Trazodone Prolonged-Release Monotherapy in Cannabis Dependent Patients during Lockdown Due to COVID-19 Pandemic: A Case Series
Abstract
:1. Introduction
2. Case Reports
2.1. Method
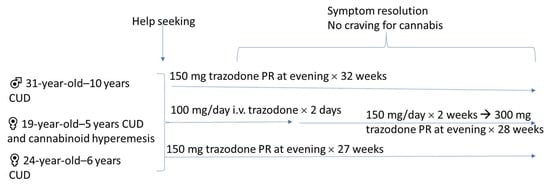
2.2. Case 1
2.3. Case 2
2.4. Case 3
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Caroppo, E.; Mazza, M.; Sannella, A.; Marano, G.; Avallone, C.; Claro, A.E.; Janiri, D.; Moccia, L.; Janiri, L.; Sani, G. Will Nothing Be the Same Again? Changes in Lifestyle during COVID-19 Pandemic and Consequences on Mental Health. Int. J. Environ. Res. Public Health 2021, 18, 8433. [Google Scholar] [CrossRef] [PubMed]
- Brotto, L.A.; Chankasingh, K.; Baaske, A.; Albert, A.; Booth, A.; Kaida, A.; Smith, L.W.; Racey, S.; Gottschlich, A.; Murray, M.C.M.; et al. The influence of sex, gender, age, and ethnicity on psychosocial factors and substance use throughout phases of the COVID-19 pandemic. PLoS ONE 2021, 16, e0259676. [Google Scholar] [CrossRef] [PubMed]
- Young-Wolff, K.C.; Ray, G.T.; Alexeeff, S.E.; Adams, S.R.; Does, M.B.; Ansley, D.; Avalos, L.A. Rates of Prenatal Cannabis Use Among Pregnant Women Before and During the COVID-19 Pandemic. JAMA 2021, 326, 1745–1747. [Google Scholar] [CrossRef] [PubMed]
- Bruno, S.; Bazzani, A.; Marantonio, S.; Cruz-Sanabria, F.; Benedetti, D.; Frumento, P.; Turchetti, G.; Faraguna, U. Poor sleep quality and unhealthy lifestyle during the lockdown: An Italian study. Sleep Med. 2022, 90, 53–64. [Google Scholar] [CrossRef] [PubMed]
- Satre, D.D.; Iturralde, E.; Ghadiali, M.; Young-Wolff, K.C.; Campbell, C.I.; Leibowitz, A.S.; Sterling, S.A. Treatment for Anxiety and Substance Use Disorders During the COVID-19 Pandemic: Challenges and Strategies. J. Addict. Med. 2020, 14, e293–e296. [Google Scholar] [CrossRef]
- Ek, J.; Jacobs, W.; Kaylor, B.; McCall, W.V. Addiction and Sleep Disorders. Adv. Exp. Med. Biol. 2021, 1297, 163–171. [Google Scholar] [CrossRef]
- Marek, G.J.; McDougle, C.J.; Price, L.H.; Seiden, L.S. A comparison of trazodone and fluoxetine: Implications for a serotonergic mechanism of antidepressant action. Psychopharmacology 1992, 109, 2–11. [Google Scholar] [CrossRef]
- Montalbano, A.; Mlinar, B.; Bonfiglio, F.; Polenzani, L.; Magnani, M.; Corradetti, R. Dual inhibitory action of trazodone on dorsal raphe serotonergic neurons through 5-HT1A receptor partial agonism and α1-adrenoceptor antagonism. PLoS ONE 2019, 14, e0222855. [Google Scholar] [CrossRef] [Green Version]
- Clements-Jewery, S. The development of cortical beta-adrenoceptor subsensitivity in the rat by chronic treatment with trazodone, doxepin and mianserine. Neuropharmacology 1978, 17, 779–781. [Google Scholar] [CrossRef]
- Wichniak, A.; Wierzbicka, A.E.; Jarema, M. Treatment of insomnia-effect of trazodone and hypnotics on sleep. Psychiatr. Pol. 2021, 55, 743–755, (In English, Polish). [Google Scholar] [CrossRef]
- Roth, B.L.; Driscol, J. PDSP Ki Database. In Psychoactive Drug Screening Program (PDSP); University of North Carolina at Chapel Hill and the United States National Institute of Mental Health: Chapel Hill, NC, USA, 14 August 2017. [Google Scholar]
