Use of Physical Activity and Exercise to Reduce Inflammation in Children and Adolescents with Obesity
Abstract
:1. Introduction
2. Methods
3. Obesity in Pediatric Age
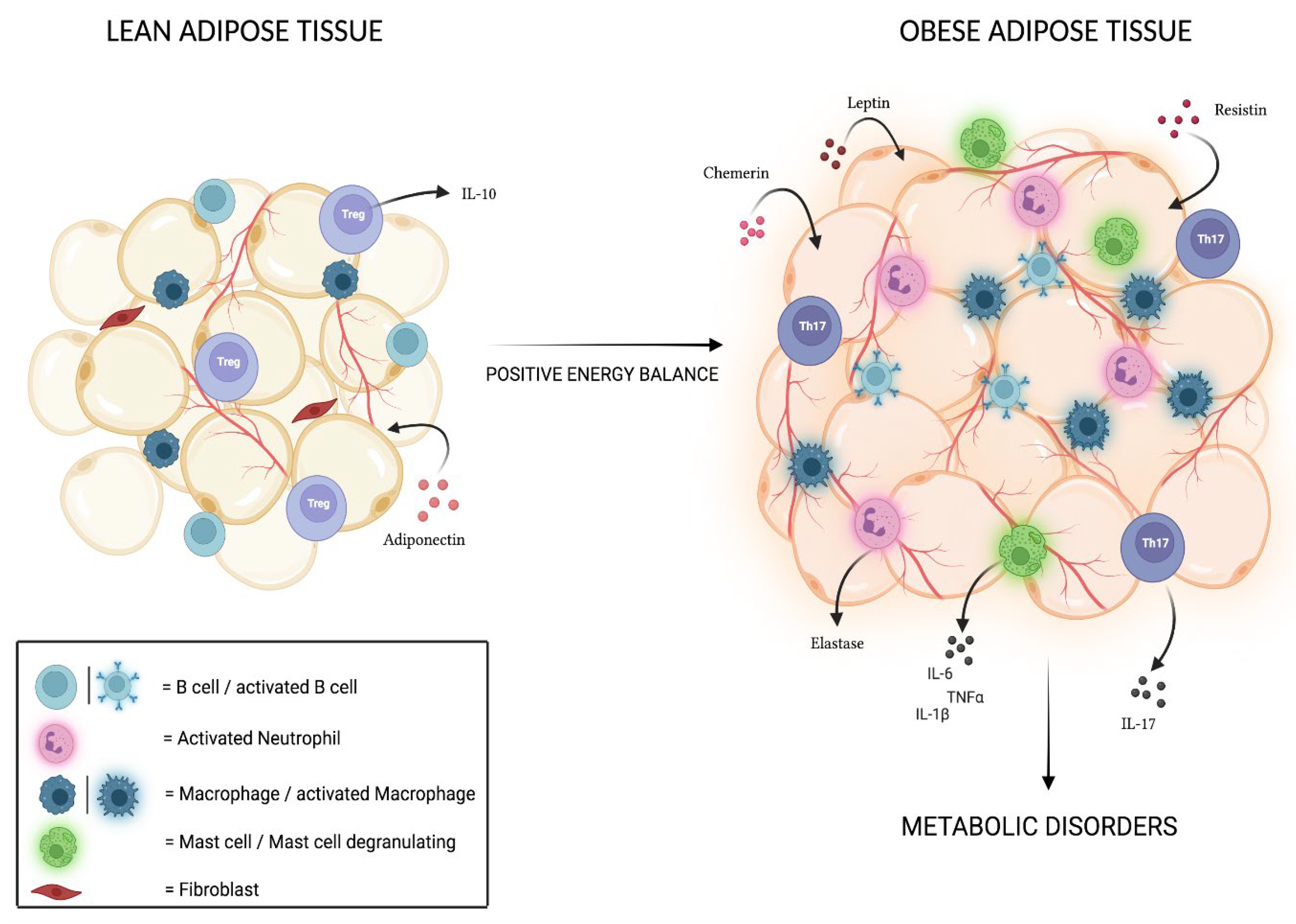
4. Adipose Tissue and Associated Inflammation
5. Physical Activity Guidelines for Children and Adolescents
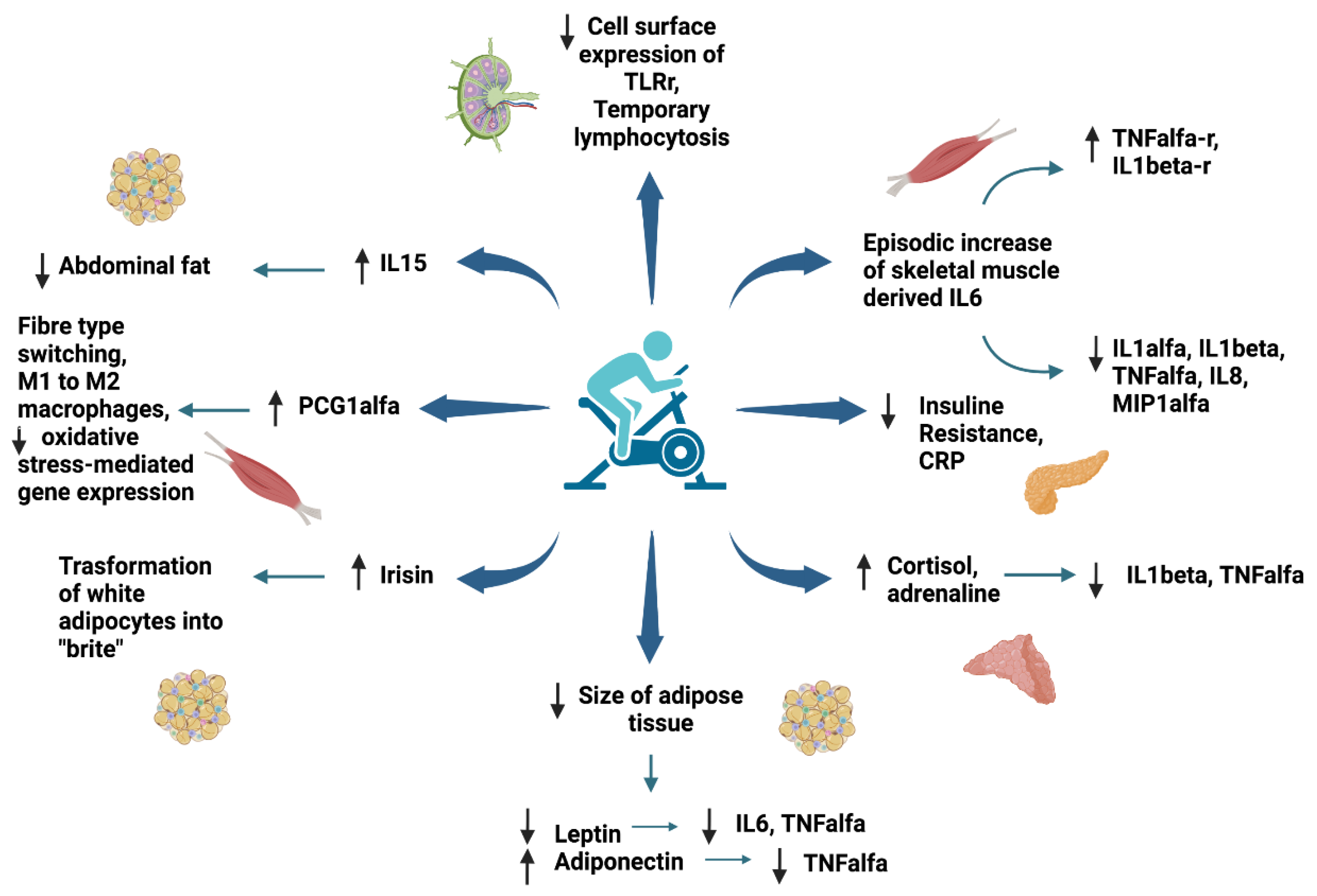
6. Anti-Inflammatory Effect of Exercise in Children and Adolescents with Obesity
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kumar, S.; Kelly, A.S. Review of Childhood Obesity: From Epidemiology, Etiology, and Comorbidities to Clinical Assessment and Treatment. Mayo Clin. Proc. 2017, 92, 251–265. [Google Scholar] [CrossRef] [Green Version]
- Ogden, C.L.; Carroll, M.D.; Kit, B.K.; Flegal, K.M. Prevalence of Childhood and Adult Obesity in the United States, 2011-2012. JAMA 2014, 311, 806–814. [Google Scholar] [CrossRef] [Green Version]
- Dietz, W.H.; Robinson, T.N. Overweight Children and Adolescents. N. Engl. J. Med. 2005, 352, 2100–2109. [Google Scholar] [CrossRef] [Green Version]
- Juonala, M.; Magnussen, C.G.; Berenson, G.S.; Venn, A.; Burns, T.L.; Sabin, M.A.; Srinivasan, S.R.; Daniels, S.R.; Davis, P.H.; Chen, W.; et al. Childhood Adiposity, Adult Adiposity, and Cardiovascular Risk Factors. N. Engl. J. Med. 2011, 365, 1876–1885. [Google Scholar] [CrossRef] [Green Version]
- Metsios, G.S.; Moe, R.H.; Kitas, G.D. Exercise and Inflammation. Best Pract. Res. Clin. Rheumatol. 2020, 34, 101504. [Google Scholar] [CrossRef]
- Calcaterra, V.; Zuccotti, G. Physical Exercise as a Non-Pharmacological Intervention for Attenuating Obesity-Related Complications in Children and Adolescents. Int. J. Environ. Res. Public Health 2022, 19, 5046. [Google Scholar] [CrossRef]
- Gleeson, M.; Bishop, N.C.; Stensel, D.J.; Lindley, M.R.; Mastana, S.S.; Nimmo, M.A. The Anti-Inflammatory Effects of Exercise: Mechanisms and Implications for the Prevention and Treatment of Disease. Nat. Rev. Immunol. 2011, 11, 607–615. [Google Scholar] [CrossRef]
- Gregory, A.T.; Denniss, A.R. An Introduction to Writing Narrative and Systematic Reviews—Tasks, Tips and Traps for Aspiring Authors. Heart Lung Circ. 2018, 27, 893–898. [Google Scholar] [CrossRef] [Green Version]
- Freedman, D.S.; Sherry, B. The Validity of BMI as an Indicator of Body Fatness and Risk Among Children. Pediatrics 2009, 124, S23–S34. [Google Scholar] [CrossRef] [Green Version]
