New Insights into Microbial Degradation of Cyanobacterial Organic Matter Using a Fractionation Procedure
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection and Pretreatment
2.2. Batch Experiment
2.2.1. Long-Term Microbial Degradation of Algal Organic Matter
2.2.2. Process Analysis of Microbial Degradation of Algal Organic Matter Using the Fractionation Procedure
2.3. Sample Analysis
2.3.1. DOC and Chromophoric Dissolved Organic Matter (CDOM) Measurement
2.3.2. Nutrient Concentrations
2.3.3. Bacterial Abundance (BA)
2.4. Statistical Analysis
3. Results and Discussion
3.1. Algal Organic Matter Analysis
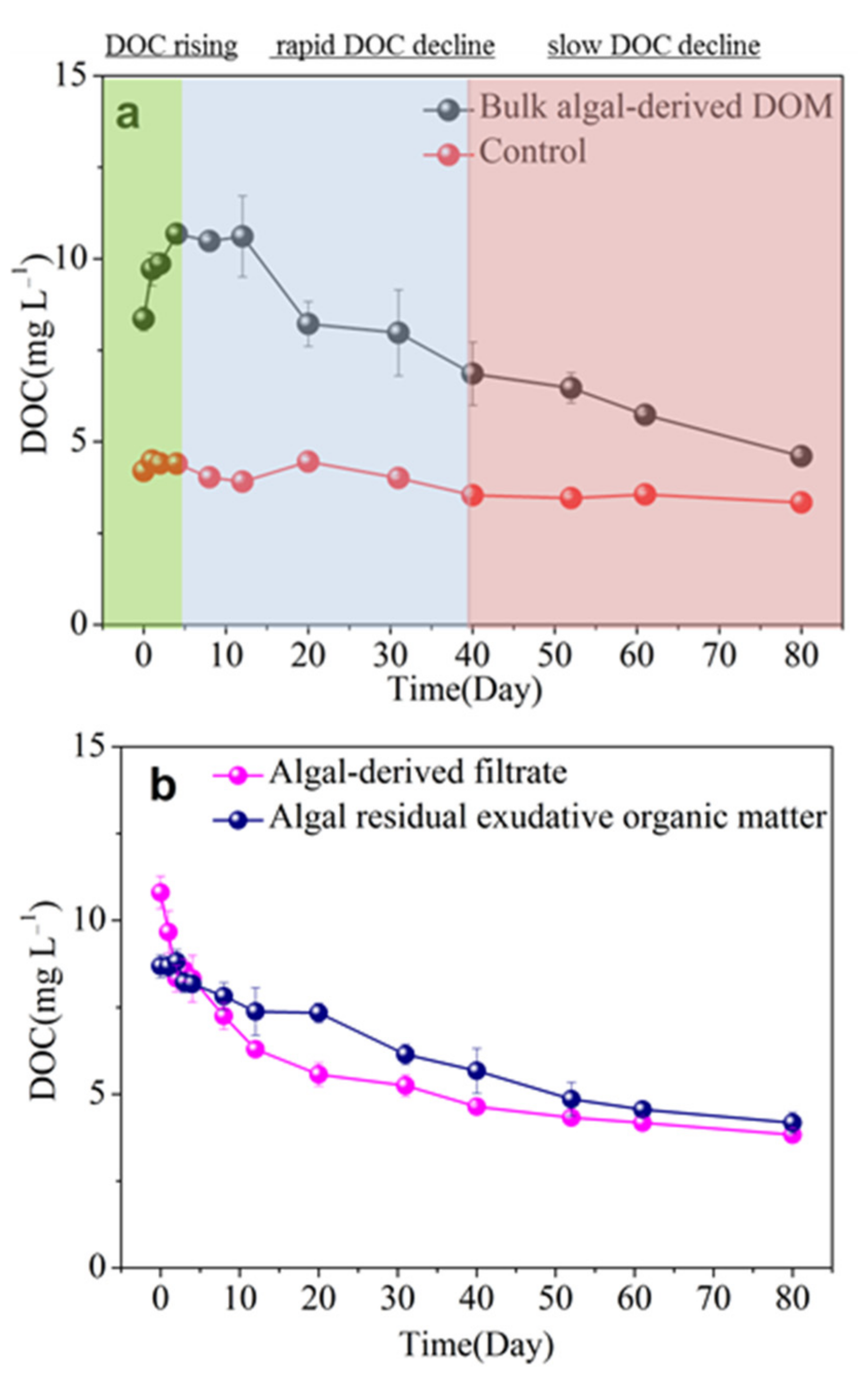
3.2. Release and Microbial Degradation of Algal-Derived DOC
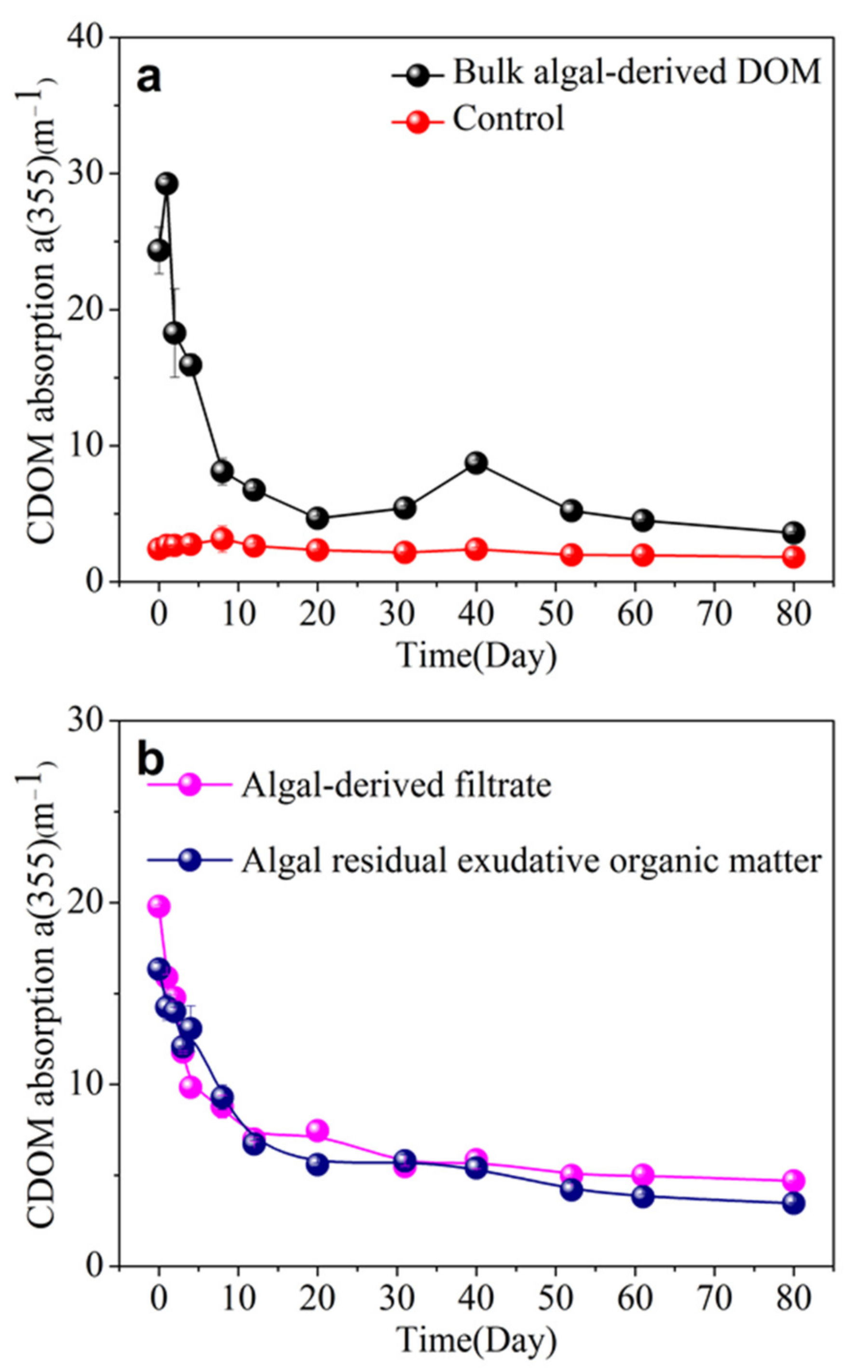
3.3. CDOM Absorption and Spectral Slope in Different Algal-Derived DOM
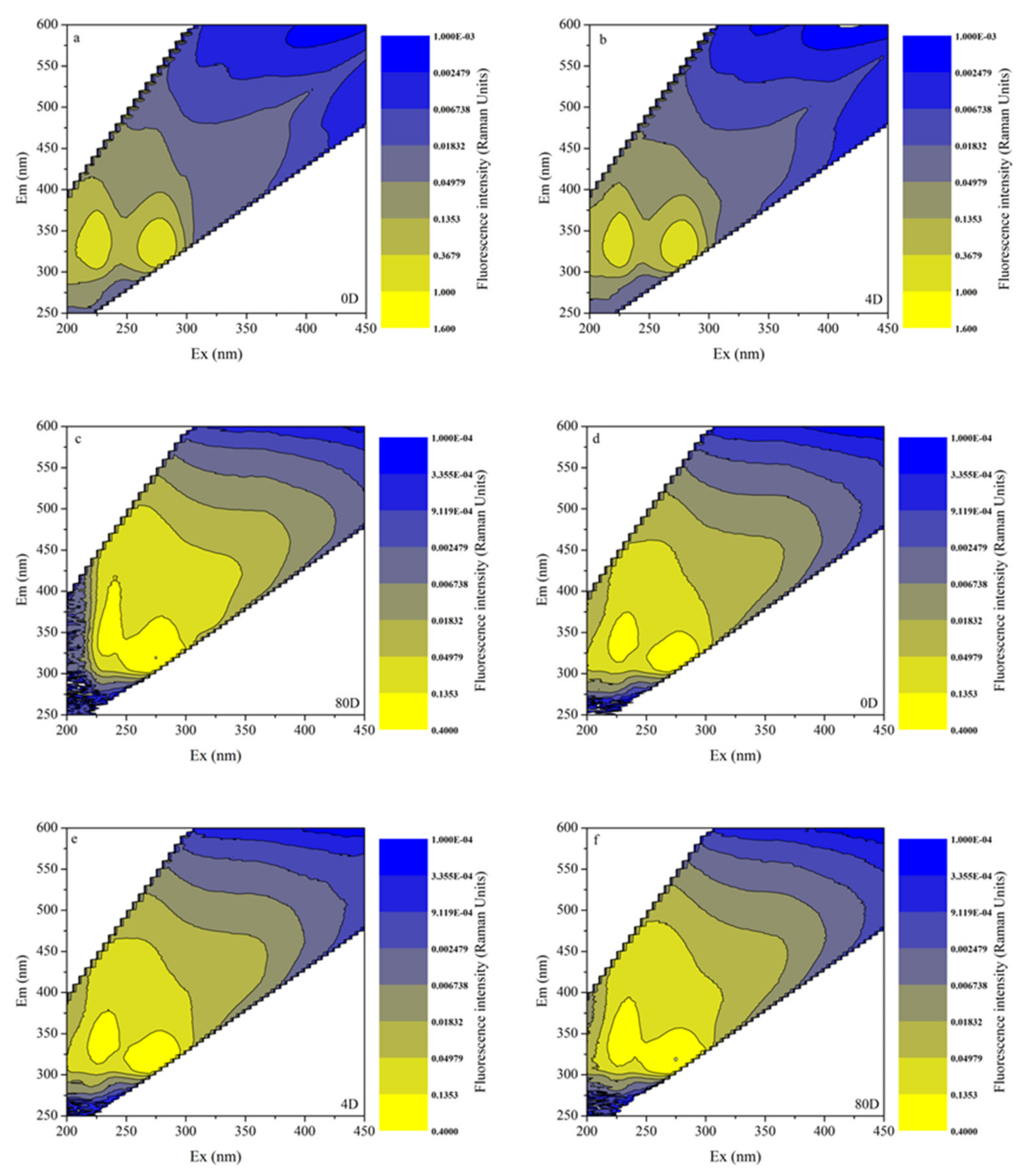
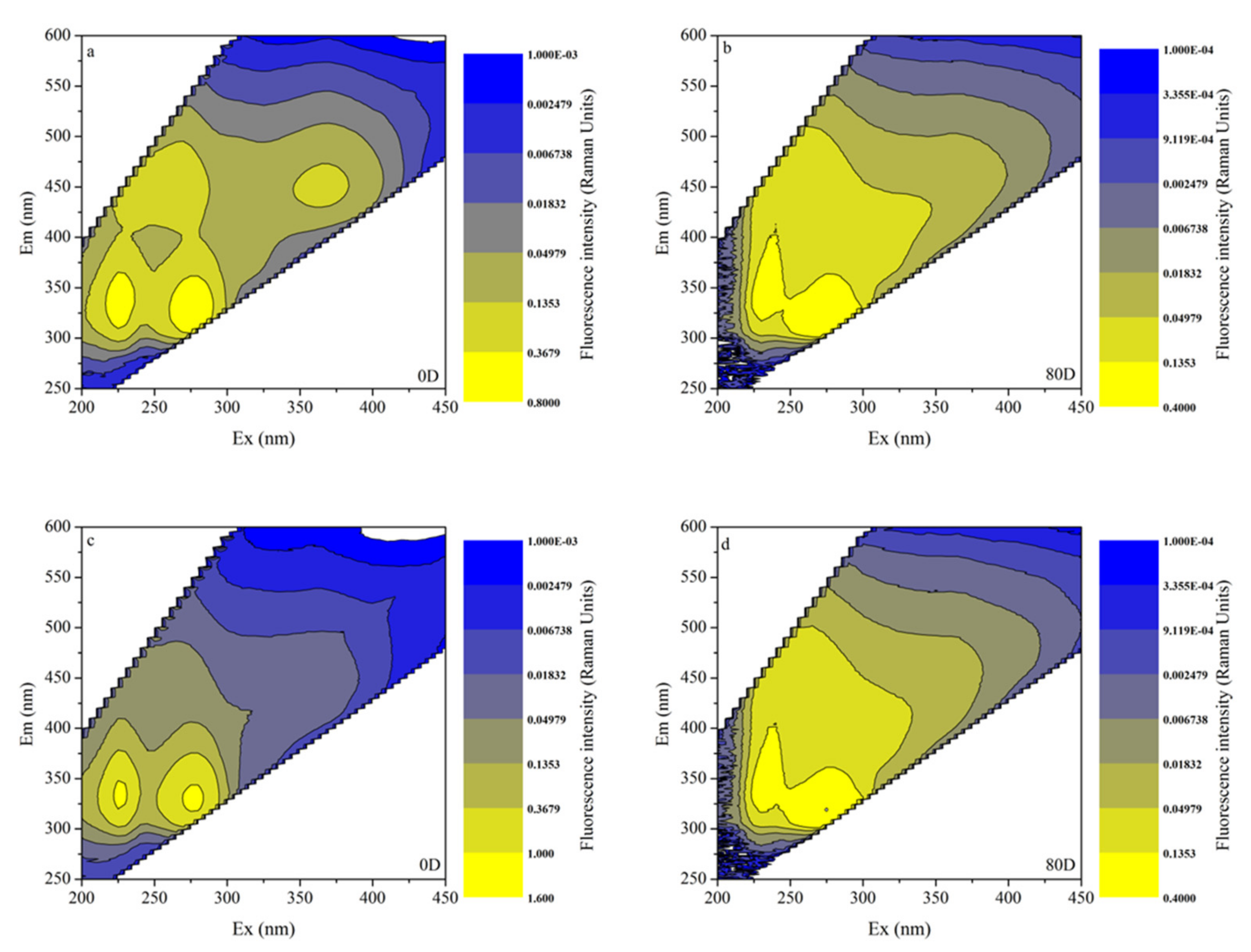
3.4. EEMs in Different Algal-Derived DOM
3.5. FDOM Components in Different Algal-Derived DOM
3.6. Variability of Nutrient Compositions in Different Algal-Derived DOM
3.7. Variability of Free-Living Bacterial Abundance in Different Algal-Derived DOM
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xiao, M.; Chen, Z.C.; Zhang, Y.; Wen, Y.A.; Shang, L.H.; Zhong, J. The Optical Characterization and Distribution of Dissolved Organic Matter in Water Regimes of Qilian Mountains Watershed. Int. J. Environ. Res. Public Health 2022, 19, 59. [Google Scholar] [CrossRef]
- Zhang, Y.L.; Deng, J.M.; Qin, B.Q.; Zhu, G.W.; Zhang, Y.J.; Jeppesen, E.; Tong, Y.D. Importance and vulnerability of lakes and reservoirs supporting drinking water in China. Fundam. Res. 2022, in press. [Google Scholar] [CrossRef]
- Yao, X.L.; Zhang, Y.L.; Zhang, L.; Zhu, G.W.; Qin, B.Q.; Zhou, Y.Q.; Xue, J.Y. Emerging role of dissolved organic nitrogen in supporting algal bloom persistence in Lake Taihu, China: Emphasis on internal transformations. Sci. Total Environ. 2020, 736, 139497. [Google Scholar] [CrossRef]
- Deng, J.M.; Qin, B.Q.; Paerl, H.W.; Zhang, Y.L.; Ma, J.R.; Chen, Y.W. Earlier and warmer springs increase cyanobacterial (Microcystis spp.) blooms in subtropical Lake Taihu, China. Freshw. Biol. 2014, 59, 1076–1085. [Google Scholar] [CrossRef]
- Hu, W.P. A review of the models for Lake Taihu and their application in lake environmental management. Ecol. Modell. 2016, 319, 9–20. [Google Scholar] [CrossRef]
- Qin, B.Q.; Paerl, H.W.; Brookes, J.D.; Liu, J.G.; Jeppesen, E.; Zhu, G.W.; Zhang, Y.L.; Xu, H.; Shi, K.; Deng, J.M. Why lake Taihu continues to be plagued with cyanobacterial blooms through 10 years (2007–2017) efforts. Sci. Bull. 2019, 64, 354–356. [Google Scholar] [CrossRef]
