Healing Process, Pain, and Health-Related Quality of Life in Patients with Venous Leg Ulcers Treated with Fish Collagen Gel: A 12-Week Randomized Single-Center Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Interventions
2.3. Ulcer Area
2.4. Quality of Life
2.5. Ethics Statement
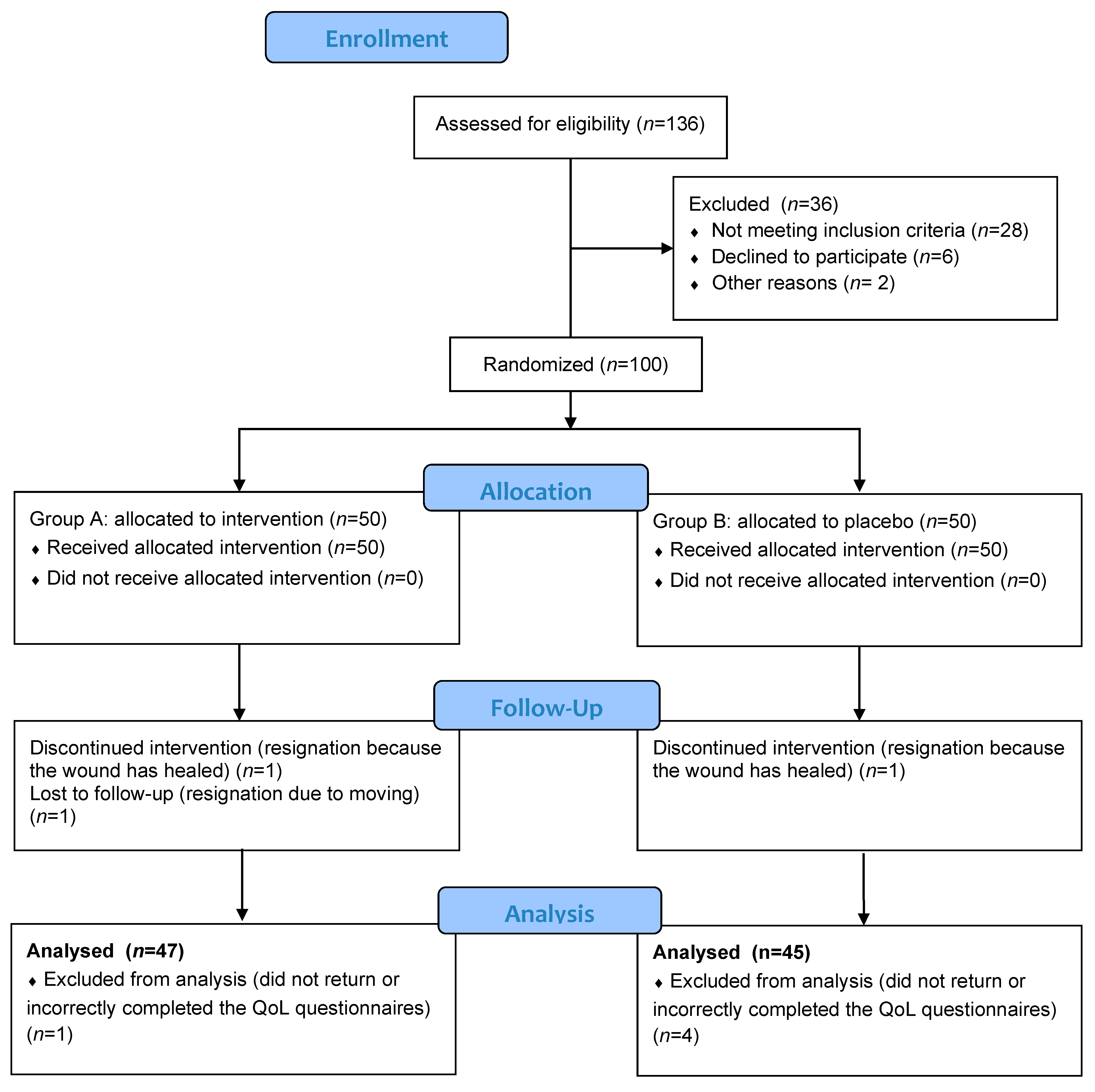
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Raffetto, J.D.; Ligi, D.; Maniscalco, R.; Khalil, R.A.; Mannello, F. Why Venous Leg Ulcers Have Difficulty Healing: Overview on Pathophysiology, Clinical Consequences, and Treatment. J. Clin. Med. 2020, 10, 29. [Google Scholar] [CrossRef]
- Abbade, L.P.F.; Lastoria, S. Venous ulcer: Epidemiology, physiopathology, diagnosis and treatment. Int. J. Dermatol. 2005, 44, 449–456. [Google Scholar] [CrossRef] [PubMed]
- Agale, S.V. Chronic Leg Ulcers: Epidemiology, Aetiopathogenesis, and Management. Ulcers 2013, 2013, 1–9. [Google Scholar] [CrossRef]
- Eberhardt, R.T.; Raffetto, J.D. Chronic Venous Insufficiency. Circulation 2014, 130, 333–346. [Google Scholar] [CrossRef] [PubMed]
- Chi, Y.-W.; Raffetto, J.D. Venous leg ulceration pathophysiology and evidence based treatment. Vasc. Med. 2015, 20, 168–181. [Google Scholar] [CrossRef] [PubMed]
- Marston, W.A.; Carlin, R.E.; Passman, M.A.; Farber, M.; Keagy, B.A. Healing rates and cost efficacy of outpatient compression treatment for leg ulcers associated with venous insufficiency. J. Vasc. Surg. 1999, 30, 491–498. [Google Scholar] [CrossRef][Green Version]
- O’Brien, J.; Finlayson, K.; Kerr, G.; Edwards, H. Evaluating the effectiveness of a self-management exercise intervention on wound healing, functional ability and health-related quality of life outcomes in adults with venous leg ulcers: A randomised controlled trial. Int. Wound J. 2016, 14, 130–137. [Google Scholar] [CrossRef]
- Mościcka, P.; Cwajda-Białasik, J.; Jawień, A.; Szewczyk, M.T. Complex treatment of venous leg ulcers including the use of oral nutritional supplementation: Results of 12-week prospective study. Adv. Dermatol. Allergol. 2022, 39, 336–346. [Google Scholar] [CrossRef]
- Szewczyk, M.T.; Jawień, A.; Migdalski, A.; Piotrowicz, R.; Grzela, T.; Brazis, P. Predicting time to healing by anatomical assessment of venous pathology. Med. Sci. Monitor. 2009, 15, CR74–CR81. [Google Scholar]
- Parker, C.N.; Finlayson, K.J.; Shuter, P.; Edwards, H.E. Risk factors for delayed healing in venous leg ulcers: A review of the literature. Int. J. Clin. Pr. 2015, 69, 967–977. [Google Scholar] [CrossRef]
- Finlayson, K.; Edwards, H.; Courtney, M. Factors associated with recurrence of venous leg ulcers: A survey and retrospective chart review. Int. J. Nurs. Stud. 2009, 46, 1071–1078. [Google Scholar] [CrossRef] [PubMed]
- Mościcka, P.; Szewczyk, M.T.; Jawien, A.; Cierzniakowska, K.; Cwajda-Białasik, J. Subjective and objective assessment of patients’ compression therapy skills as a predicator of ulcer recurrence. J. Clin. Nurs. 2016, 25, 1969–1976. [Google Scholar] [CrossRef]
- Jawień, A.; Szewczyk, M.; Mościcka, P.; Cwajda-Białasik, J.; Hancke, E. Analysis of the recurrence of venous ulceration during 5-year follow-up. Abstract. EWMA J. 2013, 13, 25. [Google Scholar]
- Green, J.; Jester, R.; McKinley, R.; Pooler, A. The impact of chronic venous leg ulcers: A systematic review. J. Wound Care 2014, 23, 601–612. [Google Scholar] [CrossRef] [PubMed]
- Szewczyk, M.T.; Mościcka, P.; Jawień, A.; Cwajda-Białasik, J.; Cierzniakowska, K.; Ślusarz, R.; Hancke, E. Quality of life in patients with leg ulcers or skin lesions—a pilot study. Adv. Dermatol. Allergol. 2015, 32, 465–469. [Google Scholar] [CrossRef]
- Jawień, A.; Szewczyk, M.T.; Kędziora-Kornatowska, K.; Mościcka, P.; Cierzniakowska, K.; Cwajda, J.; Polak, A.; Grzeszak, I. Functional and biopsychosocial restrictions among patients with a venous ulcer. Arch. Med. Sci. 2006, 2, 36–41. [Google Scholar]