- Javed, A.; Jumean, M.; Murad, M.H.; Okorodudu, D.; Kumar, S.; Somers, V.K.; Sochor, O.; Lopez-Jimenez, F. Diagnostic Performance of Body Mass Index to Identify Obesity as Defined by Body Adiposity in Children and Adolescents: A Systematic Review and Meta-Analysis: Diagnostic Performance of BMI to Identify Obesity. Pediatric Obes. 2015, 10, 234–244. [Google Scholar] [CrossRef]
- Lee, S.; Bacha, F.; Gungor, N.; Arslanian, S.A. Waist Circumference Is an Independent Predictor of Insulin Resistance in Black and White Youths. J. Pediatrics 2006, 148, 188–194. [Google Scholar] [CrossRef]
- Fernández, J.R.; Redden, D.T.; Pietrobelli, A.; Allison, D.B. Waist Circumference Percentiles in Nationally Representative Samples of African-American, European-American, and Mexican-American Children and Adolescents. J. Pediatrics 2004, 145, 439–444. [Google Scholar] [CrossRef]
- Moreno, L.; Rodríguez, G.; Guillén, J.; Rabanaque, M.; León, J.; Ariño, A. Anthropometric Measurements in Both Sides of the Body in the Assessment of Nutritional Status in Prepubertal Children. Eur. J. Clin. Nutr. 2002, 56, 1208–1215. [Google Scholar] [CrossRef] [Green Version]
- Flegal, K.M.; Wei, R.; Ogden, C.L.; Freedman, D.S.; Johnson, C.L.; Curtin, L.R. Characterizing Extreme Values of Body Mass Index–for-Age by Using the 2000 Centers for Disease Control and Prevention Growth Charts. Am. J. Clin. Nutr. 2009, 90, 1314–1320. [Google Scholar] [CrossRef]
- Gulati, A.K.; Kaplan, D.W.; Daniels, S.R. Clinical Tracking of Severely Obese Children: A New Growth Chart. Pediatrics 2012, 130, 1136–1140. [Google Scholar] [CrossRef] [Green Version]
- Skinner, A.C.; Skelton, J.A. Prevalence and Trends in Obesity and Severe Obesity Among Children in the United States, 1999-2012. JAMA Pediatr. 2014, 168, 561. [Google Scholar] [CrossRef] [Green Version]
- The GBD 2015 Obesity Collaborators Health Effects of Overweight and Obesity in 195 Countries over 25 Years. N. Engl. J. Med. 2017, 377, 13–27. [CrossRef]
- Gregg, E.W.; Shaw, J.E. Global Health Effects of Overweight and Obesity. N. Engl. J. Med. 2017, 377, 80–81. [Google Scholar] [CrossRef]
- Ng, M.; Fleming, T.; Robinson, M.; Thomson, B.; Graetz, N.; Margono, C.; Mullany, E.C.; Biryukov, S.; Abbafati, C.; Abera, S.F.; et al. Global, Regional, and National Prevalence of Overweight and Obesity in Children and Adults during 1980–2013: A Systematic Analysis for the Global Burden of Disease Study 2013. Lancet 2014, 384, 766–781. [Google Scholar] [CrossRef] [Green Version]
- WHO. Obesity and Overweight. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 20 April 2022).