- Qin, B.Q.; Zhu, G.W.; Gao, G.; Zhang, Y.L.; Li, W.; Paerl, H.W.; Carmichael, W.W. A drinking water crisis in Lake Taihu, China: Linkage to climatic variability and lake management. Environ. Manage 2010, 45, 105–112. [Google Scholar] [CrossRef]
- Qin, B.Q.; Li, W.; Zhu, G.W.; Zhang, Y.L.; Wu, T.F.; Gao, G. Cyanobacterial bloom management through integrated monitoring and forecasting in large shallow eutrophic Lake Taihu (China). J. Hazard. Mater. 2015, 287, 356–363. [Google Scholar] [CrossRef]
- Jalil, A.; Li, Y.P.; Zhang, K.; Gao, X.M.; Wang, W.C.; Khan, H.O.S.; Pan, B.Z.; Ali, S.; Acharya, K. Wind-induced hydrodynamic changes impact on sediment resuspension for large, shallow Lake Taihu, China. Int. J. Sediment. Res. 2019, 34, 205–215. [Google Scholar] [CrossRef]
- Chen, H.M.; Zhu, Y.Y.; Zhang, Y.; Chen, X.Q.; Wang, R.C.; Zhu, W. Cyanobacterial bloom expansion caused by typhoon disturbance in Lake Taihu China. Environ. Sci. Pollut. Res. 2020, 27, 42294–42303. [Google Scholar] [CrossRef]
- Dai, J.Y.; Chen, D.; Wu, S.Q.; Wu, X.F.; Gao, G.; Tang, X.M.; Shao, K.Q.; Lv, X.Y.; Xue, W.Y.; Yang, Q.Q.; et al. Dynamics of phosphorus and bacterial phoX genes during the decomposition of Microcystis blooms in a mesocosm. PLoS ONE 2018, 13, e0195205. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.C.; Li, G.W.; Li, G.B.; Li, D.H. The decline process and major pathways of Microcystis bloom in Taihu Lake, China. Chin. J. Oceanol. Limn. 2012, 30, 37–46. [Google Scholar] [CrossRef]
- Daft, M.J.; Mccord, S.B.; Stewart, W.D.P. Ecological studies on algal-lysing bacteria in fresh waters. Freshw. Biol. 1975, 5, 577–596. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Niizuma, S.; Kuroda, N.; Sakamoto, M. Occurrence of heterotrophic bacteria causing lysis of cyanobacteria in a eutrophic lake. Jpn. J. Appl. Phys. 1993, 41, 46–48. [Google Scholar] [CrossRef]
- Zhou, Y.Q.; Jeppesen, E.; Zhang, Y.L.; Niu, C.; Shi, K.; Liu, X.H.; Zhu, G.W.; Qin, B.Q. Chromophoric dissolved organic matter of black waters in a highly eutrophic Chinese lake: Freshly produced from algal scums? J. Hazard. Mater. 2015, 299, 222–230. [Google Scholar] [CrossRef]
- Kuznetsov, I.; Neumann, T.; Burchard, H. Model study on the ecosystem impact of a variable C:N:P ratio for cyanobacteria in the Baltic Proper. Ecol. Modell. 2008, 219, 107–114. [Google Scholar] [CrossRef]
- Diehnelt, C.W.; Peterman, S.M.; Budde, W.L. Liquid chromatography–tandem mass spectrometry and accurate m/z measurements of cyclic peptide cyanobacteria toxins. Trends Analyt. Chem. 2005, 24, 622–634. [Google Scholar] [CrossRef]
