Influence of Environmental Pollution and Living Conditions on Parasite Transmission among Indigenous Ecuadorians
Abstract
1. Introduction
2. Materials and Methods
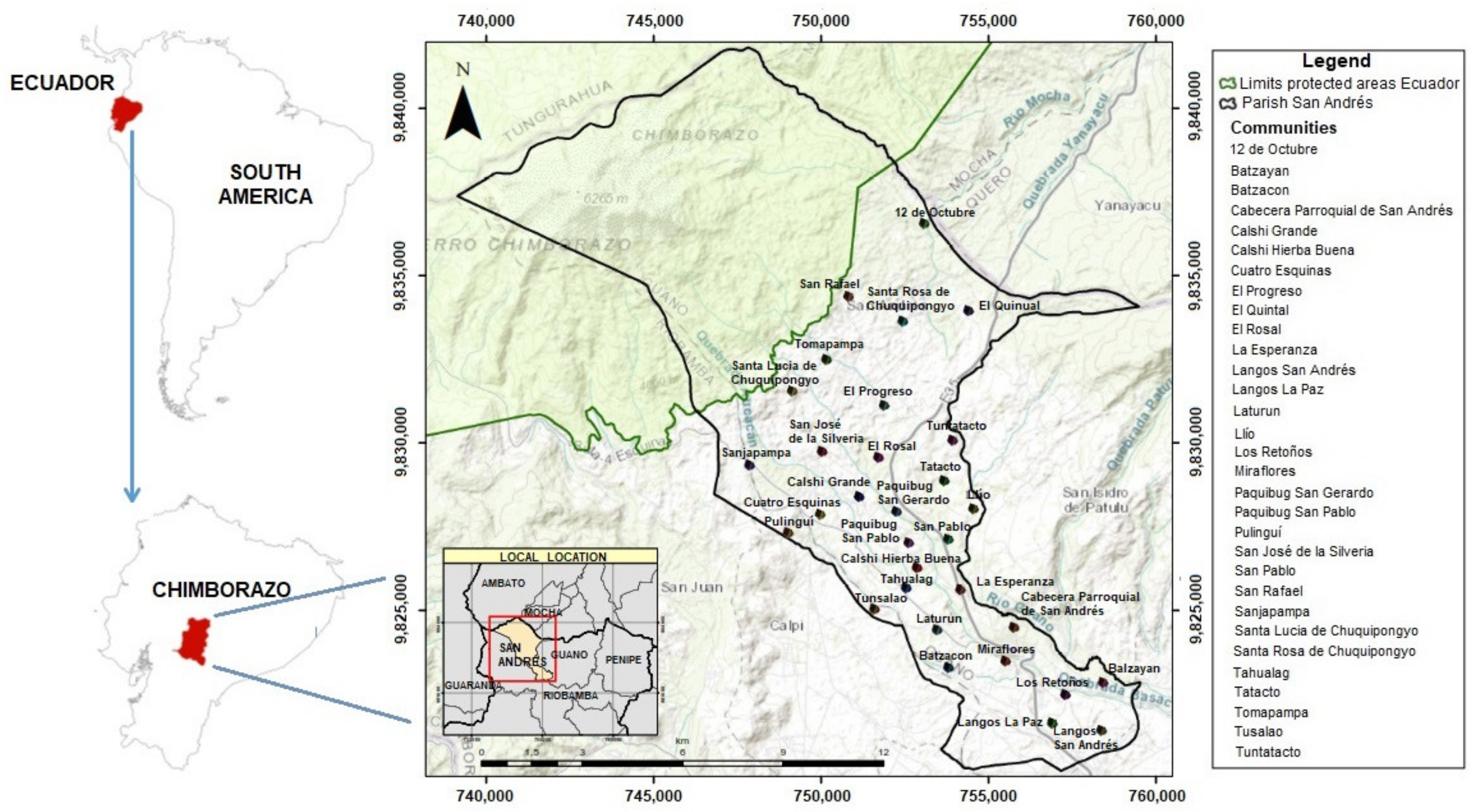
2.1. Study Area
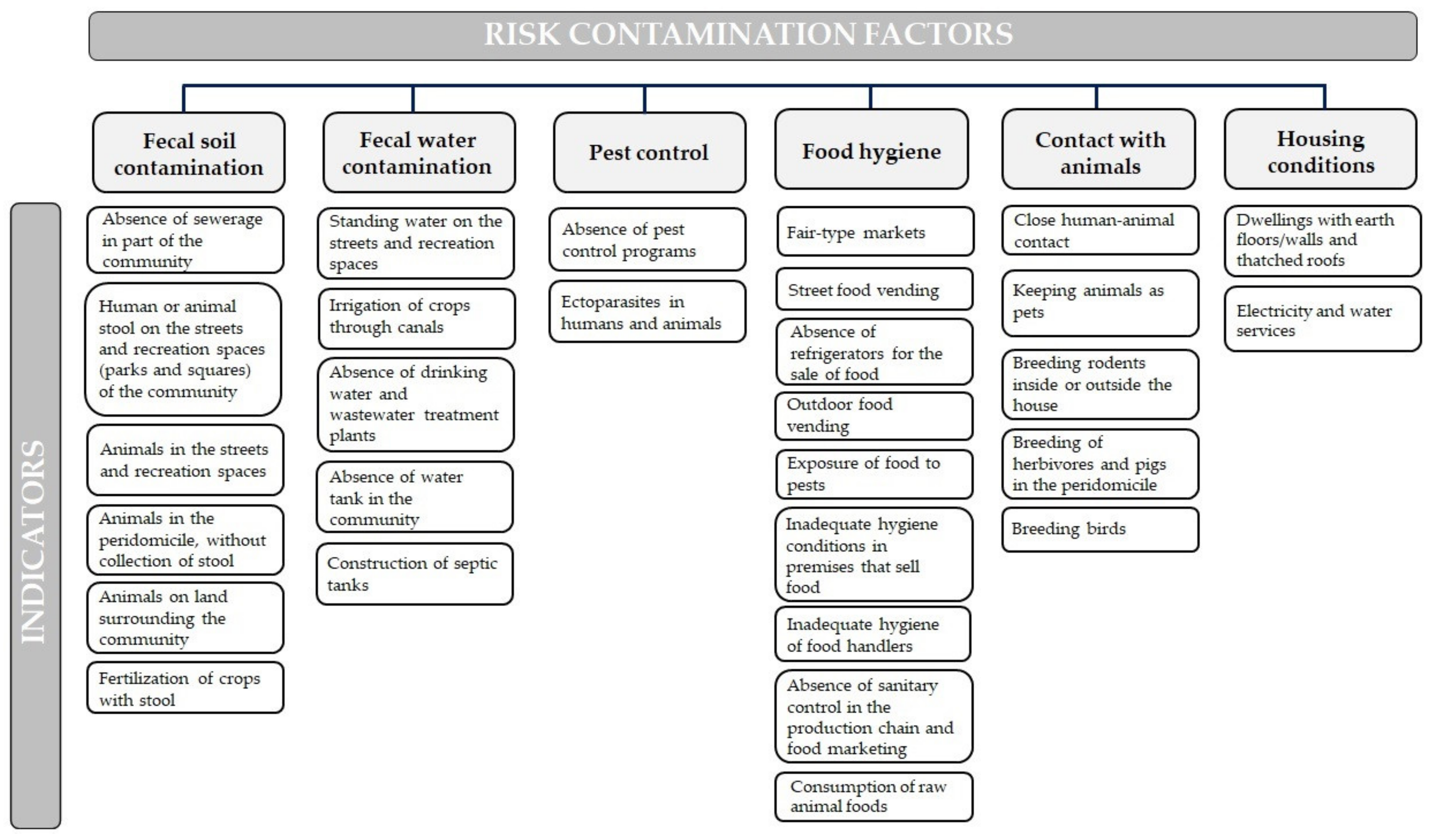
2.1.1. Fecal Soil Contamination
2.1.2. Fecal Water Contamination
2.1.3. Pest Control
2.1.4. Food Hygiene
2.1.5. Contact with Animals
2.1.6. Housing Conditions
2.2. Registry of Environmental Indicators
2.3. Sampling
2.3.1. Communities Sample
2.3.2. Arthropods, Fruits and Vegetables Sample
2.3.3. Human Sample