- Lee, E.Y.; Yoon, K.-H. Epidemic Obesity in Children and Adolescents: Risk Factors and Prevention. Front. Med. 2018, 12, 658–666. [Google Scholar] [CrossRef]
- Fan, H.; Zhang, X. Recent Trends in Overweight and Obesity in Adolescents Aged 12 to 15 Years across 21 Countries. Pediatric Obes. 2022, 17, e12839. [Google Scholar] [CrossRef]
- Nielsen, S. Trends in Food Locations and Sources among Adolescents and Young Adults. Prev. Med. 2002, 35, 107–113. [Google Scholar] [CrossRef]
- Paeratakul, S.; Ferdinand, D.P.; Champagne, C.M.; Ryan, D.H.; Bray, G.A. Fast-Food Consumption among US Adults and Children: Dietary and Nutrient Intake Profile. J. Am. Diet. Assoc. 2003, 103, 1332–1338. [Google Scholar] [CrossRef]
- Ebbeling, C.B.; Pawlak, D.B.; Ludwig, D.S. Childhood Obesity: Public-Health Crisis, Common Sense Cure. Lancet 2002, 360, 473–482. [Google Scholar] [CrossRef]
- Miller, J.L.; Couch, J.; Schwenk, K.; Long, M.; Towler, S.; Theriaque, D.W.; He, G.; Liu, Y.; Driscoll, D.J.; Leonard, C.M. Early Childhood Obesity Is Associated With Compromised Cerebellar Development. Dev. Neuropsychol. 2009, 34, 272–283. [Google Scholar] [CrossRef] [Green Version]
- Fisher, J.O.; Birch, L.L. Eating in the Absence of Hunger and Overweight in Girls from 5 to 7 y of Age. Am. J. Clin. Nutr. 2002, 76, 226–231. [Google Scholar] [CrossRef] [Green Version]
- Arluk, S.L.; Branch, J.D.; Swain, D.P.; Dowling, E.A. Childhood Obesity’s Relationship to Time Spent in Sedentary Behavior. Mil. Med. 2003, 168, 583–586. [Google Scholar] [CrossRef] [Green Version]
- Vicente-Rodríguez, G.; Rey-López, J.P.; Martín-Matillas, M.; Moreno, L.A.; Wärnberg, J.; Redondo, C.; Tercedor, P.; Delgado, M.; Marcos, A.; Castillo, M.; et al. Television Watching, Videogames, and Excess of Body Fat in Spanish Adolescents: The AVENA Study. Nutrition 2008, 24, 654–662. [Google Scholar] [CrossRef]
- Jiang, F.; Zhu, S.; Yan, C.; Jin, X.; Bandla, H.; Shen, X. Sleep and Obesity in Preschool Children. J. Pediatrics 2009, 154, 814–818. [Google Scholar] [CrossRef]
- Reilly, J.J.; Armstrong, J.; Dorosty, A.R.; Emmett, P.M.; Ness, A.; Rogers, I.; Steer, C.; Sherriff, A. Early Life Risk Factors for Obesity in Childhood: Cohort Study. BMJ 2005, 330, 1357. [Google Scholar] [CrossRef] [Green Version]
- Karnik, S.; Kanekar, A. Childhood Obesity: A Global Public Health Crisis. Int. J. Prev. Med. 2012, 3, 1–7. [Google Scholar]
- Gontariuk, M.; Krafft, T.; Rehbock, C.; Townend, D.; Van der Auwermeulen, L.; Pilot, E. The European Union and Public Health Emergencies: Expert Opinions on the Management of the First Wave of the COVID-19 Pandemic and Suggestions for Future Emergencies. Front. Public Health 2021, 9, 698995. [Google Scholar] [CrossRef]
- Tornaghi, M.; Lovecchio, N.; Vandoni, M.; Chirico, A.; Codella, R. Physical Activity Levels across COVID-19 Outbreak in Youngsters of Northwestern Lombardy. J. Sports Med. Phys. Fit. 2021, 61, 971–976. [Google Scholar] [CrossRef]
- Di Renzo, L.; Gualtieri, P.; Pivari, F.; Soldati, L.; Attinà, A.; Cinelli, G.; Leggeri, C.; Caparello, G.; Barrea, L.; Scerbo, F.; et al. Eating Habits and Lifestyle Changes during COVID-19 Lockdown: An Italian Survey. J. Transl. Med. 2020, 18, 229. [Google Scholar] [CrossRef]
- Calcaterra, V.; Verduci, E.; Vandoni, M.; Rossi, V.; Di Profio, E.; Carnevale Pellino, V.; Tranfaglia, V.; Pascuzzi, M.C.; Borsani, B.; Bosetti, A.; et al. Telehealth: A Useful Tool for the Management of Nutrition and Exercise Programs in Pediatric Obesity in the COVID-19 Era. Nutrients 2021, 13, 3689. [Google Scholar] [CrossRef]
- Vancini, R.L.; Andrade, M.S.; Viana, R.B.; Nikolaidis, P.T.; Knechtle, B.; Campanharo, C.R.V.; de Almeida, A.A.; Gentil, P.; de Lira, C.A.B. Physical Exercise and COVID-19 Pandemic in PubMed: Two Months of Dynamics and One Year of Original Scientific Production. Sports Med. Health Sci. 2021, 3, 80–92. [Google Scholar] [CrossRef]
- Coelho, M.; Oliveira, T.; Fernandes, R. State of the Art Paper Biochemistry of Adipose Tissue: An Endocrine Organ. Arch. Med. Sci. 2013, 2, 191–200. [Google Scholar] [CrossRef] [Green Version]
- Qi, Y.; Hui, X. The Shades of Grey in Adipose Tissue Reprogramming. Biosci. Rep. 2022, 42, BSR20212358. [Google Scholar] [CrossRef]
- Curat, C.A.; Miranville, A.; Sengenès, C.; Diehl, M.; Tonus, C.; Busse, R.; Bouloumié, A. From Blood Monocytes to Adipose Tissue-Resident Macrophages: Induction of Diapedesis by Human Mature Adipocytes. Diabetes 2004, 53, 1285–1292. [Google Scholar] [CrossRef] [Green Version]
- Frayn, K.N.; Karpe, F.; Fielding, B.A.; Macdonald, I.A.; Coppack, S.W. Integrative Physiology of Human Adipose Tissue. Int. J. Obes. Relat. Metab. Disord 2003, 27, 875–888. [Google Scholar] [CrossRef] [Green Version]
- Lynch, L.; O’Shea, D.; Winter, D.C.; Geoghegan, J.; Doherty, D.G.; O’Farrelly, C. Invariant NKT Cells and CD1d(+) Cells Amass in Human Omentum and Are Depleted in Patients with Cancer and Obesity. Eur. J. Immunol. 2009, 39, 1893–1901. [Google Scholar] [CrossRef]
- Wu, D.; Molofsky, A.B.; Liang, H.-E.; Ricardo-Gonzalez, R.R.; Jouihan, H.A.; Bando, J.K.; Chawla, A.; Locksley, R.M. Eosinophils Sustain Adipose Alternatively Activated Macrophages Associated with Glucose Homeostasis. Science 2011, 332, 243–247. [Google Scholar] [CrossRef] [Green Version]
- Pond, C.M. Adipose Tissue and the Immune System. Prostaglandins Leukot. Essent. Fat. Acids 2005, 73, 17–30. [Google Scholar] [CrossRef]
- Kaminski, D.A.; Randall, T.D. Adaptive Immunity and Adipose Tissue Biology. Trends Immunol. 2010, 31, 384–390. [Google Scholar] [CrossRef] [Green Version]
- Moro, K.; Yamada, T.; Tanabe, M.; Takeuchi, T.; Ikawa, T.; Kawamoto, H.; Furusawa, J.-I.; Ohtani, M.; Fujii, H.; Koyasu, S. Innate Production of T(H)2 Cytokines by Adipose Tissue-Associated c-Kit(+)Sca-1(+) Lymphoid Cells. Nature 2010, 463, 540–544. [Google Scholar] [CrossRef]
- Feuerer, M.; Herrero, L.; Cipolletta, D.; Naaz, A.; Wong, J.; Nayer, A.; Lee, J.; Goldfine, A.B.; Benoist, C.; Shoelson, S.; et al. Lean, but Not Obese, Fat Is Enriched for a Unique Population of Regulatory T Cells That Affect Metabolic Parameters. Nat. Med. 2009, 15, 930–939. [Google Scholar] [CrossRef]
- Bambace, C.; Pedrotti, M.; Ferrara, G.; Zamboni, M. Obesità, Tessuto Adiposo e Infiammazione. Biochim. Clin. 2011, 35, 4. [Google Scholar]
- Gordon, S. Alternative Activation of Macrophages. Nat. Rev. Immunol. 2003, 3, 23–35. [Google Scholar] [CrossRef]