- Merel, S.; Walker, D.; Chicana, R.; Snyder, S.; Baurès, E.; Thomas, O. State of knowledge and concerns on cyanobacterial blooms and cyanotoxins. Environ. Int. 2013, 59, 303–327. [Google Scholar] [CrossRef]
- Zurawell, R.W.; Chen, H.; Burke, J.M.; Prepas, E.E. Hepatotoxic cyanobacteria: A review of the biological importance of microcystins in freshwater environments. J. Toxicol. Environ. Health Part. B 2005, 8, 1–37. [Google Scholar] [CrossRef]
- Zhou, J.; Lao, Y.-M.; Cai, Z.-H. Draft Genome Sequence of Providencia sneebia Strain ST1, a Quorum Sensing Bacterium Associated with Marine Microalgae. J. Genom. 2016, 4, 10–12. [Google Scholar] [CrossRef][Green Version]
- Leblanc, C.; Colin, C.; Cosse, A.; Delage, L.; Barre, S.L.; Morin, P.; Fiévet, B.; Voiseux, C.; Ambroise, Y.; Verhaeghe, E.; et al. Iodine transfers in the coastal marine environment: The key role of brown algae and of their vanadium-dependent haloperoxidases. Biochimie 2006, 88, 1773–1785. [Google Scholar] [CrossRef]
- Aliyev, S.A.; Sari, A. Biogeochemical properties of bituminous deposits in the Miocene Himmetoǧlu basin (Turkey). Geochem. Int. 2011, 49, 170–180. [Google Scholar] [CrossRef]
- Bittar, T.B.; Vieira, A.A.H.; Stubbins, A.; Mopper, K. Competition between photochemical and biological degradation of dissolved organic matter from the cyanobacteria Microcystis aeruginosa. Limnol. Oceanogr. 2015, 60, 1172–1194. [Google Scholar] [CrossRef]
- Lee, Y.; Lee, B.; Hur, J.; Min, J.-O.; Ha, S.-Y.; Ra, K.; Kim, K.-T.; Shin, K.-H. Biodegradability of algal-derived organic matter in a large artificial lake by using stable isotope tracers. Environ. Sci. Pollut. Res. 2016, 23, 8358–8366. [Google Scholar] [CrossRef]
- McIntyre, A.M.; Guéguen, C. Binding interactions of algal-derived dissolved organic matter with metal ions. Chemosphere 2013, 90, 620–626. [Google Scholar] [CrossRef] [PubMed]
- Li, L.L.; Yu, T.B.; Cheng, S.Z.; Li, J.; Li, C.X.; Wang, G.H.; Tan, D.Y.; Li, L.; Zhang, H.Y.; Zhang, X.Z. Removal of cyanobacteria using novel pre-pressurized coagulation: The effect of cellular properties and algogenic organic matter characteristics. Sep. Purif. Technol. 2022, 282, 119927. [Google Scholar] [CrossRef]
- Richardson, S.; Plewa, M.; Wagner, E.; Schoeny, R.; Demarini, D. Occurrence, genotoxicity, and carcinogenicity disinfection by products in drinking water: A review and roadmap for research. Mutat. Res. 2007, 636, 178–242. [Google Scholar] [CrossRef]
- Herzsprung, P.; Tümpling, W.; Hertkorn, N.; Harir, M.; Büttner, O.; Bravidor, J.; Friese, K.; Schmitt-Kopplin, P. Variations of DOM quality in inflows of a drinking water reservoir: Linking of van Krevelen diagrams with EEMF spectra by rank correlation. Environ. Sci. Technol. 2012, 46, 5511–5518. [Google Scholar] [CrossRef]