2.4. Collection of Environmental Pollution Information
2.5. Processing and Analysis Samples
2.5.1. Arthropod Processing and Analysis
2.5.2. Vegetable Product Processing and Analysis
2.5.3. Human Stool Sample Processing and Analysis
2.6. Ethical Considerations
2.7. Statistical Analysis
3. Results
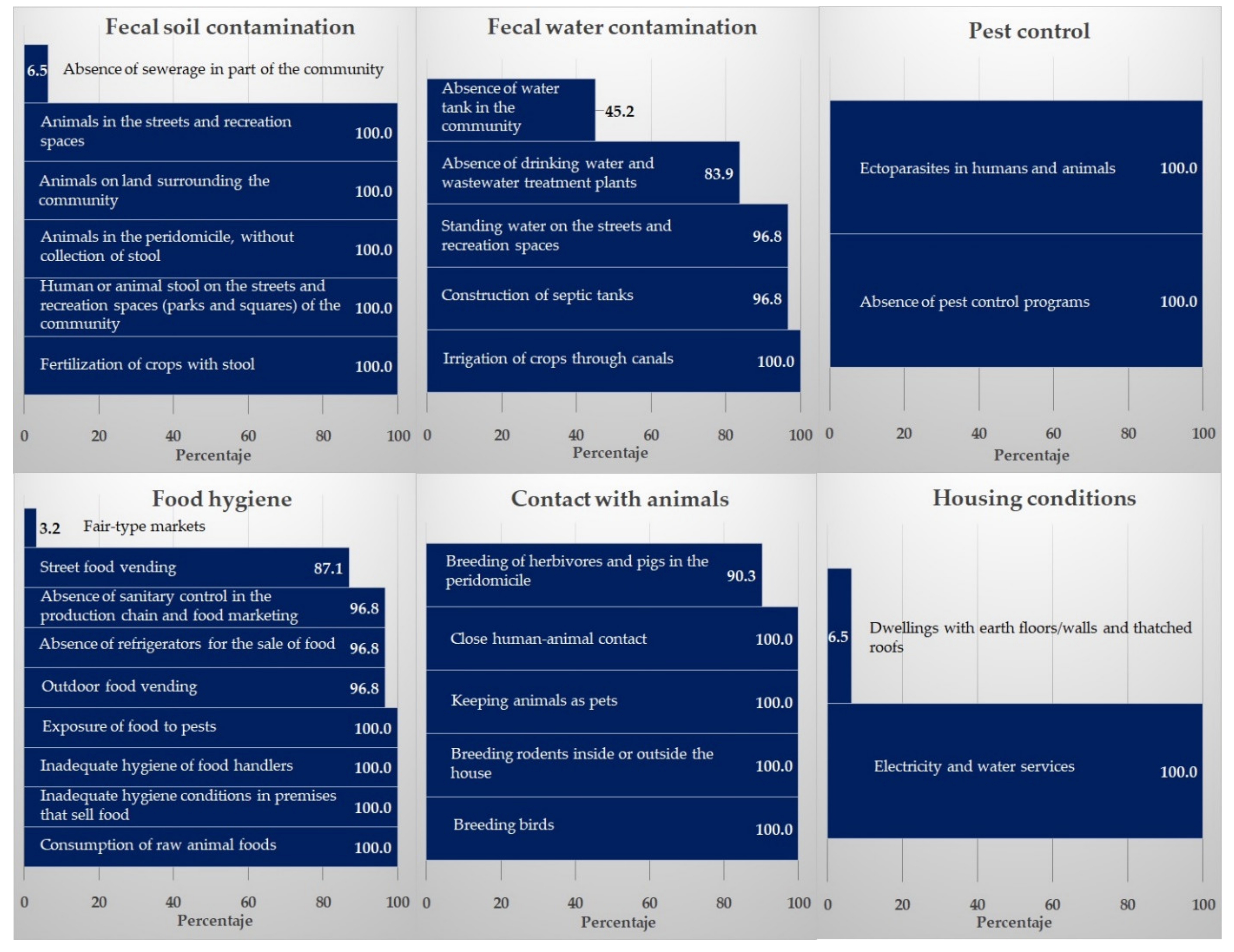
3.1. Risk Factors
3.2. Parasitic Contamination in Arthropods, Vegetables, and Humans
3.2.1. Parasitic Contamination of Arthropods
3.2.2. Parasitic Contamination of Agricultural Products (Fruit and Vegetables)
3.2.3. Parasite Frequency in Humans