- Harman-Boehm, I.; Blüher, M.; Redel, H.; Sion-Vardy, N.; Ovadia, S.; Avinoach, E.; Shai, I.; Klöting, N.; Stumvoll, M.; Bashan, N.; et al. Macrophage Infiltration into Omental versus Subcutaneous Fat across Different Populations: Effect of Regional Adiposity and the Comorbidities of Obesity. J. Clin. Endocrinol. Metab. 2007, 92, 2240–2247. [Google Scholar] [CrossRef] [Green Version]
- Lumeng, C.N.; Bodzin, J.L.; Saltiel, A.R. Obesity Induces a Phenotypic Switch in Adipose Tissue Macrophage Polarization. J. Clin. Invest. 2007, 117, 175–184. [Google Scholar] [CrossRef] [Green Version]
- Wentworth, J.M.; Naselli, G.; Brown, W.A.; Doyle, L.; Phipson, B.; Smyth, G.K.; Wabitsch, M.; O’Brien, P.E.; Harrison, L.C. Pro-Inflammatory CD11c+CD206+ Adipose Tissue Macrophages Are Associated with Insulin Resistance in Human Obesity. Diabetes 2010, 59, 1648–1656. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bourlier, V.; Zakaroff-Girard, A.; Miranville, A.; De Barros, S.; Maumus, M.; Sengenes, C.; Galitzky, J.; Lafontan, M.; Karpe, F.; Frayn, K.N.; et al. Remodeling Phenotype of Human Subcutaneous Adipose Tissue Macrophages. Circulation 2008, 117, 806–815. [Google Scholar] [CrossRef] [PubMed]
- Shaul, M.E.; Bennett, G.; Strissel, K.J.; Greenberg, A.S.; Obin, M.S. Dynamic, M2-like Remodeling Phenotypes of CD11c+ Adipose Tissue Macrophages during High-Fat Diet--Induced Obesity in Mice. Diabetes 2010, 59, 1171–1181. [Google Scholar] [CrossRef] [Green Version]
- Christiansen, T.; Richelsen, B.; Bruun, J.M. Monocyte Chemoattractant Protein-1 Is Produced in Isolated Adipocytes, Associated with Adiposity and Reduced after Weight Loss in Morbid Obese Subjects. Int. J. Obes. 2005, 29, 146–150. [Google Scholar] [CrossRef] [Green Version]
- Gerhardt, C.C.; Romero, I.A.; Cancello, R.; Camoin, L.; Strosberg, A.D. Chemokines Control Fat Accumulation and Leptin Secretion by Cultured Human Adipocytes. Mol. Cell. Endocrinol. 2001, 175, 81–92. [Google Scholar] [CrossRef]
- Kang, Y.-H.; Cho, M.-H.; Kim, J.-Y.; Kwon, M.-S.; Peak, J.-J.; Kang, S.-W.; Yoon, S.-Y.; Song, Y. Impaired Macrophage Autophagy Induces Systemic Insulin Resistance in Obesity. Oncotarget 2016, 7, 35577–35591. [Google Scholar] [CrossRef] [Green Version]
- Weisberg, S.P.; McCann, D.; Desai, M.; Rosenbaum, M.; Leibel, R.L.; Ferrante, A.W. Obesity Is Associated with Macrophage Accumulation in Adipose Tissue. J. Clin. Invest. 2003, 112, 1796–1808. [Google Scholar] [CrossRef]
- Abraham, S.N.; St John, A.L. Mast Cell-Orchestrated Immunity to Pathogens. Nat. Rev. Immunol. 2010, 10, 440–452. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Divoux, A.; Sun, J.; Zhang, J.; Clément, K.; Glickman, J.N.; Sukhova, G.K.; Wolters, P.J.; Du, J.; Gorgun, C.Z.; et al. Genetic Deficiency and Pharmacological Stabilization of Mast Cells Reduce Diet-Induced Obesity and Diabetes in Mice. Nat. Med. 2009, 15, 940–945. [Google Scholar] [CrossRef] [Green Version]
- Nijhuis, J.; Rensen, S.S.; Slaats, Y.; van Dielen, F.M.H.; Buurman, W.A.; Greve, J.W.M. Neutrophil Activation in Morbid Obesity, Chronic Activation of Acute Inflammation. Obesity 2009, 17, 2014–2018. [Google Scholar] [CrossRef]
- Talukdar, S.; Oh, D.Y.; Bandyopadhyay, G.; Li, D.; Xu, J.; McNelis, J.; Lu, M.; Li, P.; Yan, Q.; Zhu, Y.; et al. Neutrophils Mediate Insulin Resistance in Mice Fed a High-Fat Diet through Secreted Elastase. Nat. Med. 2012, 18, 1407–1412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kullo, I.J.; Hensrud, D.D.; Allison, T.G. Comparison of Numbers of Circulating Blood Monocytes in Men Grouped by Body Mass Index (<25, 25 to <30, > or =30). Am. J. Cardiol. 2002, 89, 1441–1443. [Google Scholar] [CrossRef]
- Poitou, C.; Dalmas, E.; Renovato, M.; Benhamo, V.; Hajduch, F.; Abdennour, M.; Kahn, J.-F.; Veyrie, N.; Rizkalla, S.; Fridman, W.-H.; et al. CD14dimCD16+ and CD14+CD16+ Monocytes in Obesity and during Weight Loss: Relationships with Fat Mass and Subclinical Atherosclerosis. Arter. Thromb. Vasc. Biol 2011, 31, 2322–2330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Satoh, N.; Shimatsu, A.; Himeno, A.; Sasaki, Y.; Yamakage, H.; Yamada, K.; Suganami, T.; Ogawa, Y. Unbalanced M1/M2 Phenotype of Peripheral Blood Monocytes in Obese Diabetic Patients: Effect of Pioglitazone. Diabetes Care 2010, 33, e7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schipper, H.S.; Nuboer, R.; Prop, S.; van den Ham, H.J.; de Boer, F.K.; Kesmir, Ç.; Mombers, I.M.H.; van Bekkum, K.A.; Woudstra, J.; Kieft, J.H.; et al. Systemic Inflammation in Childhood Obesity: Circulating Inflammatory Mediators and Activated CD14++ Monocytes. Diabetologia 2012, 55, 2800–2810. [Google Scholar] [CrossRef] [Green Version]
- DeFuria, J.; Belkina, A.C.; Jagannathan-Bogdan, M.; Snyder-Cappione, J.; Carr, J.D.; Nersesova, Y.R.; Markham, D.; Strissel, K.J.; Watkins, A.A.; Zhu, M.; et al. B Cells Promote Inflammation in Obesity and Type 2 Diabetes through Regulation of T-Cell Function and an Inflammatory Cytokine Profile. Proc. Natl. Acad. Sci. USA 2013, 110, 5133–5138. [Google Scholar] [CrossRef] [Green Version]
- Winer, D.A.; Winer, S.; Shen, L.; Wadia, P.P.; Yantha, J.; Paltser, G.; Tsui, H.; Wu, P.; Davidson, M.G.; Alonso, M.N.; et al. B Cells Promote Insulin Resistance through Modulation of T Cells and Production of Pathogenic IgG Antibodies. Nat. Med. 2011, 17, 610–617. [Google Scholar] [CrossRef]
- Kintscher, U.; Hartge, M.; Hess, K.; Foryst-Ludwig, A.; Clemenz, M.; Wabitsch, M.; Fischer-Posovszky, P.; Barth, T.F.E.; Dragun, D.; Skurk, T.; et al. T-Lymphocyte Infiltration in Visceral Adipose Tissue: A Primary Event in Adipose Tissue Inflammation and the Development of Obesity-Mediated Insulin Resistance. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 1304–1310. [Google Scholar] [CrossRef] [Green Version]
- Stolarczyk, E.; Vong, C.T.; Perucha, E.; Jackson, I.; Cawthorne, M.A.; Wargent, E.T.; Powell, N.; Canavan, J.B.; Lord, G.M.; Howard, J.K. Improved Insulin Sensitivity despite Increased Visceral Adiposity in Mice Deficient for the Immune Cell Transcription Factor T-Bet. Cell Metab. 2013, 17, 520–533. [Google Scholar] [CrossRef] [Green Version]
- Bellora, F.; Castriconi, R.; Dondero, A.; Reggiardo, G.; Moretta, L.; Mantovani, A.; Moretta, A.; Bottino, C. The Interaction of Human Natural Killer Cells with Either Unpolarized or Polarized Macrophages Results in Different Functional Outcomes. Proc. Natl. Acad. Sci. USA 2010, 107, 21659–21664. [Google Scholar] [CrossRef] [Green Version]
