. [Google Scholar] [CrossRef]
- Sneppen, K.; Nielsen, B.F.; Taylor, R.J.; Simonsen, L. Overdispersion in COVID-19 increases the effectiveness of limiting nonrepetitive contacts for transmission control. Proc. Natl. Acad. Sci. USA 2021, 118, e2016623118. [Google Scholar] [CrossRef]
- Britton, T.; Ball, F.; Trapman, P. A mathematical model reveals the influence of population heterogeneity on herd immunity to SARS-CoV-2. Science 2020, 369, 846–849. [Google Scholar] [CrossRef]

| No.* | Mean (95% Confidence Intervals) | |||
|---|---|---|---|---|
| In 2020 | In 2021 | In 2022 ** | ||
| The number of people who were fully vaccinated, million persons | ||||
| The United States | 51 | 0.00(0.00, 0.00) | 3.95(2.65, 5.25) ♰ | 0.22(0.13, 0.32) ♰ |
| Japan | 47 | 0.00(0.00, 0.00) | 2.09(1.48, 2.70) ♰ | 0.05(0.03, 0.07) ♰ |
| Difference | 0.00(0.00, 0.00) | 1.86(0.40, 3.32) § | 0.17(0.07, 0.27) § | |
| Vaccination rate as of the last day, % | ||||
| The United States | 51 | 0.00(0.00, 0.00) | 60.49(58.18, 62.80) ♰ | 62.64(59.30, 65.98) ♰ |
| Japan | 47 | 0.00(0.00, 0.00) | 78.60(77.87, 79.34) ♰ | 80.18(79.47, 80.90) ♰ |
| Difference | 0.00(0.00, 0.00) | −18.11(−20.59, −15.64) § | −17.54(−21.04, −14.04) § | |
| The number of people who have received a booster dose, million persons | ||||
| The United States | 51 | 0.00(0.00, 0.00) | 1.35(0.92, 1.78) ♰ | 0.52(0.30, 0.74) ♰ |
| Japan | 47 | 0.00(0.00, 0.00) | 0.02(0.02, 0.03) ♰ | 1.10(0.78, 1.41) ♰ |
| Difference | 0.00(0.00, 0.00) | 1.33(0.89, 1.77) § | −0.57(−0.96, −0.18) § | |
| Booster vaccination rate as of the last day, % | ||||
| The United States | 51 | 0.00(0.00, 0.00) | 21.25(19.59, 22.91) ♰ | 28.39(26.12, 30.65) ♰ |
| Japan | 47 | 0.00(0.00, 0.00) | 0.92(0.80, 1.04) ♰ | 42.71(41.78, 43.64) ♰ |
| Difference | 0.00(0.00, 0.00) | 20.33(18.63, 22.04) § | −14.32(−16.82, −11.83) § | |
| The number of confirmed COVID-19 cases per year, million persons | ||||
| The United States | 51 | 0.38(0.27, 0.50) | 0.66(0.46, 0.85) ♰ | 1.91(1.26, 2.57) ♰ |
| Japan | 47 | 0.00(0.00, 0.01) | 0.03(0.02, 0.05) ♰ | 0.42(0.22, 0.61) ♰ |
| Difference | 0.38(0.26, 0.50) § | 0.62(0.42, 0.82) § | 1.50(0.80, 2.19) § | |
| The number of confirmed COVID-19 cases per 1000 population per year | ||||
| The United States | 51 | 63.11(56.99, 69.22) | 104.65(99.77, 109.53) ♰ | 307.24(290.57, 323.91) ♰ |
| Japan | 47 | 1.08(0.81, 1.35) | 7.90(6.26, 9.54) ♰ | 113.39(98.05, 128.73) ♰ |
| Difference | 62.03(55.76, 68.29) § | 96.75(91.50, 102.00) § | 193.80(170.80, 216.90) § | |
| The Number of Confirmed Cases per 1000 Population per Year (95% Confidence Intervals) in 2021 | ||
|---|---|---|
| The United States | Japan | |
| No. of jurisdictions | 51 | 47 |
| The number in 1% increment of vaccination rate * | ||
| Crude model ** | −0.74(−1.29, −0.20) ♰ | −1.48(−1.95, −1.00) ♰ |
| The Number of Confirmed Cases per 1000 Population per Year (95% Confidence Intervals) in 2022 | ||
|---|---|---|
| The United States | Japan | |
| No. of jurisdictions | 51 | 47 |
| The number in 1% increment of booster vaccination rate | ||
| Crude model ** | −2.89(−4.75, −1.04) ♰ | −7.94(−12.06, −3.83) ♰ |
| Multivariable model *** | −0.40(−4.18, 3.38) | −5.61(−9.86, −1.36) ♰ |
| The number in 1% increment of vaccination rate * | ||
| Crude model ** | −2.17(−3.40, −0.93) ♰ | −10.31(−15.68, −4.94) ♰ |
| Multivariable model *** | −1.93(−4.49, 0.63) | −7.25(−12.78, −1.71) ‡ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Noda, H. A Macro-Level Association of Vaccination Rate with the Number of Confirmed COVID-19 Cases in the United States and Japan. Int. J. Environ. Res. Public Health 2022, 19, 7435. https://doi.org/10.3390/ijerph19127435
Noda H. A Macro-Level Association of Vaccination Rate with the Number of Confirmed COVID-19 Cases in the United States and Japan. International Journal of Environmental Research and Public Health. 2022; 19(12):7435. https://doi.org/10.3390/ijerph19127435
Chicago/Turabian StyleNoda, Hiroyuki. 2022. "A Macro-Level Association of Vaccination Rate with the Number of Confirmed COVID-19 Cases in the United States and Japan" International Journal of Environmental Research and Public Health 19, no. 12: 7435. https://doi.org/10.3390/ijerph19127435
APA StyleNoda, H. (2022). A Macro-Level Association of Vaccination Rate with the Number of Confirmed COVID-19 Cases in the United States and Japan. International Journal of Environmental Research and Public Health, 19(12), 7435. https://doi.org/10.3390/ijerph19127435