- Shin, J.J.; Saadabadi, A. Trazodone. 6 August 2021. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Bossini, L.; Coluccia, A.; Casolaro, I.; Benbow, J.; Amodeo, G.; De Giorgi, R.; Fagiolini, A. Off-Label Trazodone Prescription: Evidence, Benefits and Risks. Curr. Pharm. Des. 2015, 21, 3343–3351. [Google Scholar] [CrossRef] [PubMed]
- Fagiolini, A.; Comandini, A.; Catena Dell’Osso, M.; Kasper, S. Rediscovering trazodone for the treatment of major depressive disorder. CNS Drugs 2012, 26, 1033–1049. [Google Scholar] [CrossRef] [PubMed]
- Kast, K.A.; Gershengoren, L. Cannabinoid Hyperemesis Syndrome and the Consulting Psychiatrist: A Case Study of Diagnosis and Treatment for an Emerging Disorder in Psychiatric Practice. J. Psychiatr. Pract. 2018, 24, 51–55. [Google Scholar] [CrossRef] [PubMed]
- Senderovich, H.; Patel, P.; Jimenez Lopez, B.; Waicus, S. A Systematic Review on Cannabis Hyperemesis Syndrome and Its Management Options. Med. Princ. Pract. 2022, 31, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Graham, J.; Barberio, M.; Wang, G.S. Capsaicin Cream for Treatment of Cannabinoid Hyperemesis Syndrome in Adolescents: A Case Series. Pediatrics 2017, 140, e20163795. [Google Scholar] [CrossRef] [Green Version]
- Yi, X.-Y.; Ni, S.-F.; Ghadami, M.R.; Meng, H.-Q.; Chen, M.-Y.; Kuang, L.; Zhang, Y.-Q.; Zhang, L.; Zhou, X.-Y. Trazodone for the treatment of insomnia: A meta-analysis of randomized placebo-controlled trials. Sleep Med. 2018, 45, 25–32. [Google Scholar] [CrossRef]
- Janiri, L.; Hadjichristos, A.; Buonanno, A.; Rago, R.; Mannelli, P.; de Risio, S. Adjuvant trazodone in the treatment of alcoholism: An open study. Alcohol Alcohol. 1998, 33, 362–365. [Google Scholar] [CrossRef] [Green Version]
- Pozzi, G.; Conte, G.; De Risio, S. Combined use of trazodone-naltrexone versus clonidine-naltrexone in rapid withdrawal from methadone treatment. A comparative inpatient study. Drug Alcohol Depend. 2000, 59, 287–294. [Google Scholar] [CrossRef]
- Faingold, C.L.; Knapp, D.J.; Chester, J.A.; Gonzalez, L.P. Integrative neurobiology of the alcohol withdrawal syndrome—From anxiety to seizures. Alcohol. Clin. Exp. Res. 2004, 28, 268–278. [Google Scholar] [CrossRef]
- Rogawski, M.A. Update on the neurobiology of alcohol withdrawal seizures. Epilepsy Curr. 2005, 5, 225–230. [Google Scholar] [CrossRef] [Green Version]
- Eşel, E. Alkol Yoksunluğunun Nörobiyolojisi: Baskilayici ve Uyarici Nörotransmiterler [Neurobiology of alcohol withdrawal inhibitory and excitatory neurotransmitters]. Turk Psikiyatri Derg. 2006, 17, 129–137. (In Turkish) [Google Scholar] [PubMed]
- Devane, W.A.; Hanus, L.; Breuer, A.; Pertwee, R.G.; Stevenson, L.A.; Griffin, G.; Gibson, D.; Mandelbaum, A.; Etinger, A.; Mechoulam, R. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science 1992, 258, 1946–1949. [Google Scholar] [CrossRef] [PubMed]
- Glass, M.; Dragunow, M.; Faull, R.L. Cannabinoid receptors in the human brain: A detailed anatomical and quantitative autoradiographic study in the fetal, neonatal and adult human brain. Neuroscience 1997, 77, 299–318. [Google Scholar] [CrossRef]