- Cwajda-Białasik, J.; Szewczyk, M.T.; Mościcka, P.; Jawień, A.; Ślusarz, R. Influence of ulceration etiology on the global quality of life and its specific dimensions, including the control of pain, in patients with lower limb vascular insufficiency. Adv. Dermatol. Allergol. 2017, 34, 471–477. [Google Scholar] [CrossRef]
- Szewczyk, M.T.; Jawień, A.; Kędziora-Kornatowska, K.; Mościcka, P.; Cwajda, J.; Cierzniakowska, K.; Brazis, P. The nutritional status of older adults with and without venous ulcers: A comparative, descriptive study. Ostomy Wound Manag. 2008, 54, 34–42. [Google Scholar]
- Szewczyk, M.T.; Jawien, A.; Cwajda-Białasik, J.; Cierzniakowska, K.; Mościcka, P.; Hancke, E. Randomized study assessing the influence of supervised exercises on ankle joint mobility in patients with venous leg ulcerations. Arch. Med. Sci. 2010, 6, 956–963. [Google Scholar] [CrossRef] [PubMed]
- Cwajda-Białasik, J.; Szewczyk, M.T.; Mościcka, P.; Cierzniakowska, K. The locus of pain control in patients with lower limb ulcerations. J. Clin. Nurs. 2012, 21, 3346–3351. [Google Scholar] [CrossRef] [PubMed]
- Phillips, P.; Lumley, E.; Duncan, R.; Aber, A.; Woods, H.B.; Jones, G.; Michaels, J. A systematic review of qualitative research into people’s experiences of living with venous leg ulcers. J. Adv. Nurs. 2017, 74, 550–563. [Google Scholar] [CrossRef]
- Wiegand, C.; Schönfelder, U.; Abel, M.; Ruth, P.; Kaatz, M.; Hipler, U.C. Protease and pro-inflammatory cytokine concentrations are elevated in chronic compared to acute wounds and can be modulated by collagen type I in vitro. Arch. Dermatol. Res. 2010, 302, 419–428. [Google Scholar] [CrossRef] [PubMed]
- Gould, L.J. Topical Collagen-Based Biomaterials for Chronic Wounds: Rationale and Clinical Application. Adv. Wound Care New Rochelle 2016, 5, 19–31. [Google Scholar] [CrossRef] [PubMed]
- Marchand, M.; Monnot, C.; Muller, L.; Germain, S. Extracellular matrix scaffolding in angiogenesis and capillary homeostasis. Semin. Cell Dev. Biol. 2018, 89, 147–156. [Google Scholar] [CrossRef]
- Lee, C.H.; Singla, A.; Lee, Y. Biomedical applications of collagen. Int. J. Pharm. 2001, 221, 1–22. [Google Scholar] [CrossRef]
- Bain, M.A.; Thibodeaux, K.T.; Speyrer, M.S.; Carlson, E.; Koullias, G.J. Effect of Native Type I Collagen with Polyhexamethylene Biguanide Antimicrobial on Wounds: Interim Registry Results. Plast. Reconstr. Surg. Glob. Open 2019, 7, e2251. [Google Scholar] [CrossRef] [PubMed]
- Stafiej, J.M.; Szewczyk, M.T. Treatment of full-thickness pressure ulcers with a gentamicin sponge: A case report. J. Wound Ostomy Cont. Nurs. 2012, 39, 331–341. [Google Scholar] [CrossRef]
- Jafari, H.; Lista, A.; Siekapen, M.M.; Ghaffari-Bohlouli, P.; Nie, L.; Alimoradi, H.; Shavandi, A. Fish Collagen: Extraction, Characterization, and Applications for Biomaterials Engineering. Polymers 2020, 12, 2230. [Google Scholar] [CrossRef] [PubMed]
- Kawałkiewicz, W.; Janus-Kubiak, M.; Majewska, A.; Szewczyk, M.T.; Jawień, A.; Hojan-Jezierska, D.; Pankowski, E.; Kubisz, L. Evaluation of skin condition in the area of leg ulcer based on cutometry and results of the MPA program—Case report. Abstract. In Proceedings of the IV Symposium—Biophysics and Medicine, Poznań, Poland, 19–20 September 2019. [Google Scholar]
- Majewska, A.; Janus-Kubiak, M.; Kawałkiewicz, W.; Cwajda-Białasik, J.; Jawień, A.; Hojan-Jezierska, D.; Pankowski, E.; Kubisz, L. Description of skin condition in the area of the ulcer based on its electrical parameters—Case report. Abstract. In Proceedings of the IV Symposium—Biophysics and Medicine, Poznań, Poland, 19–20 September 2019. [Google Scholar]
- Kubisz, L.; Hojan-Jezierska, D.; Szewczyk, M.; Majewska, A.; Kawałkiewicz, W.; Pankowski, E.; Janus, M.; Cwajda-Białasik, J.; Mościcka, P.; Jawień, A. In vivo electrical impedance measurement in human skin assessment. Pure Appl. Chem. 2019, 91, 1481–1491. [Google Scholar] [CrossRef]