- Martín-Fontecha, A.; Thomsen, L.L.; Brett, S.; Gerard, C.; Lipp, M.; Lanzavecchia, A.; Sallusto, F. Induced Recruitment of NK Cells to Lymph Nodes Provides IFN-Gamma for T(H)1 Priming. Nat. Immunol. 2004, 5, 1260–1265. [Google Scholar] [CrossRef] [PubMed]
- Martinez, F.O.; Helming, L.; Gordon, S. Alternative Activation of Macrophages: An Immunologic Functional Perspective. Annu. Rev. Immunol. 2009, 27, 451–483. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmed, M.; Gaffen, S.L. IL-17 in Obesity and Adipogenesis. Cytokine Growth Factor Rev. 2010, 21, 449–453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Endo, Y.; Yokote, K.; Nakayama, T. The Obesity-Related Pathology and Th17 Cells. Cell Mol. Life Sci. 2017, 74, 1231–1245. [Google Scholar] [CrossRef] [PubMed]
- Touch, S.; Clément, K.; André, S. T Cell Populations and Functions Are Altered in Human Obesity and Type 2 Diabetes. Curr. Diab. Rep. 2017, 17, 81. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.; Chen, F.; Wang, J.; Zeng, Z.; Yang, Q.; Shao, S. Th17 and Treg Lymphocytes in Obesity and Type 2 Diabetic Patients. Clin. Immunol. 2018, 197, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Calcaterra, V.; Croce, S.; Vinci, F.; De Silvestri, A.; Cordaro, E.; Regalbuto, C.; Zuccotti, G.V.; Mameli, C.; Albertini, R.; Avanzini, M.A. Th17 and Treg Balance in Children With Obesity and Metabolically Altered Status. Front. Pediatr. 2020, 8, 591012. [Google Scholar] [CrossRef]
- Martinez-Sanchez, M.E.; Hiriart, M.; Alvarez-Buylla, E.R. The CD4+ T Cell Regulatory Network Mediates Inflammatory Responses during Acute Hyperinsulinemia: A Simulation Study. BMC Syst. Biol. 2017, 11, 64. [Google Scholar] [CrossRef]
- Zeng, C.; Shi, X.; Zhang, B.; Liu, H.; Zhang, L.; Ding, W.; Zhao, Y. The Imbalance of Th17/Th1/Tregs in Patients with Type 2 Diabetes: Relationship with Metabolic Factors and Complications. J. Mol. Med. 2012, 90, 175–186. [Google Scholar] [CrossRef]
- Durant, L.; Watford, W.T.; Ramos, H.L.; Laurence, A.; Vahedi, G.; Wei, L.; Takahashi, H.; Sun, H.-W.; Kanno, Y.; Powrie, F.; et al. Diverse Targets of the Transcription Factor STAT3 Contribute to T Cell Pathogenicity and Homeostasis. Immunity 2010, 32, 605–615. [Google Scholar] [CrossRef] [Green Version]
- Laurence, A.; Amarnath, S.; Mariotti, J.; Kim, Y.C.; Foley, J.; Eckhaus, M.; O’Shea, J.J.; Fowler, D.H. STAT3 Transcription Factor Promotes Instability of NTreg Cells and Limits Generation of ITreg Cells during Acute Murine Graft-versus-Host Disease. Immunity 2012, 37, 209–222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laurence, A.; Tato, C.M.; Davidson, T.S.; Kanno, Y.; Chen, Z.; Yao, Z.; Blank, R.B.; Meylan, F.; Siegel, R.; Hennighausen, L.; et al. Interleukin-2 Signaling via STAT5 Constrains T Helper 17 Cell Generation. Immunity 2007, 26, 371–381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, H.M.; Doss, H.M.; Kim, K.S. Multifaceted Physiological Roles of Adiponectin in Inflammation and Diseases. Int. J. Mol. Sci. 2020, 21, 1219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, C.; Feng, X.; Li, Q.; Wang, Y.; Li, Q.; Hua, M. Adiponectin, TNF-α and Inflammatory Cytokines and Risk of Type 2 Diabetes: A Systematic Review and Meta-Analysis. Cytokine 2016, 86, 100–109. [Google Scholar] [CrossRef]
- Hotamisligil, G.S. Foundations of Immunometabolism and Implications for Metabolic Health and Disease. Immunity 2017, 47, 406–420. [Google Scholar] [CrossRef] [Green Version]
- Gariballa, S.; Alkaabi, J.; Yasin, J.; Al Essa, A. Total Adiponectin in Overweight and Obese Subjects and Its Response to Visceral Fat Loss. BMC Endocr. Disord. 2019, 19, 55. [Google Scholar] [CrossRef]
- Bellissimo, M.P.; Hsu, E.; Hao, L.; Easley, K.; Martin, G.S.; Ziegler, T.R.; Alvarez, J.A. Relationships between Plasma Apelin and Adiponectin with Normal Weight Obesity, Body Composition, and Cardiorespiratory Fitness in Working Adults. J. Clin. Transl. Endocrinol. 2021, 24, 100257. [Google Scholar] [CrossRef]
- Obradovic, M.; Sudar-Milovanovic, E.; Soskic, S.; Essack, M.; Arya, S.; Stewart, A.J.; Gojobori, T.; Isenovic, E.R. Leptin and Obesity: Role and Clinical Implication. Front. Endocrinol. 2021, 12, 585887. [Google Scholar] [CrossRef]
- Holt, E.H.; Lupsa, B.; Lee, G.S.; Bassyouni, H.; Peery, H.E.; Goodman, H.M. Goodman’s Basic Medical Endocrinology, 5th ed.; Elsevier: Amsterdam, The Netherlands; Cambridge, MA, USA, 2021; ISBN 9780128158449. [Google Scholar]
- Balland, E.; Chen, W.; Tiganis, T.; Cowley, M.A. Persistent Leptin Signaling in the Arcuate Nucleus Impairs Hypothalamic Insulin Signaling and Glucose Homeostasis in Obese Mice. Neuroendocrinology 2019, 109, 374–390. [Google Scholar] [CrossRef]
- Abella, V.; Scotece, M.; Conde, J.; Pino, J.; Gonzalez-Gay, M.A.; Gómez-Reino, J.J.; Mera, A.; Lago, F.; Gómez, R.; Gualillo, O. Leptin in the Interplay of Inflammation, Metabolism and Immune System Disorders. Nat. Rev. Rheumatol. 2017, 13, 100–109. [Google Scholar] [CrossRef]
- Moraes-Vieira, P.M.M.; Larocca, R.A.; Bassi, E.J.; Peron, J.P.S.; Andrade-Oliveira, V.; Wasinski, F.; Araujo, R.; Thornley, T.; Quintana, F.J.; Basso, A.S.; et al. Leptin Deficiency Impairs Maturation of Dendritic Cells and Enhances Induction of Regulatory T and Th17 Cells: Immunomodulation. Eur. J. Immunol. 2014, 44, 794–806. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Su, K.; Li, Y.; Zhang, D.; Yuan, J.; Zhang, C.; Liu, Y.; Song, L.; Lin, Q.; Li, M.; Dong, J. Relation of Circulating Resistin to Insulin Resistance in Type 2 Diabetes and Obesity: A Systematic Review and Meta-Analysis. Front. Physiol. 2019, 10, 1399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tripathi, D.; Kant, S.; Pandey, S.; Ehtesham, N.Z. Resistin in Metabolism, Inflammation, and Disease. FEBS J. 2020, 287, 3141–3149. [Google Scholar] [CrossRef]
- Karczewska-Kupczewska, M.; Nikołajuk, A.; Stefanowicz, M.; Matulewicz, N.; Kowalska, I.; Strączkowski, M. Serum and Adipose Tissue Chemerin Is Differentially Related to Insulin Sensitivity. Endocr. Connect. 2020, 9, 360–369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, Y.; Liu, P.; Jiao, W.; Meng, J.; Feng, J. Gax Suppresses Chemerin/CMKLR1-induced Preadipocyte Biofunctions through the Inhibition of Akt/MTOR and ERK Signaling Pathways. J. Cell. Physiol. 2018, 233, 572–586. [Google Scholar] [CrossRef]