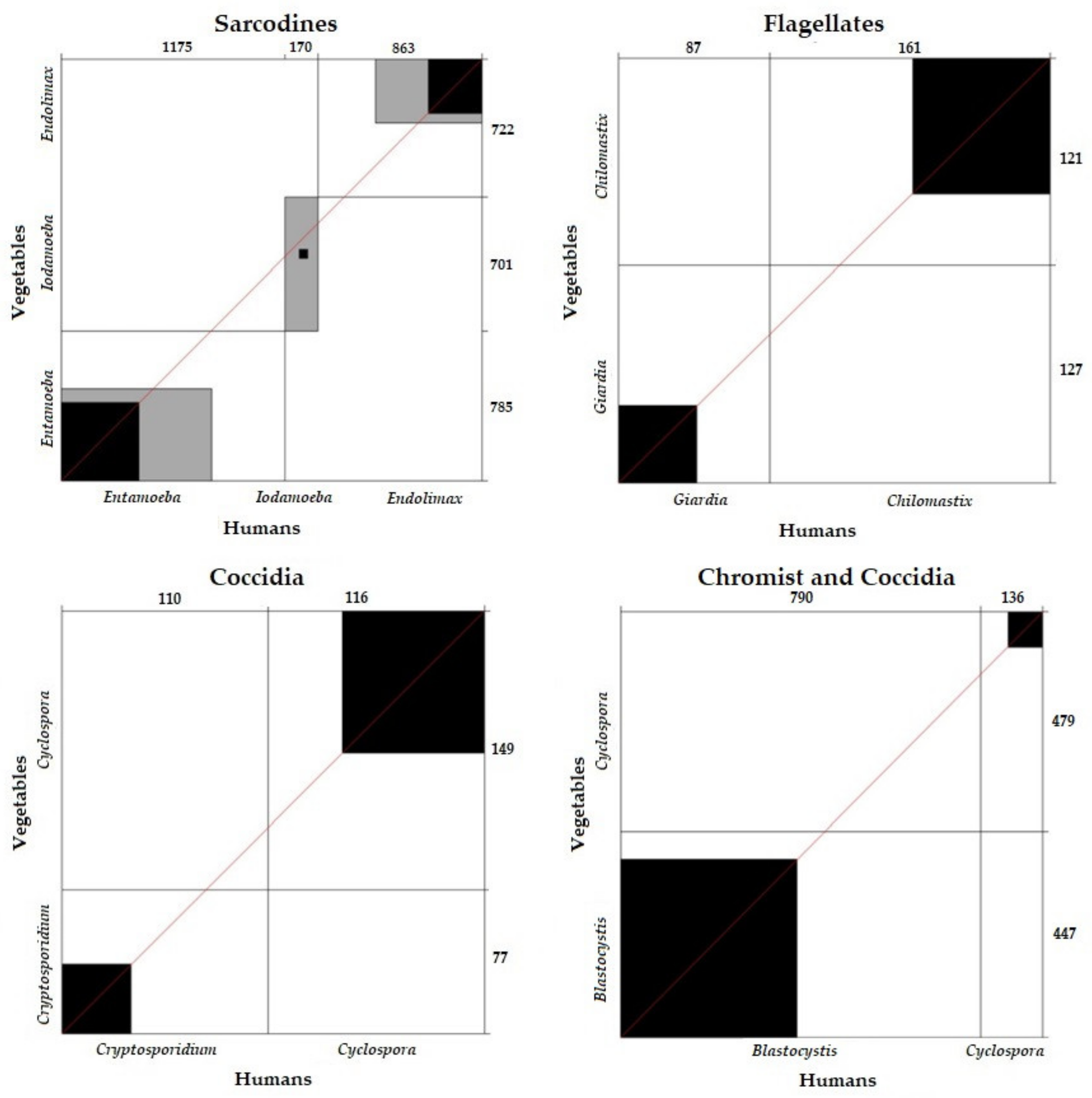
3.2.4. Parasite Correlations and Concordance between Variables (Humans, Vegetables, and Arthropods)
Correlation and Concordance of Parasite Frequency between Vegetables and Humans According to Parasite Group
Correlation and Concordance of Parasite Frequency between Humans and Arthropods According to Parasite Group
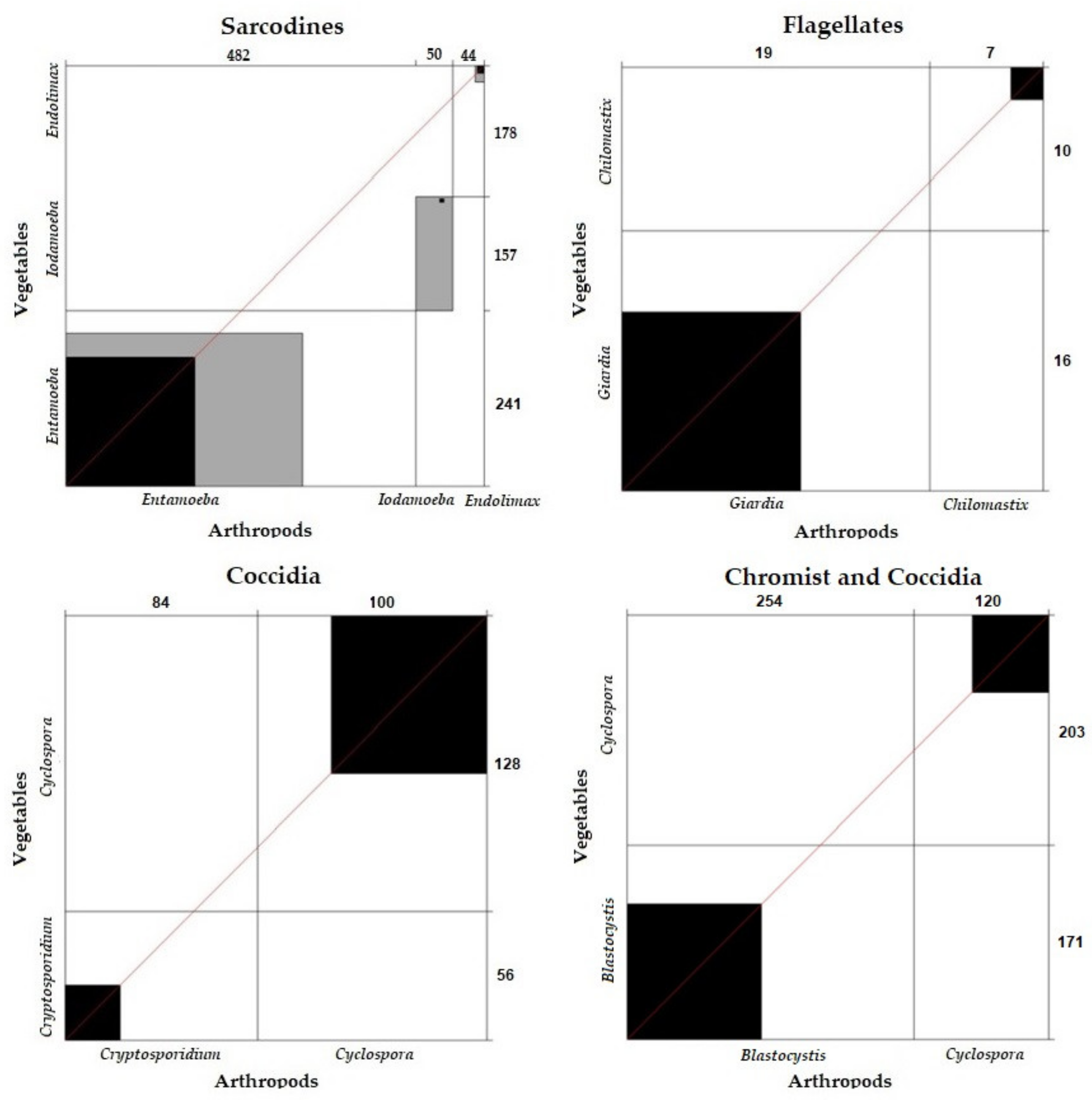
Correlation and Concordance of Parasite Frequency between Vegetables and Arthropods According to Parasite Group
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Panti-May, J.A.; Zonta, M.L.; Cociancic, P.; Barrientos-Medina, R.C.; Machain-Williams, C.; Robles, M.R.; Hernández-Betancourt, S.F. Occurrence of intestinal parasites in Mayan children from Yucatán, Mexico. Acta Trop. 2019, 195, 58–61. [Google Scholar] [CrossRef]
- Gotera, J.; Panunzio, A.; Ávila, A.; Villarroel, F.; Urdaneta, O.; Fuentes, B.; Linares, J. Saneamiento ambiental y su relación con la prevalencia de parásitos intestinales. Kasmera 2019, 47, 59–65. Available online: https://produccioncientificaluz.org/index.php/kasmera/article/view/24678 (accessed on 10 January 2022).