- Jiao, N.Z.; Cai, R.H.; Zheng, Q.; Tang, K.; Liu, J.H.; Jiao, F.L.; Wallace, D.; Chen, F.; Li, C.; Amann, R.; et al. Unveiling the enigma of refractory carbon in the ocean. Natl. Sci. Rev. 2018, 5, 459–463. [Google Scholar] [CrossRef]
- Garcia, A.C.; Bargu, S.; Dash, P.; Rabalais, N.N.; Sutor, M.; Morrison, W.; Walker, N.D. Evaluating the potential risk of microcystins to blue crab (Callinectes sapidus) fisheries and human health in a eutrophic estuary. Harmful Algae 2010, 9, 134–143. [Google Scholar] [CrossRef]
- Loftin, K.A.; Graham, J.L.; Hilborn, E.D.; Lehmann, S.C.; Meyer, M.T.; Dietze, J.E.; Griffith, C.B. Cyanotoxins in inland lakes of the United States: Occurrence and potential recreational health risks in the EPA National Lakes Assessment 2007. Harmful Algae 2016, 56, 77–90. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Zhou, Y.Q.; Tang, X.M.; Zhang, Y.L.; Jang, K.-S.; Székely, A.J.; Jeppesen, E. Resource aromaticity affects bacterial community successions in response to different sources of dissolved organic matter. Water Res. 2021, 190, 116776. [Google Scholar] [CrossRef] [PubMed]
- Kamiyama, T.; Itakura, S.; Nagasaki, K. Changes in microbial loop components: Effects of a harmful algal bloom formation and its decay. Aquat. Microb. Ecol. 2000, 21, 21–30. [Google Scholar] [CrossRef]
- Zhao, Z.; Gonsior, M.; Schmitt-Kopplin, P.; Zhan, Y.C.; Zhang, R.; Jiao, N.Z.; Chen, F. Microbial transformation of virus-induced dissolved organic matter from picocyanobacteria: Coupling of bacterial diversity and DOM chemodiversity. ISME J. 2019, 13, 2551–2565. [Google Scholar] [CrossRef]
- Kieft, B.; Li, Z.; Bryson, S.; Hettich, R.L.; Pan, C.; Mayali, X.; Mueller, R.S. Phytoplankton exudates and lysates support distinct microbial consortia with specialized metabolic and ecophysiological traits. Proc. Natl. Acad. Sci. USA 2021, 118, e2101178118. [Google Scholar] [CrossRef] [PubMed]
- Bertilsson, S.; Eiler, A.; Nordqvist, A.; Jørgensen, N.O.G. Links between bacterial production, amino-acid utilization and community composition in productive lakes. ISME J. 2007, 1, 532–544. [Google Scholar] [CrossRef] [PubMed]
- Guillemette, F.; McCallister, S.L.; Giorgio, P.A. Selective consumption and metabolic allocation of terrestrial and algal carbon determine allochthony in lake bacteria. ISME J. 2016, 10, 1373–1382. [Google Scholar] [CrossRef]