- Cwajda-Białasik, J.; Mościcka, P.; Szewczyk, M.T.; Hojan-Jezierska, D.; Kawałkiewicz, W.; Majewska, A.; Janus-Kubiak, M.; Kubisz, L.; Jawień, A. Venous leg ulcers treated with fish collagen gel in a 12-week randomized single-centre study. Adv. Dermatol. Allergol. 2021, 38. [Google Scholar] [CrossRef]
- Murphy, C.; Atkin, L.; Swanson, T.; Tachi, M.; Tan, Y.K.; De Ceniga, M.V.; Weir, D.; Wolcott, R.; Ĉernohorská, J.; Ciprandi, G.; et al. Defying hard-to-heal wounds with an early antibiofilm intervention strategy: Wound hygiene. J. Wound Care 2020, 29, S1–S26. [Google Scholar] [CrossRef] [PubMed]
- Wajszczuk, K.; Wawrzynowicz, J.; Sajna, P.; Nowotarska, A.; Szewczyk, M.T.; Jawień, A.; Mościcka, P.; Cwajda-Białasik, J. An Economic Evaluation of a New Medical Technology of Treatment of Venous Leg Ulcers with Fish Collagen. Abstract. In Proceedings of the IV Symposium—Biophysics and Medicine, Poznań, Poland, 19–20 September 2019. [Google Scholar]
- Chren, M.M.; Lasek, R.J.; Quinn, L.M.; Mostow, E.N.; Zyzanski, S.J. Skindex, a quality-of-life measure for patients with skin disease: Reliability, validity, and responsiveness. J. Investig. Derm. 1996, 107, 707–713. [Google Scholar] [CrossRef] [PubMed]
- Chren, M.M.; Lasek, R.J.; Flocke, S.A.; Zyzanski, S.J. Improved discriminative and evaluative capability of a refined version of Skindex, a quality-of-life instrument for patients with skin diseases. Arch. Dermatol. 1997, 133, 1433–1440. [Google Scholar] [CrossRef]
- Janowski, K.; Steuden, S.; Bereza, B. The Polish version of Skindex-29: Psychometric properties of an instrument to measure quality of life in dermatology. Adv. Dermatol. Allergol. 2014, 1, 12–20. [Google Scholar] [CrossRef]
- Cwajda-Białasik, J.; Mościcka, K.; Szewczyk, M.T.; Cierzniakowska, K. Usefulness of the Skindex-29 questionnaire for quality of life assessment in patients with lower-limb ulcers of vascular aetiology. Pielęgniarstwo Chir. Angiol. 2018, 2, 64–70. [Google Scholar]
- Launois, R.; Reboul-Marty, J.; Henry, B. Construction and validation of a quality of life questionnaire in Chronic Lower Limb Venous Insufficiency (CIVIQ). Qual. Life Res. 1996, 5, 539–554. [Google Scholar] [CrossRef]
- Launois, R.; Mansilha, A.; Jantet, G. International psychometric validation of the chronic venous disease quality of life questionnaire (CIVIQ-20). Eur. J. Vasc. Endovasc. Surg. 2010, 40, 783–789. [Google Scholar] [CrossRef]
- Servier, L.L. Linguistic Versions of CIVIQ-20. Available online: https://www.civiq-20.com/getting-copy/linguistic-versions-civiq-20/ (accessed on 21 January 2019).
- Rodriguez, C.S. Pain measurement in the elderly: A review. Pain Manag. Nurs. 2001, 2, 38–46. [Google Scholar] [CrossRef]
- The Personal Data Protection Act of 10 May 2018 (Journal of Laws from 2018, item 1000). Available online: https://isap.sejm.gov.pl/isap.nsf/DocDetails.xsp?id=WDU20180001000 (accessed on 1 October 2020).
- Araujo, T.A.T.; Almeida, M.C.; Avanzi, I.; Parisi, J.; Sales, A.F.S.; Na, Y.; Renno, A. Collagen membranes for skin wound repair: A systematic review. J. Biomater. Appl. 2020, 36, 95–112. [Google Scholar] [CrossRef]
- Chattopadhyay, S.; Raines, R.T. Collagen-based biomaterials for wound healing. Biopolymers 2014, 101, 821–833. [Google Scholar] [CrossRef]
- Veves, A.; Sheehan, P.; Pham, H. A Randomized, Controlled Trial of Promogran (a Collagen/Oxidized Regenerated Cellulose Dressing) vs Standard Treatment in the Management of Diabetic Foot Ulcers. Arch. Surg. 2002, 137, 822–827. [Google Scholar] [CrossRef] [PubMed]
- Gottrup, F.; Cullen, B.M.; Karlsmark, T.; Bischoff-Mikkelsen, M.; Nisbet, L.; Gibson, M.C. Randomized controlled trial on collagen/oxidized regenerated cellulose/silver treatment. Wound Repair Regen 2013, 21, 216–225. [Google Scholar] [CrossRef] [PubMed]