- Pertwee, R.G. Ligands that target cannabinoid receptors in the brain: From THC to anandamide and beyond. Addict. Biol. 2008, 13, 147–159. [Google Scholar] [CrossRef] [PubMed]
- Schreiber, S.; Backler, M.M.; Herman, I.; Shamir, D.; Rigai, T.; Bar-Hamburger, R.; Pick, C.G. Mianserin and trazodone significantly attenuate the intensity of opioid withdrawal symptoms in mice. Addict. Biol. 2003, 8, 107–114. [Google Scholar]
- Schreiber, S.; Pick, C.G. Trazodone and mirtazapine: A possible opioid involvement in their use (at low dose) for sleep? Med. Hypotheses 2020, 136, 109501. [Google Scholar] [CrossRef]
- Cuomo, I.; Kotzalidis, G.D.; de Persis, S.; Piacentino, D.; Perrini, F.; Amici, E.; De Filippis, S. Head-to-head comparison of 1-year aripiprazole long-acting injectable (LAI) versus paliperidone LAI in comorbid psychosis and substance use disorder: Impact on clinical status, substance craving, and quality of life. Neuropsychiatr. Dis. Treat. 2018, 14, 1645–1656. [Google Scholar] [CrossRef] [Green Version]
- Kotzalidis, G.D.; Lombardozzi, G.; Matrone, M.; Amici, E.; Perrini, F.; Cuomo, I.; De Filippis, S. Vortioxetine vs. Other Antidepressants in Patients with Major Depressive Episode with or Without Substance Use Disorder. Curr. Neuropharmacol. 2021, 19, 2296–2307. [Google Scholar] [CrossRef]
- De Filippis, S.; Lombardozzi, G.; Matrone, M.; Amici, E.; Trovini, G.; Perrini, F.; Di Giovanni, A.; Giovanetti, V.; Kotzalidis, G.D. Differential response to three antidepressants in patients with major depressive episode who suffered COVID-19-related trauma. Curr. Neuropharmacol. 2022. [Google Scholar] [CrossRef]
- Clelland, C.L.; Ramiah, K.; Steinberg, L.; Clelland, J.D. Analysis of the impact of antidepressants and other medications on COVID-19 infection risk in a chronic psychiatric in-patient cohort. BJPsych Open 2021, 8, e6. [Google Scholar] [CrossRef]
- De Berardis, D.; Fornaro, M.; Ventriglio, A.; Valchera, A.; Vellante, F.; Pettorruso, M.; Martinotti, G.; Fraticelli, S.; Giannantonio, M.D. Trazodone Add-on in COVID-19-related Selective Serotonin Reuptake Inhibitor-resistant Post-traumatic Stress Disorder in Healthcare Workers: Two Case Reports. Clin. Psychopharmacol. Neurosci. 2021, 19, 780–785. [Google Scholar] [CrossRef] [PubMed]
- Hirschtritt, M.E.; Slama, N.; Sterling, S.A.; Olfson, M.; Iturralde, E. Psychotropic medication prescribing during the COVID-19 pandemic. Medicine 2021, 100, e27664. [Google Scholar] [CrossRef] [PubMed]
- Barcellona, P.S. Investigations on the possible teratogenic effects of trazodone in rats and rabbits. Boll. Chim. Farm. 1970, 109, 323–332. [Google Scholar] [PubMed]
- De Gregorio, M.; Dionisio, A. A controlled clinical study of a new antidepressant (trazodone). Panminerva Med. 1971, 13, 27–30. [Google Scholar] [PubMed]
- ClinCalc.com. Trazodone. Drug Usage Statistics, United States, 2013–2019. Available online: https://clincalc.com/DrugStats/Drugs/Trazodone (accessed on 28 April 2022).