- Zhang, L.; Liao, Q.J.H.; Gao, R.; Luo, R.; Liu, C.; Zhong, J.C.; Wang, Z.D. Spatial variations in diffusive methane fluxes and the role of eutrophication in a subtropical shallow lake. Sci. Total Environ. 2021, 759, 143459. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, C.; He, K.; Shen, Q.S.; Zhong, J.C. Dramatic temporal variations in methane levels in black bloom prone areas of a shallow eutrophic lake. Sci. Total Environ. 2021, 767, 144868. [Google Scholar] [CrossRef]
- Tong, Y.D.; Xu, X.W.; Miao, Q.; Sun, J.J.; Zhang, Y.Y.; Zhang, W.; Wang, M.Z.; Wang, X.J.; Zhang, Y. Lake warming intensifies the seasonal pattern of internal nutrient cycling in the eutrophic lake and potential impacts on algal blooms. Water Res. 2021, 188, 116570. [Google Scholar] [CrossRef]
- Ai, Y.; Bi, Y.H.; Hu, Z.Y. Changes in phytoplankton communities along nutrient gradients in Lake Taihu: Evidence for nutrient reduction strategies. Chin. J. Oceanol. Limn. 2015, 33, 447–457. [Google Scholar] [CrossRef]
- Zhang, P.; Zhai, C.M.; Chen, R.Q.; Liu, C.H.; Xue, Y.R.; Jiang, J.H. The dynamics of the water bloom-forming Microcystis aeruginosa and its relationship with biotic and abiotic factors in Lake Taihu, China. Ecol. Eng. 2012, 47, 274–277. [Google Scholar] [CrossRef]
- Cruaud, P.; Vigneron, A.; Fradette, M.-S.; Dorea, C.C.; Culley, A.I.; Rodriguez, M.J.; Charette, S.J. Annual bacterial community cycle in a seasonally ice-covered river reflects environmental and climatic conditions. Limnol. Oceanogr. 2020, 65, S21–S37. [Google Scholar] [CrossRef]
- Chen, Y.W.; Fan, C.X.; Teubner, K.; Dokulil, M. Changes of nutrients and phytoplankton chlorophyll-a in a large shallow lake, Taihu, China: An 8-year investigation. Hydrobiologia 2003, 506, 273–279. [Google Scholar] [CrossRef]
- Zhou, S.Q.; Zhu., S.M.; Shao., Y.S.; Gao., N.Y. Characteristics of C-, N-DBPs formation from algal organic matter: Role of molecular weight fractions and impacts of pre-ozonation. Water Res. 2015, 72, 381–390. [Google Scholar] [CrossRef]
- Pivokonsky, M.; Kloucek, O.; Pivokonska, L. Evaluation of the production, composition and aluminum and iron complexation of algogenic organic matter. Water Res. 2006, 40, 3045–3052. [Google Scholar] [CrossRef]
- Zhang, Y.L.; Dijk, M.A.; Liu, M.L.; Zhu, G.W.; Qin, B.Q. The contribution of phytoplankton degradation to chromophoric dissolved organic matter (CDOM) in eutrophic shallow lakes: Field and experimental evidence. Water Res. 2009, 43, 4685–4697. [Google Scholar] [CrossRef]
- Coble, P.G. Characterization of marine and terrestrial DOM in seawater using excitation-emission matrix spectroscopy. Mar. Chem. 1996, 51, 325–346. [Google Scholar] [CrossRef]
- Coble, P.G.; Castillo, C.E.D.; Avril, B. Distribution and optical properties of CDOM in the Arabian Sea during the 1995 Southwest Monsoon. Deep Sea Res. Part II Top. Stud. Oceanogr. 1998, 45, 2195–2223. [Google Scholar] [CrossRef]