- Vin, F.; Teot, L.; Meaume, S. The healing properties of Promogran in venous leg ulcers. J. Wound Care 2002, 11, 335–341. [Google Scholar] [CrossRef] [PubMed]
- Guest, J.F.; Rana, K.; Singh, H.; Vowden, P. Cost-effectiveness of using a collagen-containing dressing plus compression therapy in non-healing venous leg ulcers. J. Wound Care 2018, 27, 68–78. [Google Scholar] [CrossRef]
- Romanelli, M.; Mulder, G.; Paggi, B.; Macchia, M.; Panduri, S.; Dini, V. The use of a collagen matrix in hard-to-heal venous leg ulcers. J. Wound Care 2015, 24, 543–547. [Google Scholar] [CrossRef]
- Chen, H.; Wei, X.; Zhang, C.; Zhang, W. Progress of fish collagen as novel biomedical material. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi 2018, 32, 1227–1230. [Google Scholar] [CrossRef]
- Gauza-Włodarczyk, M.; Kubisz, L.; Mielcarek, S.; Włodarczyk, D. Comparison of thermal properties of fish collagen and bovine collagen in the temperature range 298–670K. Mater Sci. Eng. C Mater Biol. Appl. 2017, 80, 468–471. [Google Scholar] [CrossRef]
- Lullove, E.; Liden, B.; Winters, C.; McEneaney, P.; Raphael, A.; Ii, J.L. A Multicenter, Blinded, Randomized Controlled Clinical Trial Evaluating the Effect of Omega-3–Rich Fish Skin in the Treatment of Chronic, Nonresponsive Diabetic Foot Ulcers. Wounds A Compend. Clin. Res. Pr. 2021, 33, 169–177. [Google Scholar] [CrossRef]
- Cwajda-Białasik, J.; Mościcka, P.; Jawień, A.; Szewczyk, M.T. Infrared thermography to prognose the venous leg ulcer healing process—preliminary results of a 12-week, prospective observational study. Wound Repair Regen. 2019, 28, 224–233. [Google Scholar] [CrossRef]
- Dias, T.Y.; Costa, I.K.; Melo, M.D.; Torres, S.M.; Maia, E.M.; Torres, G.V. Quality of life assessment of patients with and without venous ulcer. Rev. Latino-Am. Enferm. 2014, 22, 576–581. [Google Scholar] [CrossRef]
- Moura, R.M.F.; Gonçalves, G.S.; Navarro, T.P.; Britto, R.R.; Dias, R.C. Relationship between quality of life and the CEAP clinical classification in chronic venous disease. Rev. Bras. Fisioter. 2010, 14, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Edwards, H.; Finlayson, K.; Skerman, H.; Alexander, K.; Miaskowski, C.; Aouizerat, B.; Gibb, M. Identification of Symptom Clusters in Patients With Chronic Venous Leg Ulcers. J. Pain Symptom Manag. 2014, 47, 867–875. [Google Scholar] [CrossRef] [PubMed]
- Zenati, N.; Bosson, J.; Blaise, S.; Carpentier, P. Health related quality of life in chronic venous disease: Systematic literature review. J. Med. Vasc. 2017, 42, 290–300. [Google Scholar] [CrossRef] [PubMed]
- Mościcka, P.; Cwajda-Białasik, J.; Jawień, A.; Sopata, M.; Szewczyk, M.T. Occurrence and Severity of Pain in Patients with Venous Leg Ulcers: A 12-Week Longitudinal Study. J. Clin. Med. 2020, 9, 3399. [Google Scholar] [CrossRef]
- Nemeth, K.A.; Harrison, M.B.; Graham, I.D.; Burke, S. Pain in pure and mixed aetiology venous leg ulcers: A three-phase point prevalence study. J. Wound Care 2003, 12, 336–340. [Google Scholar] [CrossRef]
- Maddox, D. Effects of venous leg ulceration on patients’ quality of life. Nurs. Stand. 2012, 26, 42–49. [Google Scholar] [CrossRef]
- Hyland, M.E.; Ley, A.; Thomson, B. Quality of life of leg ulcer patients: Questionnaire and preliminary findings. J. Wound Care 1994, 3, 294–298. [Google Scholar] [CrossRef]
- Nogueira, G.S.; Zanin, C.R.; Miyazaki, M.C.O.S.; Godoy, J.M.P. Quality of Life of Patients with Chronic Venous Ulcers and Socio-Demographic Factors. Wounds 2012, 24, 289–292. [Google Scholar]
- Hareendran, A.; Doll, H.; Wild, D.J.; Moffatt, C.; Musgrove, E.; Wheatley, C.; Franks, P.J. The venous leg ulcer quality of life (VLU-QoL) questionnaire: Development and psychometric validation. Wound Repair Regen. 2007, 15, 465–473. [Google Scholar] [CrossRef]