- WHO; UNICEF. Progress on Drinking Water, Sanitation and Hygiene: 2017 Update and SDG Baselines; World Health Organization (WHO): Geneva, Switzerland; The United Nations Children’s Fund (UNICEF): Washington, DC, USA, 2017; pp. 13–60. [Google Scholar]
- Chifunda, K.; Kelly, P. Parasitic infections of the gut in children. Pediatr. Int. Child Health 2019, 39, 65–72. [Google Scholar] [CrossRef]
- World Health Organization; Food and Agriculture Organization of the United Nations. Multicriteria-Based Ranking for Risk Management of Food-Borne Parasites: Report of a Joint FAO/WHO Expert Meeting, 3–7 September 2012; FAO Headquarters: Rome, Italy, 2014; Available online: https://apps.who.int/iris/handle/10665/112672 (accessed on 10 January 2022).
- Dixon, B.R. Parasitic illnesses associated with the consumption of fresh produce—An emerging issue in developed countries. Curr. Opin. Food Sci. 2016, 8, 104–109. [Google Scholar] [CrossRef]
- Serpa-Andrade, C.A.; Velecela-Abambari, S.G.; Balladares-Rengel, M.F. Prevalencia de parasitismo intestinal en los niños de la escuela José María Astudillo de la parroquia Sinincay. Rev. Pan. Med. 2014, 8, 14–19. Available online: http://186.5.103.99/bitstream/reducacue/5541/3/PREVALENCIA%20DE%20PARASISTISMO%20INTESTINAL%20EN%20NI%C3%91OS.pdf (accessed on 15 January 2022).
- Durán-Pincay, Y.; Rivero-Rodríguez, Z.; Bracho-Mora, A. Prevalencia de parasitosis intestinales en niños del Cantón Paján, Ecuador. Kasmera 2019, 47, 44–49. Available online: https://produccioncientificaluz.org/index.php/kasmera/article/view/24676 (accessed on 10 January 2022).
- Murillo-Zavala, A.M.; Rodríguez de Rivero, Z.C.; Bracho-Mora, A.M. Parasitosis intestinales y factores de riesgo de enteroparasitosis en escolares de la zona urbana del cantón Jipijapa, Ecuador. Kasmera 2020, 48, e48130858. [Google Scholar] [CrossRef]
- Escobar-Arrieta, S.; Cando-Brito, V.; Espinoza, C.; Guevara, L. Parasitosis intestinal en una población de 5 a 14 años que acuden a unidades educativas escuelas colegios públicos de la ciudad de Riobamba. Eur. Sci. J. 2017, 13, 30. [Google Scholar] [CrossRef][Green Version]
- Gobierno Autónomo Descentralizado Parroquial Rural San Andrés. Diagnóstico del GAD Parroquia Rural San Andrés. Ecuador: GAD. 2015. Available online: http://app.sni.gob.ec/sni-link/sni/PORTAL_SNI/data_sigad_plus/sigadplusdiagnostico/1865016750001_DIAGNOSTICO%20San%20Andres%202015_15-05-2015_18-28-18.pdf (accessed on 22 July 2021).
- Dawson, D. Foodborne protozoan parasites. Int. J. Food Microbiol. 2005, 103, 207–227. [Google Scholar] [CrossRef]
- Machado-Moreira, B.; Richards, K.; Brennan, F.; Abram, F.; Burgess, C.M. Microbial contamination of fresh produce: What, where, and how? Compr. Rev. Food Sci. Food Saf. 2019, 18, 1727–1750. [Google Scholar] [CrossRef]
- Calvopiña, M.; Atherton, R.; Romero-Álvarez, D.; Castañeda, B.; Valverde-Muñoz, G.; Cevallos, W.; Izurieta, R. Identification of intestinal parasite infections and associated risk factors in indigenous communities of Ecuador. Int. J. Acad. Med. 2019, 5, 171–179. [Google Scholar] [CrossRef]
- Jacobsen, K.H.; Ribeiro, P.S.; Quist, B.K.; Rydbeck, B.V. Prevalence of Intestinal Parasites in Young Quichua Children in the Highlands of Rural Ecuador. J. Health Popul. Nutr. 2007, 25, 399–405. [Google Scholar]
- Harhay, M.O.; Horton, J.; Olliaro, P.L. Epidemiology and control of human gastrointestinal parasites in children. Expert. Rev. Anti. Infect. Ther. 2010, 8, 219–234. [Google Scholar] [CrossRef]
- INEN. Norma Técnica Ecuatoriana NTE INEN 1 108: Agua Potable. Requisitos; Instituto Ecuatoriano de Normalización: Quito, Ecuador, 2011; pp. 2–8. [Google Scholar]
- González-Ramírez, L.C.; Falconí-Ontaneda, F.A.; Yaucén-Rodríguez, M.; Romero-Zapata, C.F.; Parra-Mayorga, P.; García-Ríos, C.A.; Prato-Moreno, J.G. Dispersión hídrica de enteroparásitos en una zona agropecuaria de gran altitud, en Los Andes Ecuatorianos. Kasmera 2020, 48, e48231698. [Google Scholar] [CrossRef]
- González-Ramírez, L.C.; Vázquez, C.J.; Chimbaina, M.B.; Djabayan-Djibeyan, P.; Prato-Moreno, J.G.; Trelis, M.; Fuentes, M.V. Ocurrence of enteroparasites with zoonotic potential in animals of the rural area of San Andres, Chimborazo, Ecuador. Vet. Parasit. Reg. Stud. Rep. 2021, 26, 100630. [Google Scholar] [CrossRef]
- Castro, R.; Pérez, R. Saneamiento Rural y Salud: Guía Para Acciones a Nivel Local; Organización Mundial de la Salud y Organización Panamericana de la Salud: Guatemala, Guatemala, 2009; pp. 10–26. [Google Scholar]
- Uakarn, C.; Chaokromthong, K.; Sintao, N. Sample size estimation using Yamane and Cochran and Krejcie and Morgan and Green formulas and Cohen statistical power analysis by G*Power and comparisons. APHEIT Int. J. 2021, 10, 76–88. [Google Scholar]
- Instituto Nacional de Estadísticas y Censos (INEC). Available online: http//www.inec.gob.ec (accessed on 30 January 2022).