- Zhou, Y.Q.; Zhou, L.; Zhang, Y.L.; Zhu, G.W.; Qin, B.Q.; Jang, K.-S.; Spencer, R.G.M.; Kothawala, D.N.; Jeppesen, E.; Brookes, J.D.; et al. Unraveling the Role of Anthropogenic and Natural Drivers in Shaping the Molecular Composition and Biolability of Dissolved Organic Matter in Non-pristine Lakes. Environ. Sci. Technol. 2022, 56, 4655–4664. [Google Scholar] [CrossRef]
- Zhang, Y.L.; Qin, B.Q. Variations in Spectral Slope in Lake Taihu, a Large Subtropical Shallow Lake in China. J. Great Lakes Res. 2007, 33, 483–496. [Google Scholar] [CrossRef]
- Ebina, J.; Tsutsui, T.; Shirai, T. Simultaneous determination of total nitrogen and total phosphorus in water using peroxodisulfate oxidation. Water Res. 1983, 17, 1721–1726. [Google Scholar] [CrossRef]
- Gong, Y.; Tang, X.M.; Shao, K.Q.; Hu, Y.; Gao, G. Dynamics of bacterial abundance and the related environmental factors in large shallow eutrophic Lake Taihu. J. Freshw. Ecol. 2016, 32, 1248506. [Google Scholar] [CrossRef]
- Chen, S.N.; Yan, M.M.; Huang, T.L.; Zhang, H.; Liu, K.W.; Huang, X.; Li, N.; Miao, Y.T.; Sekar, R. Disentangling the drivers of Microcystis decomposition: Metabolic profile and co-occurrence of bacterial community. Sci. Total Environ. 2020, 739, 140062. [Google Scholar] [CrossRef] [PubMed]
- Xie, R.; Wang, Y.; Chen, Q.; Guo, W.D.; Jiao, N.Z.; Zheng, Q. Coupling Between Carbon and Nitrogen Metabolic Processes Mediated by Coastal Microbes in synechococcus-derived organic matter addition incubations. Front. Microbiol. 2020, 11, 1041. [Google Scholar] [CrossRef]
- Lin, H.; Xu, H.C.; Cai, Y.H.; Belzile, C.; Macdonald, R.W.; Guo, L.D. Dynamic changes in size-fractionated dissolved organic matter composition in a seasonally ice-covered Arctic River. Limnol. Oceanogr. 2021, 66, 3085–3099. [Google Scholar] [CrossRef]
- Tanaka, K.; Kuma, K.; Hamasaki, K.; Yamashita, Y. Accumulation of humic-like fluorescent dissolved organic matter in the Japan Sea. Sci. Rep. 2014, 4, 5292. [Google Scholar] [CrossRef]
- Zuo, Y.-T.; Wu, J.; Cheng, S.; Cai, M.-H.; Han, Y.-Z.; Ji, W.-X.; Li, Y.; Huo, Z.-L.; Korshin, G.; Li, W.-T.; et al. Identification of pterins as characteristic humic-like fluorophores released from cyanobacteria and their behavior and fate in natural and engineered water systems. Chem. Eng. J. 2022, 428, 131154. [Google Scholar] [CrossRef]
- Song, W.J.; Zhao, C.X.; Zhang, D.Y.; Mu, S.Y.; Pan, X.L. Different Resistance to UV-B Radiation of Extracellular Polymeric Substances of Two Cyanobacteria from Contrasting Habitats. Front. Microbiol. 2016, 7, 1208. [Google Scholar] [CrossRef] [PubMed]