- Helfer, G.; Wu, Q.-F. Chemerin: A Multifaceted Adipokine Involved in Metabolic Disorders. J. Endocrinol. 2018, 238, R79–R94. [Google Scholar] [CrossRef] [PubMed]
- Hills, A.P.; King, N.A.; Armstrong, T.P. The Contribution of Physical Activity and Sedentary Behaviours to the Growth and Development of Children and Adolescents: Implications for Overweight and Obesity. Sports Med. 2007, 37, 533–545. [Google Scholar] [CrossRef]
- Hills, A.P.; Okely, A.D.; Baur, L.A. Addressing Childhood Obesity through Increased Physical Activity. Nat. Rev. Endocrinol. 2010, 6, 543–549. [Google Scholar] [CrossRef]
- Strong, W.B.; Malina, R.M.; Blimkie, C.J.R.; Daniels, S.R.; Dishman, R.K.; Gutin, B.; Hergenroeder, A.C.; Must, A.; Nixon, P.A.; Pivarnik, J.M.; et al. Evidence Based Physical Activity for School-Age Youth. J. Pediatrics 2005, 146, 732–737. [Google Scholar] [CrossRef]
- World Health Organization. Global Action Plan on Physical Activity 2018–2030: More Active People for a Healthier World; World Health Organization: Geneva, Switzerland, 2019; ISBN 9241514183. [Google Scholar]
- Bull, F.C.; Al-Ansari, S.S.; Biddle, S.; Borodulin, K.; Buman, M.P.; Cardon, G.; Carty, C.; Chaput, J.-P.; Chastin, S.; Chou, R.; et al. World Health Organization 2020 Guidelines on Physical Activity and Sedentary Behaviour. Br. J. Sports Med. 2020, 54, 1451–1462. [Google Scholar] [CrossRef]
- McMurray, R.G.; Berry, D.C.; Schwartz, T.A.; Hall, E.G.; Neal, M.N.; Li, S.; Lam, D. Relationships of Physical Activity and Sedentary Time in Obese Parent-Child Dyads: A Cross-Sectional Study. BMC Public Health 2015, 16, 124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Whiting, S.; Buoncristiano, M.; Gelius, P.; Abu-Omar, K.; Pattison, M.; Hyska, J.; Duleva, V.; Musić Milanović, S.; Zamrazilová, H.; Hejgaard, T.; et al. Physical Activity, Screen Time, and Sleep Duration of Children Aged 6–9 Years in 25 Countries: An Analysis within the WHO European Childhood Obesity Surveillance Initiative (COSI) 2015–2017. Obes. Facts 2020, 14, 32–44. [Google Scholar] [CrossRef] [PubMed]
- Fuemmeler, B.F.; Anderson, C.B.; Mâsse, L.C. Parent-Child Relationship of Directly Measured Physical Activity. Int. J. Behav. Nutr. Phys. Act. 2011, 8, 17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hinkley, T.; Crawford, D.; Salmon, J.; Okely, A.D.; Hesketh, K. Preschool Children and Physical Activity. Am. J. Prev. Med. 2008, 34, 435–441.e7. [Google Scholar] [CrossRef]
- Nettle, H.; Sprogis, E. Pediatric Exercise: Truth and/or Consequences. Sports Med. Arthrosc. Rev. 2011, 19, 75–80. [Google Scholar] [CrossRef]
- Haverly, K.; Davison, K.K. Personal Fulfillment Motivates Adolescents to Be Physically Active. Arch. Pediatr. Adolesc. Med. 2005, 159, 1115. [Google Scholar] [CrossRef] [Green Version]
- Trost, S.G.; Pate, R.R.; Ward, D.S.; Saunders, R.; Riner, W. Correlates of Objectively Measured Physical Activity in Preadolescent Youth. Am. J. Prev. Med. 1999, 17, 120–126. [Google Scholar] [CrossRef]
- Riddoch, C.J.; Bo Andersen, L.; Wedderkopp, N.; Harro, M.; Klasson-Heggebø, L.; Sardinha, L.B.; Cooper, A.R.; Ekelund, U. Physical Activity Levels and Patterns of 9- and 15-Yr-Old European Children. Med. Sci. Sports Exerc. 2004, 36, 86–92. [Google Scholar] [CrossRef] [Green Version]
- Garaulet, M.; Martínez, A.; Victoria, F.; Pérez–Llamas, F.; Ortega, R.M.; Zamora, S. Differences in Dietary Intake and Activity Level Between Normal-Weight and Overweight or Obese Adolescents. J. Pediatric Gastroenterol. Nutr. 2000, 30, 253–258. [Google Scholar] [CrossRef]
- Raistenskis, J.; Sidlauskiene, A.; Strukcinskiene, B.; Uğur Baysal, S.; Buckus, R. Physical Activity and Physical Fitness in Obese, Overweight, and Normal-Weight Children. Turk. J. Med. Sci. 2016, 46, 443–450. [Google Scholar] [CrossRef]
- Lovecchio, N.; Zago, M. Fitness Differences According to BMI Categories: A New Point of View. J. Sports Med. Phys. Fitness 2019, 59, 298–303. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.J.; Capio, C.M.; Abernethy, B.; Sit, C.H.P. Moderate-to-Vigorous Physical Activity and Sedentary Behavior in Children with and without Developmental Coordination Disorder: Associations with Fundamental Movement Skills. Res. Dev. Disabil. 2021, 118, 104070. [Google Scholar] [CrossRef] [PubMed]
- Babic, M.J.; Morgan, P.J.; Plotnikoff, R.C.; Lonsdale, C.; White, R.L.; Lubans, D.R. Physical Activity and Physical Self-Concept in Youth: Systematic Review and Meta-Analysis. Sports Med. 2014, 44, 1589–1601. [Google Scholar] [CrossRef] [PubMed]
- Stodden, D.F.; Goodway, J.D.; Langendorfer, S.J.; Roberton, M.A.; Rudisill, M.E.; Garcia, C.; Garcia, L.E. A Developmental Perspective on the Role of Motor Skill Competence in Physical Activity: An Emergent Relationship. Quest 2008, 60, 290–306. [Google Scholar] [CrossRef]
- Vandoni, M.; Calcaterra, V.; Carnevale Pellino, V.; De Silvestri, A.; Marin, L.; Zuccotti, G.V.; Tranfaglia, V.; Giuriato, M.; Codella, R.; Lovecchio, N. “Fitness and Fatness” in Children and Adolescents: An Italian Cross-Sectional Study. Children 2021, 8, 762. [Google Scholar] [CrossRef] [PubMed]
- Booth, F.W.; Roberts, C.K.; Laye, M.J. Lack of Exercise Is a Major Cause of Chronic Diseases. In Comprehensive Physiology; Terjung, R., Ed.; Wiley: Hoboken, NJ, USA, 2012; pp. 1143–1211. ISBN 9780470650714. [Google Scholar]
- Nimmo, M.A.; Leggate, M.; Viana, J.L.; King, J.A. The Effect of Physical Activity on Mediators of Inflammation. Diabetes Obes. Metab. 2013, 15, 51–60. [Google Scholar] [CrossRef]
- Beavers, K.M.; Brinkley, T.E.; Nicklas, B.J. Effect of Exercise Training on Chronic Inflammation. Clin. Chim. Acta 2010, 411, 785–793. [Google Scholar] [CrossRef] [Green Version]
- Bruunsgaard, H. Physical Activity and Modulation of Systemic Low-Level Inflammation. J. Leukoc. Biol. 2005, 78, 819–835. [Google Scholar] [CrossRef] [Green Version]
- Aadland, E.; Kvalheim, O.M.; Hansen, B.H.; Kriemler, S.; Ried-Larsen, M.; Wedderkopp, N.; Sardinha, L.B.; Møller, N.C.; Hallal, P.C.; Anderssen, S.A.; et al. The Multivariate Physical Activity Signature Associated with Metabolic Health in Children and Youth: An International Children’s Accelerometry Database (ICAD) Analysis. Prev. Med. 2020, 141, 106266. [Google Scholar] [CrossRef]
- Ekelund, U. Moderate to Vigorous Physical Activity and Sedentary Time and Cardiometabolic Risk Factors in Children and Adolescents. JAMA 2012, 307, 704. [Google Scholar] [CrossRef] [Green Version]