- Garcia, L.; Bruckner, D.; Brewer, T.; Shimizu, R. Techniques for the recovery and identification of Cryptosporidium oocysts from stool specimens. J. Clin. Microbiol. 1983, 18, 185–190. [Google Scholar] [CrossRef]
- Ash, L.R.; Orihel, T.C.; Savioli, L. Bench Aids for the Diagnosis of Intestinal Parasites; World Health Organization: Geneva, Switzerland, 1994; Available online: http://apps.who.int/iris/bitstream/handle/10665/37323/9789241544764_eng.pdf?sequence=1 (accessed on 15 November 2021).
- Knight, W.B.; Hiatt, R.A.; Cline, B.L.; Ritchie, L.S. A modification of the formol-ether concentration technique for increased sensitivity in detecting Schistosoma mansoni. Am. J. Trop. 1976, 55, 818–823. [Google Scholar] [CrossRef]
- Pagano, M.; Gauvreau, K. Principles of Biostatistics, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2018; pp. 323–373. [Google Scholar]
- Gera, T.; Shah, D.; Sachdev, H.S. Impact of water, sanitation and hygiene interventions on growth, non-diarrheal morbidity and mortality in children residing in low- and middle-income countries: A systematic review. Indian Pediatr. 2018, 55, 381–393. Available online: https://pubmed.ncbi.nlm.nih.gov/29428924/ (accessed on 27 February 2022). [CrossRef]
- Fontán-Sainz, M.; Gómez-Couso, H.; Fernández-Ibáñez, P.; Ares-Mazás, E. Evaluation of the solar water disinfection process (SODIS) against Cryptosporidium parvum using a 25 L static solar reactor fitted with a compound parabolic collector (CPC). Am. J. Trop. Med. Hyg. 2012, 86, 223–228. [Google Scholar] [CrossRef]
- Fuenmayor-Boscán, A.D.; Hernández, I.M.; Valero, K.J.; Paz, A.M.; Sandrea, L.B.; Rivero, Z. Association between Helicobacter pylori and intestinal parasites in an Añu indigenous community of Venezuela. Indian. J. Gastroenterol. 2016, 35, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Esteban, G.; González, C.; Bargues, M.D.; Anglés, R.; Sánchez, C.; Náquira, C.; Mas-Coma, S. High fascioliasis infection in children linked to a man-made irrigation zone in Peru. Trop. Med. Int. Health 2002, 7, 339–348. [Google Scholar] [CrossRef]
- Adenusi, A.A.; Adewoga, T.O. Human intestinal parasites in non-biting synanthropic flies in Ogun State, Nigeria. Travel. Med. Infect. Dis. 2013, 11, 181–189. [Google Scholar] [CrossRef]
- Galán-Puchades, M.T.; Sanxis-Furió, J.; Pascual, J.; Bueno-Marí, R.; Franco, S.; Peracho, V.; Montalvo, T.; Fuentes, M.V. First survey on zoonotic helminthosis in urban brown rats (Rattus norvegicus) in Spain and associated public health considerations. Vet. Parasitol. 2018, 259, 49–52. [Google Scholar] [CrossRef] [PubMed]
- Bekele, F.; Tefera, T.; Biresaw, G.; Yohannes, T. Parasitic contamination of raw vegetables and fruits collected from selected local markets in Arba Minch town, Southern Ethiopia. Infect. Dis. Poverty 2017, 6, 19. [Google Scholar] [CrossRef]
- Punsawad, C.; Phasuk, N.; Thongtup, K.; Nagavirochana, S.; Viriyavejakul, P. Prevalence of parasitic contamination of raw vegetables in Nakhon Si Thammarat province, southern Thailand. BMC Health Serv. Res. 2019, 19, 34. [Google Scholar] [CrossRef]
- Eshetu, L.; Dabsu, R.; Tadele, G. Prevalence of intestinal parasites and its risk factors among food handlers in food services in Nekemte town, west Oromia, Ethiopia. Res. Rep. Trop. Med. 2019, 10, 25–30. [Google Scholar] [CrossRef]