- Ograczyk, A.; Miniszewska, J.; Kępska, A.; Zalewska-Janowska, A. Itch, disease coping strategies and quality of life in psoriasis patients. Adv. Dermatol. Allergol. 2014, 5, 299–304. [Google Scholar] [CrossRef]
- Jockenhöfer, F.; Knust, C.; Benson, S.; Schedlowski, M.; Dissemond, J. Influence of placebo effects on quality of life and wound healing in patients with chronic venous leg ulcers. J. Dtsch. Dermatol. Ges. 2019, 18, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Mathias, S.D.; Prebil, L.A.; Boyko, W.L.; Fastenau, J. Health-related quality of life in venous leg ulcer patients successfully treated with Apligraf: A pilot study. Adv. Skin Wound Care 2000, 13, 76–78. [Google Scholar] [PubMed]
- Ricci, E.; Cutting, K. Evaluating a native collagen matrix dressing in the treatment of chronic wounds of different aetiologies: A case series. J. Wound Care 2016, 25, 670–678. [Google Scholar] [CrossRef] [PubMed]


| Characteristic | Group A (n = 47) | Group B (n = 45) | |||
|---|---|---|---|---|---|
| Mean ± SD (Range) | Median | Mean ± SD (Range) | Median | ||
| Age (Years) | 64.5 ± 12.00 (35–88) | 64.0 | 63.6 ± 11.66 (39–87) | 64.0 | |
| Gender * | |||||
| Female | 25 (53.2) | 23 (48.9) | |||
| Male | 22 (46.8) | 24 (51.1) | |||
| Duration of CVI (years) | 17.7 ± 11.9 (1–50) | 17.0 | 16.7 ± 13.7 (1–52) | 13.0 | |
| Duration of VLU (months) | 72.5 ± 102.5 (4–440) | 20.0 | 40.8 ± 62.3 (2–360) | 20.0 | |
| CEAP-C6 (score) | 11.6 ± 1.63 (7–15) | 12.0 | 11.6 ± 1.73 (7–16) | 11.5 | |
| BMI | 31.0 ± 7.2 (19.4–58,9) | 30.7 | 30.8 ± 6.6 (20.6–58.9) | 30.5 | |
| Initial wound size (cm2) | 18.3 ± 15.1 (5–50) | 11.5 | 15.4 ± 14.6 (5–50) | 8.6 | |
| ABI right | 1.09 ± 0.12 (0.90–1.33) | 1.06 | 1.14 ± 0.14 (0.80–1.5) | 1.14 | |
| ABI left | 1.07 ± 0.12 (0.83–1.40) | 1.06 | 1.11 ± 0.14 (0.72–1.5) | 1.10 | |
| Domain | Group A, n = 47 | Group B, n = 45 | |||
|---|---|---|---|---|---|
| Mean ± SD | Median [IQR] | Mean ± SD | Median [IQR] | p | |
| HR (cm2/week)12 weeks later | 0.68 ± 0.61 | 0.56 [0.42, 0.78] | 0.51 ± 0.68 | 0.43 [0.24, 0.68] | 0.598 |
| 13 (27.7%) * | 11 (24.4%) * | 0.675 | |||
| HR (cm2/week) 24 weeks later | 0.42 ± 0.44 | 0.33 [0.22, 0.49] | 0.33 ± 0.31 | 0.24 [0.21, 0.41] | 0.219 |
| 25 (53.2%) * | 18 (40.0%) * | 0.223 | |||
| I. SKINDEX-29, Initial Assessment | |||||
| PS | 17.79 ± 5.13 | 17.00 [14.00, 21.00] | 15.69 ± 5.21 | 16.00 [12.25, 21.75] | 0.420 |
| PF | 23.96 ± 8.77 | 21.00 [18.00, 29.00] | 21.73 ± 7.04 | 20.00 [16.00, 28.00] | 0.497 |
| ES | 21.79 ± 7.35 | 21.00 [16.75, 25.00] | 19.88 ± 5.60 | 20.00 [17.00, 25.00] | 0.691 |
| TS | 63.53 ± 18.92 | 60.00 [49.50, 72.25] | 57.31 ± 15.61 | 57.00 [47.00, 74.00] | 0.479 |
| II. SKINDEX-29, 12 Weeks Later | |||||
| PS | 14.49 ± 4.49 | 14.00 [11.00, 17.00] | 15.16 ± 5.09 | 16.00 [10.00, 19.00] | 0.578 |
| PF | 19.32 ± 7.32 | 15.00 [12.75, 21.25] | 17.51 ± 5.85 | 16.00 [12.00, 21.00] | 0.968 |
| ES | 18.98 ± 7.08 | 14.00 [11.00, 18.25] | 16.18 ± 4.83 | 15.00 [11.00, 19.00] | 0.651 |
| TS | 52.79 ± 17.34 | 47.00 [39.00, 56.25] | 52.87 ± 14.93 | 50.00 [38.00, 64.00] | 0.516 |
| III. SKINDEX-29, 24 Weeks Later | |||||
| PS | 13.47 ± 5.12 | 11.50 [9.00, 15.25] | 13.29 ± 4.57 | 13.00 [9.00, 15.00] | 0.951 |
| PF | 18.30 ± 8.05 | 14.00 [11.00, 18.50] | 16.16 ± 4.98 | 14.00 [11.00, 18.00] | 0.951 |
| ES | 18.23 ± 7.06 | 12.50 [10.00, 20.00] | 15.00 ± 4.73 | 14.00 [11.00, 19.00] | 0.811 |
| TS | 50.00 ± 18.53 | 41.50 [35.00, 54.75] | 48.40 ± 13.91 | 43.00 [35.00, 55.00] | 0.673 |
| CIVIQ, Initial Assessment | |||||
| Pain | 11.13 ± 3.30 | 11.00 [8.75, 13.25] | 10.80 ± 3.47 | 11.00 [8.00, 13.00] | 0.533 |
| PhD | 11.28 ± 3.66 | 12.00 [8.00, 14.25] | 11.24 ± 3.61 | 12.00 [8.00, 14.00] | 0.811 |
| PsD | 20.13 ± 6.83 | 18.50 [15.00, 23.50] | 18.84 ± 6.46 | 8.00 [6.00, 9.00] | 0.437 |