- Omori, Y.; Hama, T.; Ishii, M.; Saito, S. Vertical change in the composition of marine humic-like fluorescent dissolved organic matter in the subtropical western North Pacific and its relation to photoreactivity. Mar. Chem. 2011, 124, 38–47. [Google Scholar] [CrossRef]
- Bianchi, T.S. The role of terrestrially derived organic carbon in the coastal ocean: A changing paradigm and the priming effect. Proc. Natl. Acad. Sci. USA 2011, 108, 19473–19481. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, T.S.; Thornton, D.C.O.; Yvon-Lewis, S.A.; King, G.M.; Eglinton, T.I.; Shields, M.R.; Ward, N.D.; Curtis, J. Positive priming of terrestrially derived dissolved organic matter in a freshwater microcosm system. Geophys. Res. Lett. 2015, 42, 5460–5467. [Google Scholar] [CrossRef]
- Sebastian, M.; Auguet, J.-C.; Restrepo-Ortiz, C.X.; Sala, M.M.; Marrase, C.; Gasol, J.M. Deep ocean prokaryotic communities are remarkably malleable when facing long-term starvation. Environ. Microbiol. 2018, 20, 713–723. [Google Scholar] [CrossRef]
- Zhang, Y.L.; Zhou, Y.Q.; Shi, K.; Qin, B.Q.; Yao, X.L.; Zhang, Y.B. Optical properties and composition changes in chromophoric dissolved organic matter along trophic gradients: Implications for monitoring and assessing lake eutrophication. Water Res. 2018, 131, 255–263. [Google Scholar] [CrossRef]
- Li, W.; Qin, B.Q.; Zhu, G.W. Forecasting short-term cyanobacterial blooms in Lake Taihu, China, using a coupled hydrodynamic–algal biomass model. Ecohydrology 2014, 7, 794–802. [Google Scholar] [CrossRef]
- Alderkamp, A.-C.; Buma, A.G.J.; Rijssel, M. The carbohydrates of Phaeocystis and their degradation in the microbial food web. Biogeochemistry 2007, 83, 99–118. [Google Scholar] [CrossRef]
- Rix, L.; Goeij, J.M.; Oevelen, D.; Struck, U.; Al-Horani, F.A.; Wild, C.; Naumann, M.S. Differential recycling of coral and algal dissolved organic matter via the sponge loop. Funct. Ecol. 2017, 31, 778–789. [Google Scholar] [CrossRef]
- Zhang, Z.H.; Tang, L.L.; Liang, Y.T.; Li, G.; Li, H.M.; Rivkin, R.B.; Jiao, N.Z.; Zhang, Y.Y. The relationship between two Synechococcus strains and heterotrophic bacterial communities and its associated carbon flow. J. Appl. Phycol. 2021, 33, 953–966. [Google Scholar] [CrossRef]
- Gao, X.Q.; Heath, R.T. Relationship Between Labile Dissolved Organic Carbon (LDOC), Bacterioplankton Cell Phosphorus Quota, and Bacterial Phosphate Uptake Rate in Lakes. J. Great Lakes Res. 2005, 31 (Suppl. S2), 125–137. [Google Scholar] [CrossRef]
- Barber, C.B.; Dobkin, D.P.; Huhdanpaa, H.T. The quick hull algorithm for convex hulls. ACM T Math. Software 1996, 22, 469–483. [Google Scholar] [CrossRef]