- Rajoo, Y.; Ambu, S.; Lim, Y.A.L.; Rajoo, K.; Tey, S.C.; Lu, C.W.; Romano, N. Neglected intestinal parasites, malnutrition and associated key factors: A population based cross-sectional study among indigenous communities in Sarawak, Malaysia. PLoS ONE 2017, 12, e0170174. [Google Scholar] [CrossRef]






| Parasites | Flies | Spiders | Total | Dif.Prop | EE (Dif.Prop) | 95% | RR | 95% CI | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ns = 186 | ns = 71 | ns = 300 * | ||||||||||
| np | % | np | % | np | % | Ll | Ul | RR | ||||
| Blastocystis sp. | 75 | 40.3 | 6 | 8.5 | 81 | 27.0 | 0.3187 | 0.0488 | 0.2230 | 0.4144 | 15.9 | 15.2–16.5 |
| Entamoeba spp. | 149 | 80.1 | 6 | 8.5 | 155 | 51.7 | 0.7166 | 0.0441 | 0.6301 | 0.8030 | 9.5 | 8.7–10.2 |
| Endolimax nana | 3 | 1.6 | 0 | 0 | 3 | 1.0 | - | - | - | - | - | - |
| Iodamoeba butschlii | 5 | 2.7 | 0 | 0 | 5 | 1.7 | - | - | - | - | - | - |
| Giardia spp. | 6 | 3.2 | 0 | 0 | 6 | 2.0 | - | - | - | - | - | - |
| Cyclospora spp. | 8 | 4.3 | 0 | 0 | 8 | 2.7 | - | - | - | - | - | - |
| Eimeria spp. | 18 | 9.7 | 0 | 0 | 18 | 6.0 | - | - | - | - | - | - |
| Protozoa | 149 | 80.1 | 9 | 12.7 | 158 | 52.7 | 0.6743 | 0.0492 | 0.5780 | 0.7706 | 6.3 | 5.7–6.9 |
| Toxocara spp. | 2 | 1.1 | 0 | 0 | 2 | 0.7 | - | - | - | - | - | - |
| Strongylida | 1 | 0.5 | 0 | 0 | 1 | 0.3 | - | - | - | - | - | - |
| Helminths | 3 | 1.6 | 0 | 0 | 3 | 1.0 | - | - | - | - | - | - |
| Total | 149 | 80.1 | 9 | 12.7 | 158 | 52.7 | ||||||
| Parasites | Fruits | Vegetables | Total | Dif.Prop | EE (Dif.Prop) | 95% CI | RR | 95% CI | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ns = 146 | ns = 174 | ns = 320 | ||||||||||
| np | % | np | % | np | % | Ll | Ul | RR | ||||
| Blastocystis sp. | 48 | 32.9 | 44 | 25.3 | 92 | 28.8 | 0.0759 | 0.0510 | −0.0240 | 0.1758 | 1.3001 | 0.96–1.65 |
| Entamoeba spp. | 18 | 12.3 | 28 | 16.1 | 46 | 14.4 | −0.0376 | 0.0389 | −0.1140 | 0.0387 | 0.7661 | 0.22–1.32 |
| Endolimax nana | 11 | 7.5 | 7 | 4.0 | 18 | 5.6 | 0.0351 | 0.0264 | −0.0167 | 0.0869 | 1.8728 | 0.95–2.79 |
| Iodamoeba butschlii | 0 | 0 | 1 | 0.6 | 1 | 0.3 | −0.0057 | 0.0057 | - | - | - | - |
| Giardia spp. | 0 | 0 | 5 | 2.9 | 5 | 1.6 | −0.0287 | 0.0127 | - | - | - | - |
| Chilomastix spp. | 2 | 1.4 | 2 | 1.1 | 4 | 1.3 | 0.0022 | 0.0126 | −0.0224 | 0.0268 | 1.1918 | 0.00–3.14 |
| Balantidium spp. | 0 | 0 | 8 | 4.6 | 8 | 2.5 | −0.0460 | 0.0159 | - | - | - | - |
| Cryptosporidium spp. | 18 | 12.3 | 24 | 13.8 | 42 | 13.1 | −0.0146 | 0.0377 | −0.0886 | 0.0593 | 0.8938 | 0.32–1.46 |
| Cyclospora spp. | 32 | 21.9 | 60 | 34.5 | 92 | 28.8 | −0.1256 | 0.0497 | −0.2231 | −0.0282 | 0.6356 | 0.26–1.00 |
| Cystoisospora spp. | 0 | 0 | 2 | 1.1 | 2 | 0.6 | −0.0115 | 0.0081 | −0.0273 | 0.0043 | 0.0000 | - |
| Eimeria spp. | 28 | 19.2 | 37 | 21.3 | 65 | 20.31 | −0.0209 | 0.0450 | −0.1090 | 0.0673 | 0.9019 | 0.46–1.34 |
| Protozoa | 98 | 67.1 | 120 | 69.0 | 218 | 68.1 | −0.0184 | 0.0524 | −0.1210 | - | - | - |
| Ascaris spp. | 0 | 0 | 1 | 0.6 | 1 | 0.3 | −0.0057 | 0.0057 | - | - | - | - |
| Strongylida | 0 | 0 | 45 | 25.9 | 45 | 14.1 | −0.2586 | 0.0332 | - | - | - | - |
| Helminths | 0 | 0 | 46 | 26.4 | 46 | 14.4 | −0.2644 | 0.0334 | - | - | - | - |
| Total | 98 | 67.1 | 128 | 73.6 | 226 | 70.6 | ||||||
| Parasites | Communities of San Andrés | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | Total | ||||||||
| ns = 110 | ns = 122 | ns = 57 | ns = 32 | ns = 51 | ns = 24 | ns = 396 | ||||||||
| np = 107 | % | np = 122 | % | np = 55 | % | np = 32 | % | np = 49 | % | np =24 | % | np = 389 | % | |