| ScD | 7.89 ± 2.80 | 7.50 [6.00, 10.00] | 7.87 ± 2.74 | 8.00 [6.00, 9.00] | 0.749 |
| TS | 50.43 ± 13.40 | 50.00 [38.50, 60.50] | 48.76 ± 13.79 | 50.00 [38.50, 60.00] | 0.444 |
| GIS | 38.03 ± 16.75 | 37.50 [23.12, 50.62] | 35.95 ± 17.01 | 38.75 [22.50, 50.000] | |
| CIVIQ, 12 Weeks Later | |||||
| Pain | 8.74 ± 3.54 | 8.00 [6.00, 11.00] | 8.78 ± 3.32 | 7.00 [6.00, 11.00] | 0.667 |
| PhD | 10.28 ± 3.28 | 10.50 [6.75, 13.00] | 10.47 ± 3.92 | 11.00 [6.00, 13.00] | 0.854 |
| PsD | 16.94 ± 6.02 | 15.00 [11.75, 21.25] | 17.67 ± 5.40 | 17.00 [13.00, 21.00] | 0.548 |
| ScD | 7.30 ± 2.67 | 7.00 [5.00, 9.00] | 7.02 ± 2.46 | 6.00 [5.00, 8.00] | 0.358 |
| TS | 43.26 ± 13.52 | 41.00 [31.50, 51.50] | 43.93 ± 12.11 | 42.00 [34.00, 53.00] | 0.860 |
| GIS | 29.06 ± 16.90 | 26.25 [16.87, 40.00] | 29.91 ± 14.96 | 28.75 [20.00, 42.50] | |
| CIVIQ, 24 Weeks Later | |||||
| Pain | 8.00 ± 3.21 | 7.00 [5.00, 9.00] | 7.87 ± 3.69 | 7.00 [5.00, 9.00] | 0.816 |
| PhD | 10.15 ± 3.62 | 10.00 [6.00, 12.25] | 10.24 ± 4.87 | 10.00 [6.00, 12.00] | 0.845 |
| PsD | 16.44 ± 6.31 | 13.00 [11.00, 20.00] | 16.89 ± 3.18 | 15.00 [11.00, 20.00] | 0.685 |
| ScD | 7.13 ± 2.57 | 7.00 [5.00, 9.00] | 6.96 ± 2.59 | 6.00 [4.00, 8.00] | 0.279 |
| TS | 41.04 ± 13.54 | 37.00 [28.75, 50.25] | 41.96 ± 11.97 | 39.00 [30.00, 49.00] | 0.877 |
| GIS | 26.30 ± 17.03 | 21.25 [12.50, 38.12] | 27.45 ± 18.13 | 25.00 [15.00, 37.50] | |
| Item | Group A | Group B | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean ± SD | Mean ± SD | Mean ± SD | I–II | I–III | Mean ± SD | Mean ± SD | Mean ± SD | I–II | I–III | ||
| 1 | My skin hurts ** | 2.47 ± 1.40 | 2.11 ± 1.20 | 1.70 ± 1.00 | 0.36 | 0.77 | 2.40 ± 1.34 | 2.24 ± 1.25 | 1.89 ± 1.15 | 0.16 | 0.51 |
| 2 | My skin condition affects how well I sleep ** | 2.43 ± 1.30 | 1.85 ± 1.08 | 1.68 ± 1.07 | 0.57 | 0.74 | 2.47 ± 1.39 | 2.13 ± 1.20 | 1.87 ± 1.04 | 0.33 | 0.60 |
| 3 | I worry that my skin condition may be serious ** | 3.26 ± 1.19 | 2.83 ± 1.32 | 2.74 ± 1.28. | 0.43 | 0.51 | 3.00 ± 1.07 | 2.89 ± 1.07 | 2.82 ± 1.03 | 0.11 | 0.18 |
| 4 | My skin condition makes it hard to work or do hobbies * | 2.47 ± 1.27 | 2.00 ± 1.12 | 1.74 ± 0.99 | 0.47 | 0.72 | 2.51 ± 1.25 | 2.18 ± 1.07 | 2.09 ± 1.04 | 0.33 | 0.42 |
| 5 | My skin condition affects my social life * | 2.22 ± 1.23 | 1.62 ± 0.95 | 1.55 ± 0.93 | 0.60 | 0.66 | 1.82 ± 1.03 | 1.51 ± 0.76 | 1.31 ± 0.60 | 0.31 | 0.51 |
| 6 | My skin condition makes me feel depressed * | 2.51 ± 1.16 | 2.09 ± 1.10 | 2.02 ± 1.13 | 0.43 | 0.49 | 2.44 ± 1.22 | 1.80 ± 0.92 | 1.84 ± 0.85 | 0.64 | 0.60 |
| 7 | My skin condition burns or strings ** | 2.83 ± 1.22 | 2.43 ± 1.17 | 2.02 ± 1.15 | 0.40 | 0.81 | 2.56 ± 1.20 | 2.27 ± 1.23 | 2.09 ± 1.02 | 0.29 | 0.47 |
| 8 | I tend to stay at home because of my skin condition | 2.11 ± 1.17 | 1.55 ± 0.97 | 1.53 ± 0.95 | 0.55 | 0.57 | 2.09 ± 1.20 | 1.56 ± 0.92 | 1.42 ± 0.81 | 0.53 | 0.62 |
| 9 | I worry about getting scars from my skin condition ** | 1.68 ± 1.04 | 1.60 ± 1.10 | 1.40 ± 0.74 | 0.08 | 0.28 | 1.47 ± 0.76 | 1.76 ± 1.03 | 1,58 ± 0.87 | -0.29 | -0.11 |
| 10 | My skin itches | 3.00 ± 1.25 | 2.72 ± 1.23 | 2.53 ± 1.27 | 0.28 | 0.47 | 3.04 ± 1.30 | 2.71 ± 1.10 | 2.50 ± 1.11 | 0.33 | 0.54 |
| 11 | My skin condition affects how close I can be with those I love | 1.68 ± 0.91 | 1.51 ± 0.86 | 1.34 ± 0.73 | 0.17 | 0.34 | 1,58 ± 1.06 | 1.36 ± 0.68 | 1.33 ± 0.67 | 0.22 | 0.25 |
| 12 | I am ashamed of my skin condition * | 2.02 ± 1.15 | 1.98 ± 1.34 | 1.70 ± 1.02 | 0.04 | 0.32 | 1.87 ± 1.08 | 1.93 ± 1.01 | 1.78 ± 0.97 | -0.07 | 0.09 |
| 13 | I worry that my skin condition may get worse | 2.77 ± 1.24 | 2.68 ± 1.14 | 2.72 ± 1.14 | 0.09 | 0.04 | 2.69 ± 0.95 | 2.78 ± 1.22 | 2.67 ± 1.22 | -0.09 | 0.02 |
| 14 | I tend to do things by myself because of my skin condition ** | 2.17 ± 1.19 | 1.89 ± 1.20 | 1.74 ± 1.15 | 0.28 | 0.43 | 2.27 ± 1.25 | 1.67 ± 1.13 | 1.47 ± 0.97 | 0.60 | 0.80 |