- Zhang, Y.L.; Zhang, E.L.; Yin, Y.; van Dijk, M.A.; Feng, L.Q.; Shi, Z.Q.; Liu, M.L.; Qin, B.Q. Characteristics and sources of chromophoric dissolved organic matter in lakes of the Yungui Plateau, China, differing in trophic state and altitude. Limnol. Oceanogr. 2010, 55, 2645–2659. [Google Scholar] [CrossRef]
- Zhang, Y.L.; Liu, X.H.; Wang, M.Z.; Qin, B.Q. Compositional differences of chromophoric dissolved organic matter derived from phytoplankton and macrophytes. Org. Geochem. 2013, 55, 26–37. [Google Scholar] [CrossRef]

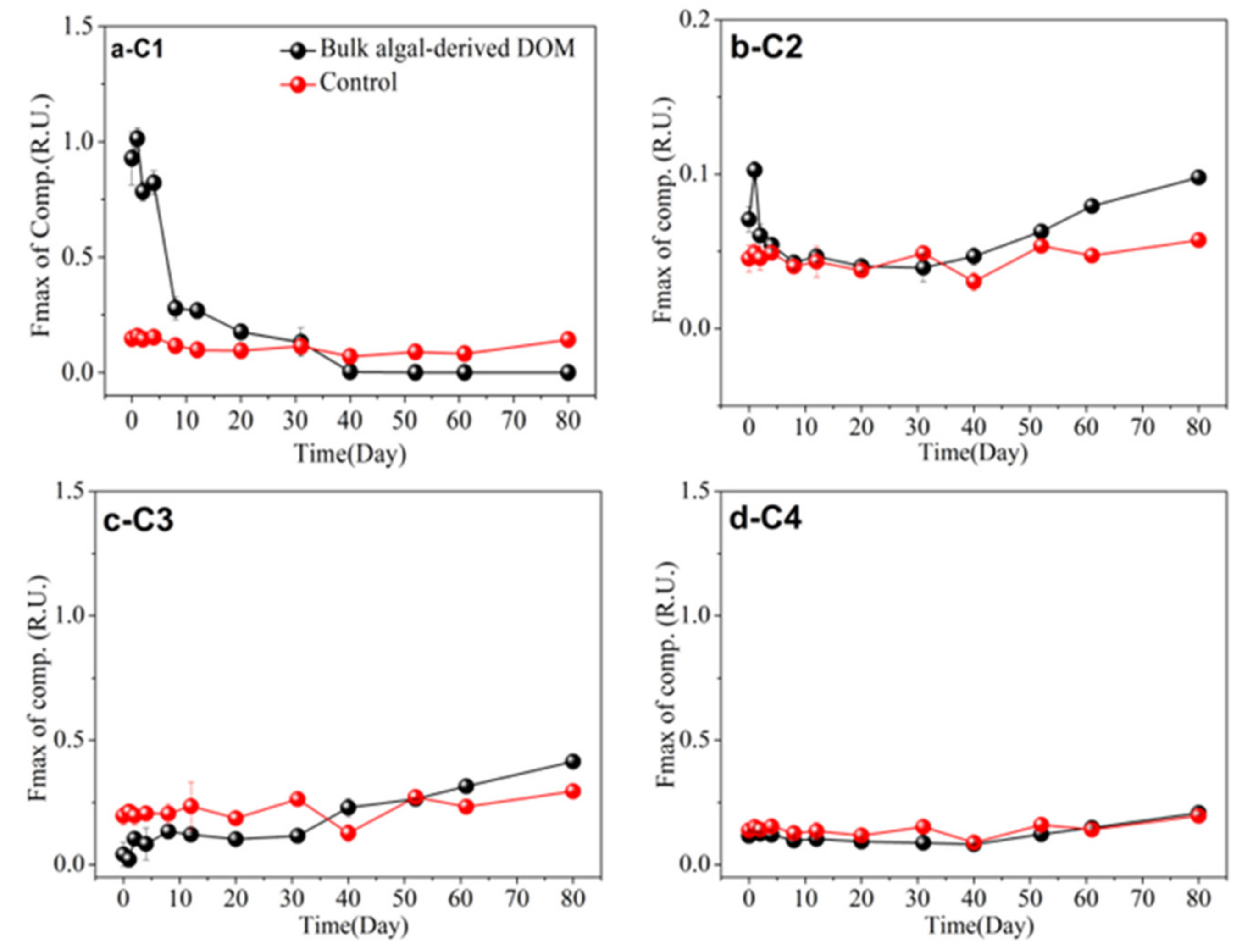

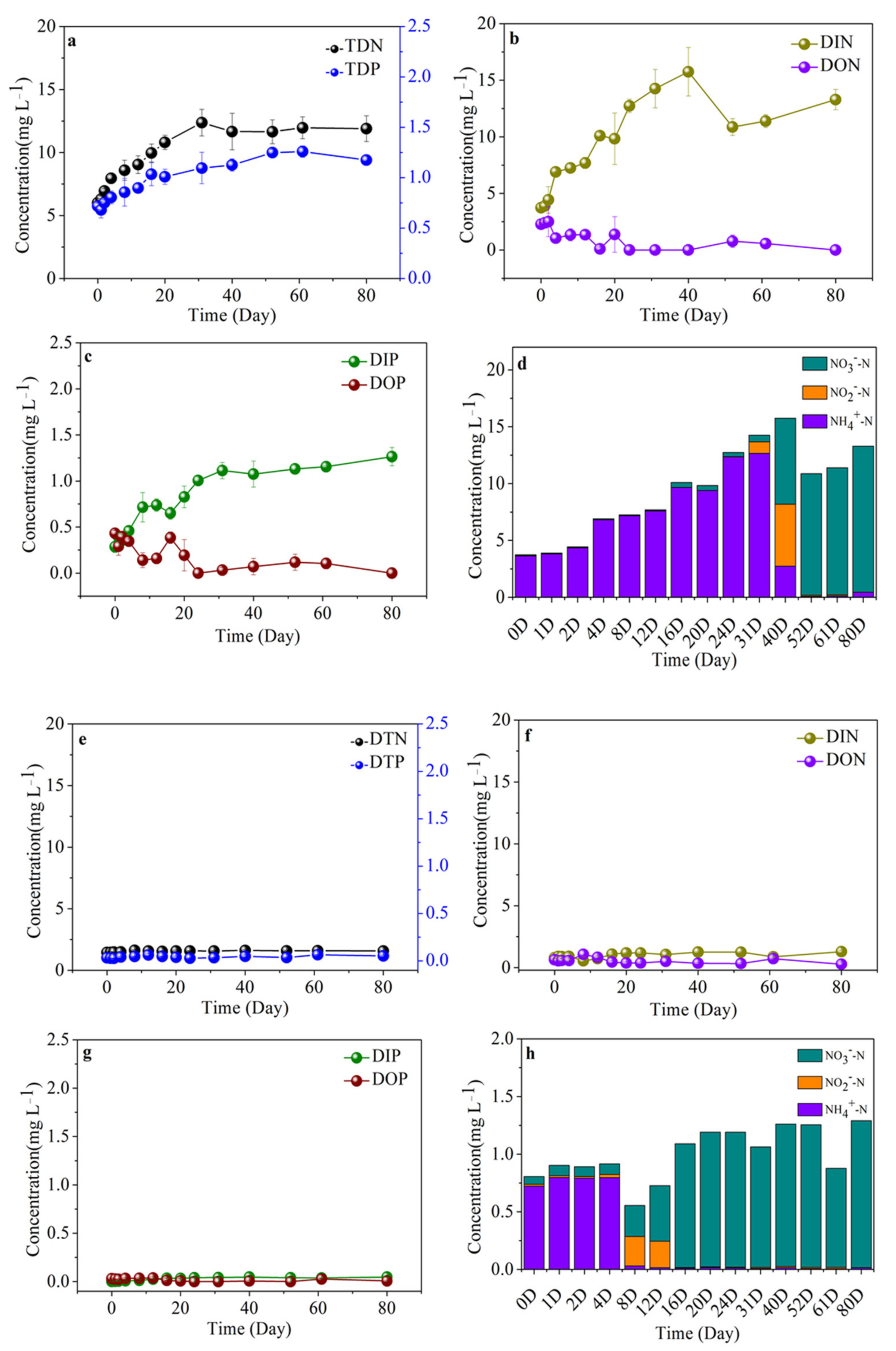

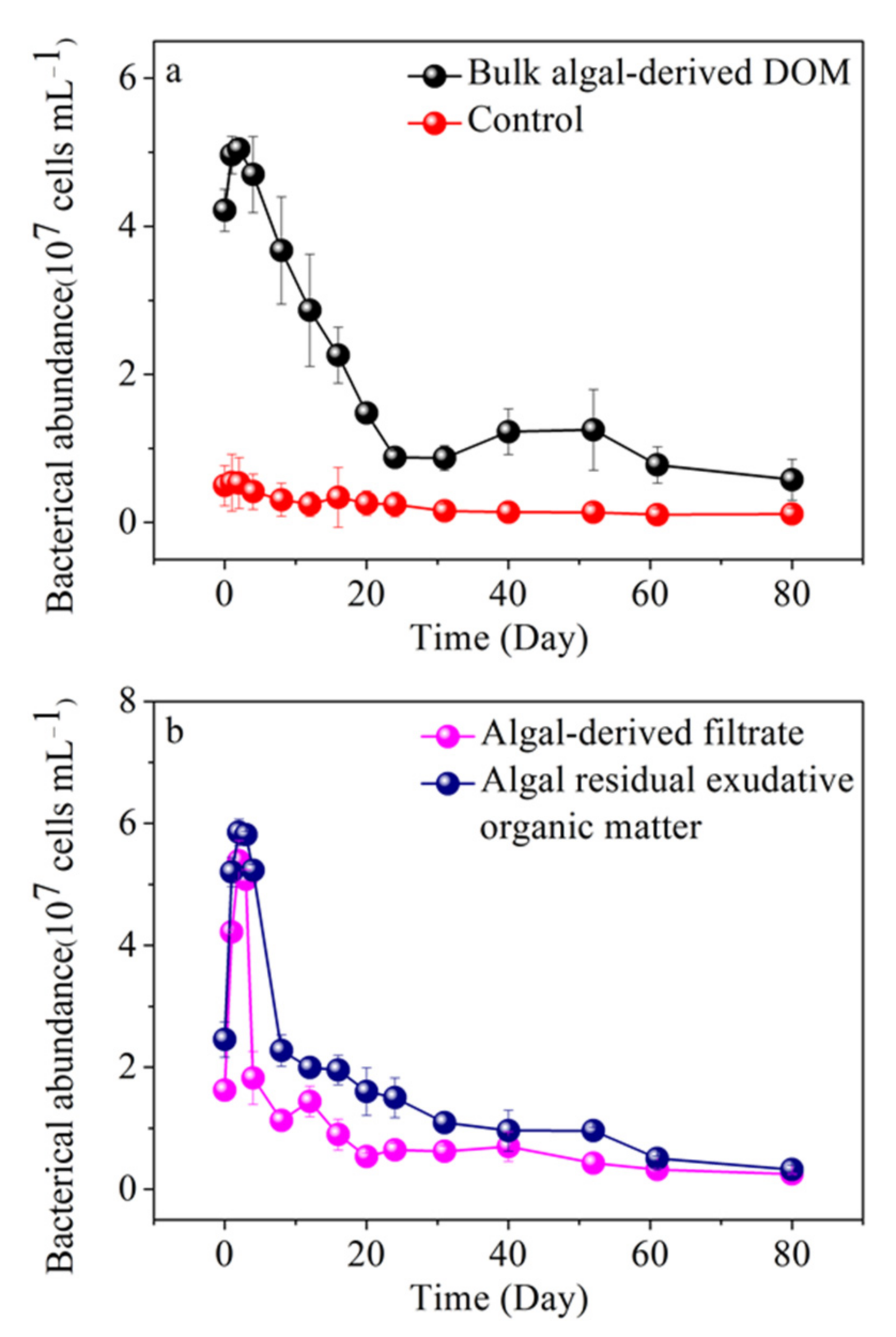
| Parameter | Concentration (Mean ± Deviation) | Parameter | Concentration (Mean ± Deviation) | |
|---|---|---|---|---|
| Bulk algal-derived DOM | DOC (mg L−1) | 4.15 ± 0.56 | C1 (R.U.) | 0.78 ± 0.13 |
| DON (mg L−1) | 1.63 ± 0.17 | C2 (R.U.) | 0.03 ± 0.01 | |
| DOP (mg L−1) | 0.40 ± 0.03 | C3 (R.U.) | ND | |
| a (355) (m−1) | 21.93 ± 1.80 | C4 (R.U.) | ND | |
| Algal-derived filtrate | DOC (mg L−1) | 6.61 ± 0.73 | C1 (R.U.) | 0.43 ± 0.04 |
| DON (mg L−1) | 1.66 ± 0.13 | C2 (R.U.) | 0.22 ± 0.02 | |
| DOP (mg L−1) | 0.22 ± 0.01 | C3 (R.U.) | 0.04 ± 0.04 | |
| a (355) (m−1) | 17.38 ± 0.16 | C4 (R.U.) | 0.03 ± 0.03 | |
| Algal residual exudative organic matter | DOC (mg L−1) | 4.48 ± 0.58 | C1 (R.U.) | 0.87 ± 0.03 |
| DON (mg L−1) | 3.00 ± 0.86 | C2 (R.U.) | 0.02 ± 0.03 | |
| DOP (mg L−1) | 0.20 ± 0.07 | C3 (R.U.) | 0.18 ± 0.10 | |
| a (355) (m−1) | 13.91 ± 0.29 | C3 (R.U.) | 0.01 ± 0.04 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, J.; Zhou, Y.; Zhang, Y. New Insights into Microbial Degradation of Cyanobacterial Organic Matter Using a Fractionation Procedure. Int. J. Environ. Res. Public Health 2022, 19, 6981. https://doi.org/10.3390/ijerph19126981
Chen J, Zhou Y, Zhang Y. New Insights into Microbial Degradation of Cyanobacterial Organic Matter Using a Fractionation Procedure. International Journal of Environmental Research and Public Health. 2022; 19(12):6981. https://doi.org/10.3390/ijerph19126981
Chicago/Turabian StyleChen, Jing, Yongqiang Zhou, and Yunlin Zhang. 2022. "New Insights into Microbial Degradation of Cyanobacterial Organic Matter Using a Fractionation Procedure" International Journal of Environmental Research and Public Health 19, no. 12: 6981. https://doi.org/10.3390/ijerph19126981
APA StyleChen, J., Zhou, Y., & Zhang, Y. (2022). New Insights into Microbial Degradation of Cyanobacterial Organic Matter Using a Fractionation Procedure. International Journal of Environmental Research and Public Health, 19(12), 6981. https://doi.org/10.3390/ijerph19126981