| Blastocystis sp. | 91 | 82.7 | 103 | 84.4 | 53 | 93.0 | 29 | 90.6 | 45 | 88.2 | 22 | 91.7 | 343 | 86.6 |
| Entamoeba histolytica * | 31 | 28.2 | 39 | 32.0 | 18 | 31.6 | 12 | 37.5 | 15 | 29.4 | 9 | 37.5 | 124 | 31.3 |
| Entamoeba coli | 71 | 64.5 | 69 | 56.6 | 21 | 36.8 | 21 | 65.6 | 31 | 60.8 | 15 | 62.5 | 228 | 57.6 |
| Entamoeba hartmanni | 22 | 20.0 | 47 | 38.5 | 25 | 43.9 | 7 | 21.9 | 14 | 27.5 | 5 | 20.8 | 120 | 30.3 |
| Endolimax nana | 75 | 68.2 | 88 | 72.1 | 43 | 75.4 | 20 | 62.5 | 37 | 72.5 | 12 | 50.0 | 275 | 69.4 |
| Iodamoeba butschlii | 77 | 70.0 | 17 | 13.9 | 4 | 7.0 | 4 | 12.5 | 9 | 17.6 | 4 | 16.7 | 115 | 29.0 |
| Giardia duodenalis | 11 | 10.0 | 8 | 6.6 | 4 | 7.0 | 4 | 12.5 | 12 | 23.5 | 1 | 4.2 | 40 | 10.1 |
| Chilomastix mesnili | 27 | 24.5 | 21 | 17.2 | 8 | 14.0 | 9 | 28.1 | 10 | 19.6 | 2 | 8.3 | 77 | 19.4 |
| Retortamona intestinalis | 2 | 1.8 | 1 | 0.8 | 0 | 0 | 1 | 3.1 | 0 | 0 | 0 | 0 | 4 | 1.0 |
| Cryptosporidium spp. | 4 | 3.6 | 5 | 4.1 | 1 | 1.8 | 2 | 6.3 | 0 | 0 | 2 | 8.3 | 14 | 3.5 |
| Cyclospora cayetanesis | 2 | 1.8 | 10 | 8.2 | 3 | 5.3 | 0 | 0 | 2 | 3.9 | 0 | 0 | 17 | 4.3 |
| Protozoa | 107 | 97.3 | 122 | 100 | 55 | 96.5 | 32 | 100 | 49 | 96.1 | 24 | 100 | 389 | 98.2 |
| Ascaris lumbricoides | 2 | 1.8 | 4 | 3.3 | 2 | 3.5 | 0 | 0 | 8 | 15.7 | 1 | 4.2 | 17 | 4.3 |
| Trichuris trichiura | 1 | 0.9 | 2 | 1.6 | 2 | 3.5 | 0 | 0 | 0 | 0 | 1 | 4.2 | 6 | 1.5 |
| Ancylostomidae ** | 0 | 0 | 1 | 0.8 | 1 | 1.8 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0.5 |
| Enterobius vermicularis | 1 | 0.9 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0.3 |
| Hymenolepis nana | 0 | 0 | 3 | 2.5 | 7 | 12.3 | 0 | 0 | 3 | 5.9 | 2 | 8.3 | 15 | 3.8 |
| Helminths | 4 | 3.6 | 8 | 6.6 | 9 | 15.8 | 0 | 0 | 9 | 17.6 | 3 | 12.5 | 33 | 8.3 |
| Total | 107 | 97.3 | 122 | 100 | 55 | 96.5 | 32 | 100 | 49 | 96.1 | 24 | 100 | 389 | 98.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-Ramírez, L.C.; Robalino-Flores, X.; De la Torre, E.; Parra-Mayorga, P.; Prato, J.G.; Trelis, M.; Fuentes, M.V. Influence of Environmental Pollution and Living Conditions on Parasite Transmission among Indigenous Ecuadorians. Int. J. Environ. Res. Public Health 2022, 19, 6901. https://doi.org/10.3390/ijerph19116901
González-Ramírez LC, Robalino-Flores X, De la Torre E, Parra-Mayorga P, Prato JG, Trelis M, Fuentes MV. Influence of Environmental Pollution and Living Conditions on Parasite Transmission among Indigenous Ecuadorians. International Journal of Environmental Research and Public Health. 2022; 19(11):6901. https://doi.org/10.3390/ijerph19116901
Chicago/Turabian StyleGonzález-Ramírez, Luisa Carolina, Ximena Robalino-Flores, Eliana De la Torre, Paúl Parra-Mayorga, José Gregorio Prato, María Trelis, and Màrius Vicent Fuentes. 2022. "Influence of Environmental Pollution and Living Conditions on Parasite Transmission among Indigenous Ecuadorians" International Journal of Environmental Research and Public Health 19, no. 11: 6901. https://doi.org/10.3390/ijerph19116901
APA StyleGonzález-Ramírez, L. C., Robalino-Flores, X., De la Torre, E., Parra-Mayorga, P., Prato, J. G., Trelis, M., & Fuentes, M. V. (2022). Influence of Environmental Pollution and Living Conditions on Parasite Transmission among Indigenous Ecuadorians. International Journal of Environmental Research and Public Health, 19(11), 6901. https://doi.org/10.3390/ijerph19116901