| 15 | I am angry about my skin condition | 1.81 ± 1.06 | 1.51 ± 0.88 | 1.49 ± 0.78 | 0.30 | 0.32 | 1.87 ± 1.12 | 1.47 ± 0.84 | 1.42 ± 0.81 | 0.40 | 0.44 |
| 16 | Water bothers my skin condition (bathing, washing) ** | 1.85 ± 1.18 | 1.30 ± 0.69 | 1.40 ± 0.80 | 0.55 | 0.45 | 1.49 ± 0.89 | 1.42 ± 0.94 | 1.29 ± 0.73 | 0.07 | 0.20 |
| 17 | My skin condition makes showing affection difficult | 1.74 ± 1.11 | 1.47 ± 0.95 | 1.40 ± 0.77 | 0.28 | 0.34 | 1.53 ± 0.99 | 1.31 ± 0.76 | 1.24 ± 0.61 | 0.22 | 0.29 |
| 18 | My skin is irritated | 2.62 ± 1.23 | 2.06 ± 0.96 | 2.15 ± 1.06 | 0.55 | 0.47 | 2.73 ± 1.23 | 2.29 ± 1.18 | 2.09 ± 1.18 | 0.44 | 0.64 |
| 19 | My skin condition affects my interactions with others | 1.51 ± 0.88 | 1.23 ± 0.56 | 1.36 ± 0.76 | 0.28 | 0.15 | 1.51 ± 1.01 | 1.36 ± 0.74 | 1.33 ± 0.71 | 0.16 | 0.18 |
| 20 | I am embarrassed by my skin condition * | 2.13 ± 1.10 | 1.60 ± 0.92 | 1.60 ± 0.85 | 0.53 | 0.53 | 2.16 ± 1.13 | 1.84 ± 1.00 | 1.71 ± 0.92 | 0.31 | 0.44 |
| 21 | My skin condition is a problem for the people I love | 1.87 ± 1.15 | 1.53 ± 0.95 | 1.43 ± 0.90 | 0.34 | 0.45 | 1.93 ± 1.23 | 1.31 ± 0.68 | 1.36 ± 0.74 | 0.62 | 0.58 |
| 22 | I am frustrated by my skin condition | 1.81 ± 1.04 | 1.74 ± 0.99 | 1.68 ± 0.96 | 0.06 | 0.13 | 1.96 ± 1.11 | 1.62 ± 0.86 | 1.53 ± 0.84 | 0.33 | 0.42 |
| 23 | My skin is sensitive ** | 2.98 ± 1.11 | 2.38 ± 0.99 | 2.28 ± 1.02 | 0.60 | 0.70 | 2.91 ± 1.29 | 2.80 ± 1.16 | 2.47 ± 1.08 | 0.11 | 0.44 |
| 24 | My skin condition affects my desire to be with people | 1.68 ± 1.02 | 1.45 ± 0.75 | 1.34 ± 0.73 | 0.23 | 0.34 | 1.60 ± 1.10 | 1.29 ± 0.59 | 1.27 ± 0.62 | 0.31 | 0.33 |
| 25 | I am humiliated by my skin condition | 1.66 ± 0.96 | 1.36 ± 0.76 | 1.34 ± 0.64 | 0.30 | 0.32 | 1.64 ± 1.05 | 1.36 ± 0.74 | 1.29 ± 0.69 | 0.29 | 0.36 |
| 26 | My skin condition bleeds | 2.04 ± 1.04 | 1.49 ± 0.80 | 1.38 ± 0.80 | 0.55 | 0.66 | 1.82 ± 1.07 | 1.42 ± 0.72 | 1.38 ± 0.68 | 0.40 | 0.44 |
| 27 | I am annoyed by my skin condition | 2.15 ± 1.23 | 1.60 ± 0.92 | 1.53 ± 0.86 | 0.55 | 0.62 | 1.96 ± 1.04 | 1.51 ± 0.84 | 1.49 ± 0.84 | 0.44 | 0.47 |
| 28 | My skin condition interferes with my sex life | 1.47 ± 1.00 | 1.26 ± 0.87 | 1.11 ± 0.60 | 0.21 | 0.36 | 1.62 ± 1.17 | 1.24 ± 0.68 | 1.24 ± 0.83 | 0.38 | 0.38 |
| 29 | My skin condition makes me tired | 2.62 ± 1.13 | 1.96 ± 1.08 | 2.06 ± 1.15 | 0.66 | 0.55 | 2.40 ± 1.12 | 1.84 ± 1.07 | 1.80 ± 1.06 | 0.56 | 0.60 |
| Statement | Group A | Group B | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean ± SD | Mean ± SD | Mean ± SD | I–II | I–III | Mean ± SD | Mean ± SD | Mean ± SD | I–II | I–III | ||
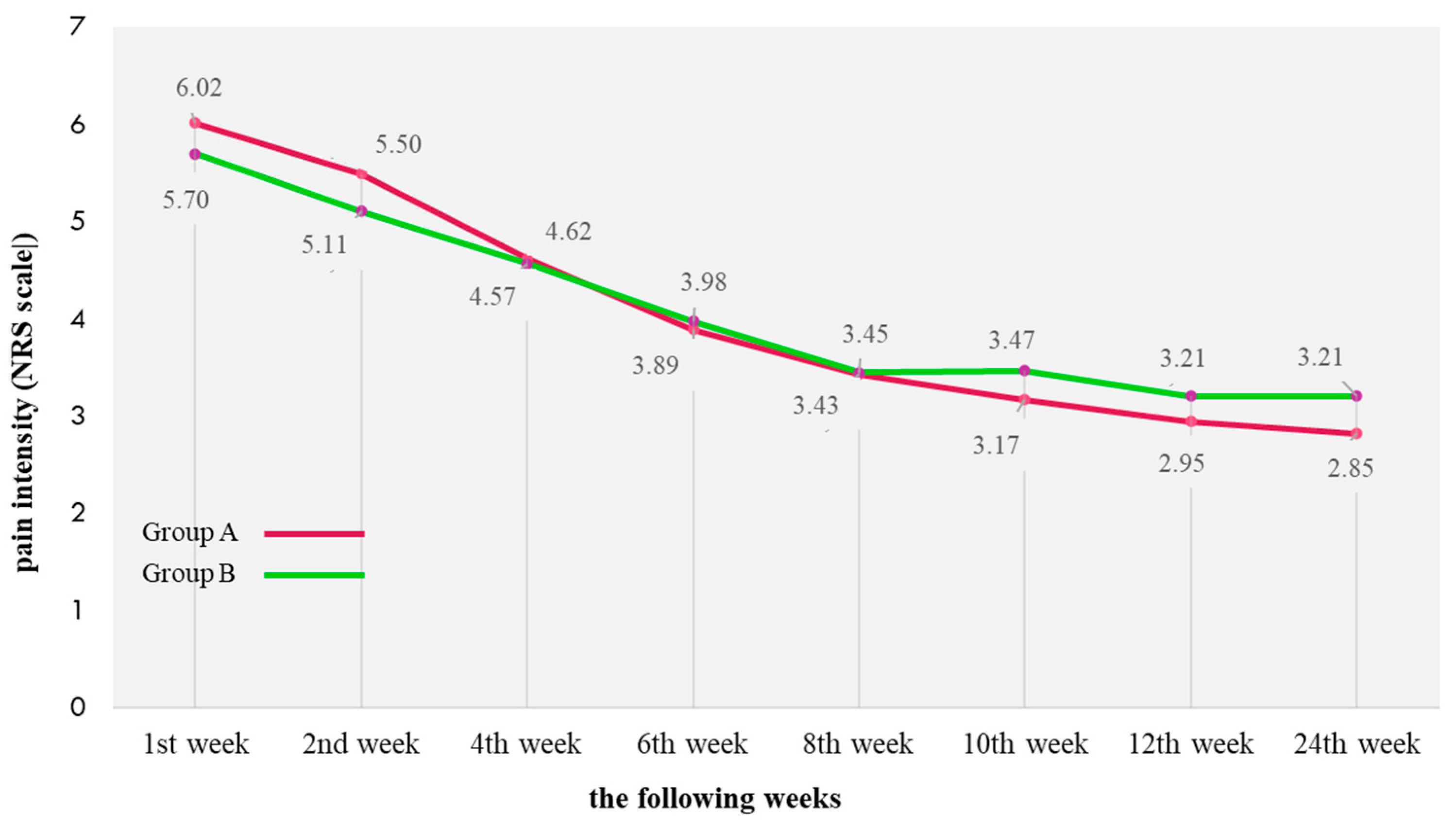
| 1 | Have you had any pain in your ankles or legs, and how severe has this pain been? * | 3.17 ± 1.13 | 2.36 ± 1.26 | 1.72 ± 0.97 | 0.81 | 1.45 | 2.93 ± 1.21 | 2.20 ± 1.08 | 1.91 ± 1.14 | 0.73 | 1.02 |
| 2 | How much trouble have you experienced at work or during your usual daily activities because of your leg problems? * | 2.77 ± 0.96 | 2.17 ± 1.03 | 2.09 ± 0.97 | 0.60 | 0.68 | 2.49 ± 1.10 | 2.24 ± 0.98 | 1.93 ± 0.96 | 0.24 | 0.56 |
| 3 | How much trouble have you experienced at work or during your usual daily activities because of your leg problems? | 2.51 ± 1.28 | 1.79 ± 1.14 | 1.53 ± 1.02 | 0.72 | 0.98 | 2.71 ± 1.22 | 1.93 ± 1.05 | 1.62 ± 1.01 | 0.78 | 1.09 |
| 4 | How much trouble have you had remaining standing for a long time? * | 2.68 ± 1.12 | 1.43 ± 1.21 | 2.26 ± 1.19 | 0.26 | 0.43 | 2.67 ± 1.26 | 2.40 ± 1.10 | 2.40 ± 1.18 | 0.27 | 0.27 |
| 5 | Climbing several flights of stairs? | 2.74 ± 1.15 | 2.28 ± 1.14 | 2.45 ± 1.08 | 0.47 | 0.30 | 2.51 ± 1.12 | 2.36 ± 1.15 | 2.27 ± 1.05 | 0.16 | 0.24 |
| 6 | Crouching, kneeling down | 3.38 ± 1.17 | 3.23 ± 1.24 | 2.94 ± 1.19 | 0.15 | 0.45 | 3.47 ± 1.22 | 3.29 ± 1.34 | 3.27 ± 1.30 | 0.18 | 0.20 |