- Haapala, E.A.; Väistö, J.; Ihalainen, J.K.; Tomaselli González, C.; Leppänen, M.H.; Veijalainen, A.; Sallinen, T.; Eloranta, A.-M.; Ekelund, U.; Schwab, U.; et al. Associations of Physical Activity, Sedentary Time, and Diet Quality with Biomarkers of Inflammation in Children. Eur. J. Sport Sci. 2021, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Lavie, C.J.; Church, T.S.; Milani, R.V.; Earnest, C.P. Impact of Physical Activity, Cardiorespiratory Fitness, and Exercise Training on Markers of Inflammation. J. Cardiopulm. Rehabil. Prev. 2011, 31, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Fedewa, M.V.; Hathaway, E.D.; Ward-Ritacco, C.L. Effect of Exercise Training on C Reactive Protein: A Systematic Review and Meta-Analysis of Randomised and Non-Randomised Controlled Trials. Br. J. Sports Med. 2017, 51, 670–676. [Google Scholar] [CrossRef]
- Kasapis, C.; Thompson, P.D. The Effects of Physical Activity on Serum C-Reactive Protein and Inflammatory Markers. J. Am. Coll. Cardiol. 2005, 45, 1563–1569. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pedersen, B.K.; Febbraio, M.A. Muscles, Exercise and Obesity: Skeletal Muscle as a Secretory Organ. Nat. Rev. Endocrinol. 2012, 8, 457–465. [Google Scholar] [CrossRef]
- Pedersen, B.K.; Febbraio, M.A. Muscle as an Endocrine Organ: Focus on Muscle-Derived Interleukin-6. Physiol. Rev. 2008, 88, 1379–1406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brandt, C.; Pedersen, B.K. The Role of Exercise-Induced Myokines in Muscle Homeostasis and the Defense against Chronic Diseases. J. Biomed. Biotechnol. 2010, 2010, 520258. [Google Scholar] [CrossRef]
- Fischer, C.P. Interleukin-6 in Acute Exercise and Training: What Is the Biological Relevance? Exerc. Immunol. Rev. 2006, 12, 6–33. [Google Scholar]
- Pedersen, B.K. Muscular Interleukin-6 and Its Role as an Energy Sensor. Med. Sci. Sports Exerc. 2012, 44, 392–396. [Google Scholar] [CrossRef]
- Petersen, A.M.W.; Pedersen, B.K. The Anti-Inflammatory Effect of Exercise. J. Appl. Physiol. 2005, 98, 1154–1162. [Google Scholar] [CrossRef] [Green Version]
- Starkie, R.; Ostrowski, S.R.; Jauffred, S.; Febbraio, M.; Pedersen, B.K. Exercise and IL-6 Infusion Inhibit Endotoxin-induced TNF-α Production in Humans. FASEB J. 2003, 17, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Bergmann, M.; Gornikiewicz, A.; Sautner, T.; Waldmann, E.; Weber, T.; Mittlböck, M.; Roth, E.; Függer, R. Attenuation of Catecholamine-Induced Immunosuppression in Whole Blood from Patients with Sepsis. Shock 1999, 12, 421–427. [Google Scholar] [CrossRef] [PubMed]
- Steensberg, A.; Fischer, C.P.; Keller, C.; Møller, K.; Pedersen, B.K. IL-6 Enhances Plasma IL-1ra, IL-10, and Cortisol in Humans. Am. J. Physiol. Endocrinol. Metab. 2003, 285, E433–E437. [Google Scholar] [CrossRef] [PubMed]
- Carbó, N.; López-Soriano, J.; Costelli, P.; Alvarez, B.; Busquets, S.; Baccino, F.M.; Quinn, L.S.; López-Soriano, F.J.; Argilés, J.M. Interleukin-15 Mediates Reciprocal Regulation of Adipose and Muscle Mass: A Potential Role in Body Weight Control. Biochim. Et Biophys. Acta (BBA)—Gen. Subj. 2001, 1526, 17–24. [Google Scholar] [CrossRef]
- Nielsen, A.R.; Hojman, P.; Erikstrup, C.; Fischer, C.P.; Plomgaard, P.; Mounier, R.; Mortensen, O.H.; Broholm, C.; Taudorf, S.; Krogh-Madsen, R.; et al. Association between Interleukin-15 and Obesity: Interleukin-15 as a Potential Regulator of Fat Mass. J. Clin. Endocrinol. Metab. 2008, 93, 4486–4493. [Google Scholar] [CrossRef] [Green Version]
- Walsh, N.P.; Gleeson, M.; Shephard, R.J.; Gleeson, M.; Woods, J.A.; Bishop, N.C.; Fleshner, M.; Green, C.; Pedersen, B.K.; Hoffman-Goetz, L.; et al. Position Statement. Part One: Immune Function and Exercise. Exerc. Immunol. Rev. 2011, 17, 6–63. [Google Scholar]
- Sirico, F.; Bianco, A.; D’Alicandro, G.; Castaldo, C.; Montagnani, S.; Spera, R.; Di Meglio, F.; Nurzynska, D. Effects of Physical Exercise on Adiponectin, Leptin, and Inflammatory Markers in Childhood Obesity: Systematic Review and Meta-Analysis. Child. Obes. 2018, 14, 207–217. [Google Scholar] [CrossRef] [Green Version]
- Tenório, T.R.S.; Balagopal, P.B.; Andersen, L.B.; Ritti-Dias, R.M.; Hill, J.O.; Lofrano-Prado, M.C.; Prado, W.L. Effect of Low- Versus High-Intensity Exercise Training on Biomarkers of Inflammation and Endothelial Dysfunction in Adolescents With Obesity: A 6-Month Randomized Exercise Intervention Study. Pediatric Exerc. Sci. 2018, 30, 96–105. [Google Scholar] [CrossRef]
- Merlin, M.; de Oliveira, H.H.; Passos, M.E.P.; Momesso, C.M.; dos Santos de Oliveira, L.C.; Santana, J.E.; Levada-Pires, A.C.; Hatanaka, E.; Massao-Hirabara, S.; Guaré, R.; et al. Relationship between Children Physical Activity, Inflammatory Mediators and Lymphocyte Activation: Possible Impact of Social Isolation (COVID-19). Sport Sci. Health 2021, 17, 431–439. [Google Scholar] [CrossRef]
- Stewart, L.K.; Flynn, M.G.; Campbell, W.W.; Craig, B.A.; Robinson, J.P.; McFarlin, B.K.; Timmerman, K.L.; Coen, P.M.; Felker, J.; Talbert, E. Influence of Exercise Training and Age on CD14+ Cell-Surface Expression of Toll-like Receptor 2 and 4. Brain Behav. Immun. 2005, 19, 389–397. [Google Scholar] [CrossRef]
- Da Luz Scheffer, D.; Latini, A. Exercise-Induced Immune System Response: Anti-Inflammatory Status on Peripheral and Central Organs. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2020, 1866, 165823. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, S.; Li, G.; Xiao, J. Exercise Regulates the Immune System. In Physical Exercise for Human Health; Xiao, J., Ed.; Springer: Singapore, 2020; Volume 1228, pp. 395–408. ISBN 9789811517914. [Google Scholar]
- Hojman, P. Exercise Protects from Cancer through Regulation of Immune Function and Inflammation. Biochem. Soc. Trans. 2017, 45, 905–911. [Google Scholar] [CrossRef] [PubMed]
- Lancaster, G.I.; Febbraio, M.A. The Immunomodulating Role of Exercise in Metabolic Disease. Trends Immunol. 2014, 35, 262–269. [Google Scholar] [CrossRef] [PubMed]
- Apostolopoulos, V.; Borkoles, E.; Polman, R.; Stojanovska, L. Physical and Immunological Aspects of Exercise in Chronic Diseases. Immunotherapy 2014, 6, 1145–1157. [Google Scholar] [CrossRef] [PubMed]
- Mach, N.; Fuster-Botella, D. Endurance Exercise and Gut Microbiota: A Review. J. Sport Health Sci. 2017, 6, 179–197. [Google Scholar] [CrossRef]