- Georgotas, A.; Forsell, T.L.; Mann, J.J.; Kim, M.; Gershon, S. Trazodone hydrochloride: A wide spectrum antidepressant with a unique pharmacological profile. A review of its neurochemical effects, pharmacology, clinical efficacy, and toxicology. Pharmacotherapy 1982, 2, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Carson, C.C., III; Mino, R.D. Priapism associated with trazodone therapy. J. Urol. 1988, 139, 369–370. [Google Scholar] [CrossRef]
- Kolla, B.P.; Schneekloth, T.D.; Biernacka, J.M.; Frye, M.A.; Mansukhani, M.P.; Hall-Flavin, D.K.; Karpyak, V.M.; Loukianova, L.L.; Lesnick, T.G.; Mrazek, D. Trazodone and alcohol relapse: A retrospective study following residential treatment. Am. J. Addict. 2011, 20, 525–529. [Google Scholar] [CrossRef]
- Kryszkowski, W.; Bobińska, K.; Talarowska, M.; Orzechowska, A.; Florkowski, A.; Gałecki, P. Trazodon w terapii uzaleznień [Trazodone in the treatment of addiction]. Pol. Merkur Lekarski 2011, 31, 384–387. (In Polish) [Google Scholar]
- Angarita, G.A.; Emadi, N.; Hodges, S.; Morgan, P.T. Sleep abnormalities associated with alcohol, cannabis, cocaine, and opiate use: A comprehensive review. Addict. Sci. Clin. Pract. 2016, 11, 9. [Google Scholar] [CrossRef] [Green Version]
- Everitt, H.; Baldwin, D.S.; Stuart, B.; Lipinska, G.; Mayers, A.; Malizia, A.L.; Manson, C.C.; Wilson, S. Antidepressants for insomnia in adults. Cochrane Database Syst. Rev. 2018, 5, CD010753. [Google Scholar] [CrossRef]
- Soe, K.K.; Lee, M.Y. Arrhythmias in Severe Trazodone Overdose. Am. J. Case Rep. 2019, 20, 1949–1955. [Google Scholar] [CrossRef] [PubMed]
- Kurtz, S.P.; Buttram, M.E.; Margolin, Z.R.; Wogenstahl, K. The diversion of nonscheduled psychoactive prescription medications in the United States, 2002 to 2017. Pharmacoepidemiol. Drug Saf. 2019, 28, 700–706. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mazza, M.; Caroppo, E.; Marano, G.; Kotzalidis, G.D.; Avallone, C.; Camardese, G.; Janiri, D.; Moccia, L.; Simonetti, A.; Janiri, L.; et al. Trazodone Prolonged-Release Monotherapy in Cannabis Dependent Patients during Lockdown Due to COVID-19 Pandemic: A Case Series. Int. J. Environ. Res. Public Health 2022, 19, 7397. https://doi.org/10.3390/ijerph19127397
Mazza M, Caroppo E, Marano G, Kotzalidis GD, Avallone C, Camardese G, Janiri D, Moccia L, Simonetti A, Janiri L, et al. Trazodone Prolonged-Release Monotherapy in Cannabis Dependent Patients during Lockdown Due to COVID-19 Pandemic: A Case Series. International Journal of Environmental Research and Public Health. 2022; 19(12):7397. https://doi.org/10.3390/ijerph19127397
Chicago/Turabian StyleMazza, Marianna, Emanuele Caroppo, Giuseppe Marano, Georgios D. Kotzalidis, Carla Avallone, Giovanni Camardese, Delfina Janiri, Lorenzo Moccia, Alessio Simonetti, Luigi Janiri, and et al. 2022. "Trazodone Prolonged-Release Monotherapy in Cannabis Dependent Patients during Lockdown Due to COVID-19 Pandemic: A Case Series" International Journal of Environmental Research and Public Health 19, no. 12: 7397. https://doi.org/10.3390/ijerph19127397
APA StyleMazza, M., Caroppo, E., Marano, G., Kotzalidis, G. D., Avallone, C., Camardese, G., Janiri, D., Moccia, L., Simonetti, A., Janiri, L., & Sani, G. (2022). Trazodone Prolonged-Release Monotherapy in Cannabis Dependent Patients during Lockdown Due to COVID-19 Pandemic: A Case Series. International Journal of Environmental Research and Public Health, 19(12), 7397. https://doi.org/10.3390/ijerph19127397