| 7 | Walking at a brisk pace | 2.98 ± 1.19 | 2.94 ± 1.24 | 2.79 ± 1.20 | 0.04 | 0.19 | 3.16 ± 1.22 | 2.93 ± 1.27 | 2.87 ± 1.20 | 0.22 | 0.29 |
| 8 | Travelling by car, bus, plane | 2.15 ± 1.12 | 1.85 ± 1.00 | 1.85 ± 1.02 | 0.30 | 0.30 | 2.22 ± 1.04 | 1.73 ± 1.03 | 1.76 ± 0.93 | 0.49 | 0.47 |
| 9 | Performing household tasks (e.g., standing and moving around in the kitchen, carrying a child in your arms, ironing, cleaning the floor or dusting the furniture) * | 2.17 ± 1.05 | 1.83 ± 0.96 | 1.74 ± 0.90 | 0.34 | 0.43 | 2.11 ± 0.98 | 1.89 ± 0.91 | 1.84 ± 0.95 | 0.22 | 0.27 |
| 10 | Going out for the evening, going to a wedding, a party, a cocktail party | 2.32 ± 1.16 | 1.96 ± 1.04 | 1.94 ± 1.01 | 0.36 | 0.38 | 2.21 ± 1.14 | 1.73 ± 0.99 | 1.71 ± 0.99 | 0.47 | 0.49 |
| 11 | Playing a sport, exerting yourself physically | 3.43 ± 1.17 | 3.49 ± 1.28 | 3.42 ± 1.36 | −0.06 | 0.00 | 3.44 ± 1.34 | 3.56 ± 1.22 | 3.49 ± 1.29 | −0.11 | −0.04 |
| 12 | I have felt nervous/tense * | 2.15 ± 1.06 | 1.85 ± 1.00 | 1.55 ± 0.95 | 0.30 | 0.60 | 2.04 ± 1.11 | 1.77 ± 1.10 | 1.08 ± 1.04 | 0.27 | 0.24 |
| 13 | I have become tired quickly ** | 2.66 ± 1.09 | 2.19 ± 1.06 | 1.83 ± 1.07 | 0.47 | 0.83 | 2.58 ± 1.01 | 2.44 ± 1.12 | 2.20 ± 1.01 | 0.13 | 0.38 |
| 14 | I have felt I am a burden | 1.62 ± 0.99 | 1.45 ± 0.85 | 1.30 ± 0.75 | 0.17 | 0.32 | 1.58 ± 0.87 | 1.36 ± 0.71 | 1.24 ± 0.57 | 0.22 | 0.33 |
| 15 | I have had to be cautious all the time | 2.79 ± 1.12 | 2.36 ± 1.07 | 2.43 ± 1.04 | 0.43 | 0.36 | 2.87 ± 1.25 | 2.73 ± 1.25 | 2.93 ± 1.05 | 0.13 | −0.07 |
| 16 | I have felt embarrassed about showing my legs ** | 2.79 ± 1.37 | 2.34 ± 1.27 | 2.17 ± 1.19 | 0.45 | 0.62 | 2.16 ± 1.19 | 2.24 ± 1.30 | 2.13 ± 1.10 | −0.09 | 0.02 |
| 17 | I have become irritated easily ** | 2.68 ± 1.32 | 2.17 ± 1.29 | 1.98 ± 1.21 | 0.51 | 0.70 | 2.33 ± 1.13 | 2.31 ± 1.12 | 2.24 ± 1.09 | 0.02 | 0.09 |
| 18 | I have felt as if I am handicapped * | 1.98 ± 1.24 | 1.64 ± 1.05 | 1.62 ± 0.99 | 0.34 | 0.36 | 1.82 ± 1.21 | 1.71 ± 1.12 | 1.60 ± 0.91 | 0.11 | 0.22 |
| 19 | I have found it hard to get going in the morning | 1.66 ± 1.20 | 1.40 ± 0.77 | 1.70 ± 1.00 | 0.26 | 0.04 | 1.69 ± 1.06 | 1.67 ± 1.00 | 1.44 ± 0.84 | 0.02 | 0.24 |
| 20 | I have not felt like going out | 1.81 ± 1.04 | 1.57 ± 0.96 | 1.64 ± 1.03 | 0.24 | 0.17 | 1.78 ± 1.06 | 1.47 ± 0.81 | 1.29 ± 0.63 | 0.31 | 0.49 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mościcka, P.; Cwajda-Białasik, J.; Szewczyk, M.T.; Jawień, A. Healing Process, Pain, and Health-Related Quality of Life in Patients with Venous Leg Ulcers Treated with Fish Collagen Gel: A 12-Week Randomized Single-Center Study. Int. J. Environ. Res. Public Health 2022, 19, 7108. https://doi.org/10.3390/ijerph19127108
Mościcka P, Cwajda-Białasik J, Szewczyk MT, Jawień A. Healing Process, Pain, and Health-Related Quality of Life in Patients with Venous Leg Ulcers Treated with Fish Collagen Gel: A 12-Week Randomized Single-Center Study. International Journal of Environmental Research and Public Health. 2022; 19(12):7108. https://doi.org/10.3390/ijerph19127108
Chicago/Turabian StyleMościcka, Paulina, Justyna Cwajda-Białasik, Maria Teresa Szewczyk, and Arkadiusz Jawień. 2022. "Healing Process, Pain, and Health-Related Quality of Life in Patients with Venous Leg Ulcers Treated with Fish Collagen Gel: A 12-Week Randomized Single-Center Study" International Journal of Environmental Research and Public Health 19, no. 12: 7108. https://doi.org/10.3390/ijerph19127108
APA StyleMościcka, P., Cwajda-Białasik, J., Szewczyk, M. T., & Jawień, A. (2022). Healing Process, Pain, and Health-Related Quality of Life in Patients with Venous Leg Ulcers Treated with Fish Collagen Gel: A 12-Week Randomized Single-Center Study. International Journal of Environmental Research and Public Health, 19(12), 7108. https://doi.org/10.3390/ijerph19127108