- Asimakos, A.; Toumpanakis, D.; Karatza, M.-H.; Vasileiou, S.; Katsaounou, P.; Mastora, Z.; Vassilakopoulos, T. Immune Cell Response to Strenuous Resistive Breathing: Comparison with Whole Body Exercise and the Effects of Antioxidants. Int. J. Chronic Obstr. Pulm. Dis. 2018, 13, 529–545. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Lv, Y.; Li, G.; Xiao, J. MicroRNAs in Heart and Circulation during Physical Exercise. J. Sport Health Sci. 2018, 7, 433–441. [Google Scholar] [CrossRef]
- Freidenreich, D.J.; Volek, J.S. Immune Responses to Resistance Exercise. Exerc. Immunol. Rev. 2012, 18, 8–41. [Google Scholar]
- Bigley, A.B.; Simpson, R.J. NK Cells and Exercise: Implications for Cancer Immunotherapy and Survivorship. Discov. Med. 2015, 19, 433–445. [Google Scholar]
- Simpson, R.J.; McFarlin, B.K.; McSporran, C.; Spielmann, G.; ó Hartaigh, B.; Guy, K. Toll-like Receptor Expression on Classic and pro-Inflammatory Blood Monocytes after Acute Exercise in Humans. Brain Behav. Immun. 2009, 23, 232–239. [Google Scholar] [CrossRef]
- Deckx, N.; Wens, I.; Nuyts, A.H.; Lee, W.-P.; Hens, N.; Koppen, G.; Goossens, H.; Van Damme, P.; Berneman, Z.N.; Eijnde, B.O.; et al. Rapid Exercise-Induced Mobilization of Dendritic Cells Is Potentially Mediated by a Flt3L- and MMP-9-Dependent Process in Multiple Sclerosis. Mediat. Inflamm. 2015, 2015, 158956. [Google Scholar] [CrossRef] [PubMed]
- Timmons, B.W.; Cieslak, T. Human Natural Killer Cell Subsets and Acute Exercise: A Brief Review. Exerc. Immunol. Rev. 2008, 14, 8–23. [Google Scholar] [PubMed]
- Janeway, C.; Murphy, K.P.; Travers, P.; Walport, M. Janeway’s Immuno Biology; Garland Science: New York, NY, USA; London, UK, 2008; ISBN 0815341237. [Google Scholar]
- Peake, J.M.; Neubauer, O.; Walsh, N.P.; Simpson, R.J. Recovery of the Immune System after Exercise. J. Appl. Physiol. 2017, 122, 1077–1087. [Google Scholar] [CrossRef] [PubMed]
- Thomas, N.E.; Williams, D.R.R. Inflammatory Factors, Physical Activity, and Physical Fitness in Young People: Inflammatory Factors, Activity, and Fitness in Young People. Scand. J. Med. Sci. Sports 2008, 18, 543–556. [Google Scholar] [CrossRef] [PubMed]
- Plaisance, E.P.; Grandjean, P.W. Physical Activity and High-Sensitivity C-Reactive Protein. Sports Med. 2006, 36, 443–458. [Google Scholar] [CrossRef]
- AVENA Study group; Wärnberg, J.; Nova, E.; Moreno, L.A.; Romeo, J.; Mesana, M.I.; Ruiz, J.R.; Ortega, F.B.; Sjöström, M.; Bueno, M.; et al. Inflammatory Proteins Are Related to Total and Abdominal Adiposity in a Healthy Adolescent Population: The AVENA Study. Am. J. Clin. Nutr. 2006, 84, 505–512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- the AVENA group; Wärnberg, J.; Moreno, L.A.; Mesana, M.I.; Marcos, A. Inflammatory Mediators in Overweight and Obese Spanish Adolescents. The AVENA Study. Int. J. Obes. 2004, 28, S59–S63. [Google Scholar] [CrossRef] [Green Version]
- Trayhurn, P.; Wood, I.S. Adipokines: Inflammation and the Pleiotropic Role of White Adipose Tissue. Br. J. Nutr. 2004, 92, 347–355. [Google Scholar] [CrossRef] [Green Version]
- Ruiz, J.R.; Ortega, F.B.; Warnberg, J.; Sjöström, M. Associations of Low-Grade Inflammation with Physical Activity, Fitness and Fatness in Prepubertal Children; the European Youth Heart Study. Int. J. Obes. 2007, 31, 1545–1551. [Google Scholar] [CrossRef] [Green Version]
- Ruiz, J.R.; Rizzo, N.S.; Hurtig-Wennlöf, A.; Ortega, F.B.; Wàrnberg, J.; Sjöström, M. Relations of Total Physical Activity and Intensity to Fitness and Fatness in Children: The European Youth Heart Study. Am. J. Clin. Nutr. 2006, 84, 299–303. [Google Scholar] [CrossRef]
- Gutin, B.; Yin, Z.; Humphries, M.C.; Barbeau, P. Relations of Moderate and Vigorous Physical Activity to Fitness and Fatness in Adolescents. Am. J. Clin. Nutr. 2005, 81, 746–750. [Google Scholar] [CrossRef] [PubMed]
- Platat, C.; Wagner, A.; Klumpp, T.; Schweitzer, B.; Simon, C. Relationships of Physical Activity with Metabolic Syndrome Features and Low-Grade Inflammation in Adolescents. Diabetologia 2006, 49, 2078–2085. [Google Scholar] [CrossRef] [Green Version]
- Cook, D.G.; Mendall, M.A.; Whincup, P.H.; Carey, I.M.; Ballam, L.; Morris, J.E.; Miller, G.J.; Strachan, D.P. C-Reactive Protein Concentration in Children: Relationship to Adiposity and Other Cardiovascular Risk Factors. Atherosclerosis 2000, 149, 139–150. [Google Scholar] [CrossRef]
- Kohl, H.W.; Fulton, J.E.; Caspersen, C.J. Assessment of Physical Activity among Children and Adolescents: A Review and Synthesis. Prev. Med. 2000, 31, S54–S76. [Google Scholar] [CrossRef]
- Rowlands, A.V.; Eston, R.G. The Measurement and Interpretation of Children’s Physical Activity. J. Sports Sci. Med. 2007, 6, 270–276. [Google Scholar] [PubMed]
- Han, Y.; Liu, Y.; Zhao, Z.; Zhen, S.; Chen, J.; Ding, N.; Ma, Y.; Wen, D. Does Physical Activity-Based Intervention Improve Systemic Proinflammatory Cytokine Levels in Overweight or Obese Children and Adolescents? Insights from a Meta-Analysis of Randomized Control Trials. Obes. Facts 2019, 12, 653–668. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calcaterra, V.; Vandoni, M.; Rossi, V.; Berardo, C.; Grazi, R.; Cordaro, E.; Tranfaglia, V.; Carnevale Pellino, V.; Cereda, C.; Zuccotti, G. Use of Physical Activity and Exercise to Reduce Inflammation in Children and Adolescents with Obesity. Int. J. Environ. Res. Public Health 2022, 19, 6908. https://doi.org/10.3390/ijerph19116908
Calcaterra V, Vandoni M, Rossi V, Berardo C, Grazi R, Cordaro E, Tranfaglia V, Carnevale Pellino V, Cereda C, Zuccotti G. Use of Physical Activity and Exercise to Reduce Inflammation in Children and Adolescents with Obesity. International Journal of Environmental Research and Public Health. 2022; 19(11):6908. https://doi.org/10.3390/ijerph19116908
Chicago/Turabian StyleCalcaterra, Valeria, Matteo Vandoni, Virginia Rossi, Clarissa Berardo, Roberta Grazi, Erika Cordaro, Valeria Tranfaglia, Vittoria Carnevale Pellino, Cristina Cereda, and Gianvincenzo Zuccotti. 2022. "Use of Physical Activity and Exercise to Reduce Inflammation in Children and Adolescents with Obesity" International Journal of Environmental Research and Public Health 19, no. 11: 6908. https://doi.org/10.3390/ijerph19116908
APA StyleCalcaterra, V., Vandoni, M., Rossi, V., Berardo, C., Grazi, R., Cordaro, E., Tranfaglia, V., Carnevale Pellino, V., Cereda, C., & Zuccotti, G. (2022). Use of Physical Activity and Exercise to Reduce Inflammation in Children and Adolescents with Obesity. International Journal of Environmental Research and Public Health, 19(11), 6908. https://doi.org/10.3390/ijerph19116908









