An Assessment of Airborne Bacteria and Fungi in the Female Dormitory Environment: Level, Impact Factors and Dose Rate
Abstract
:1. Introduction
2. Materials and Methods
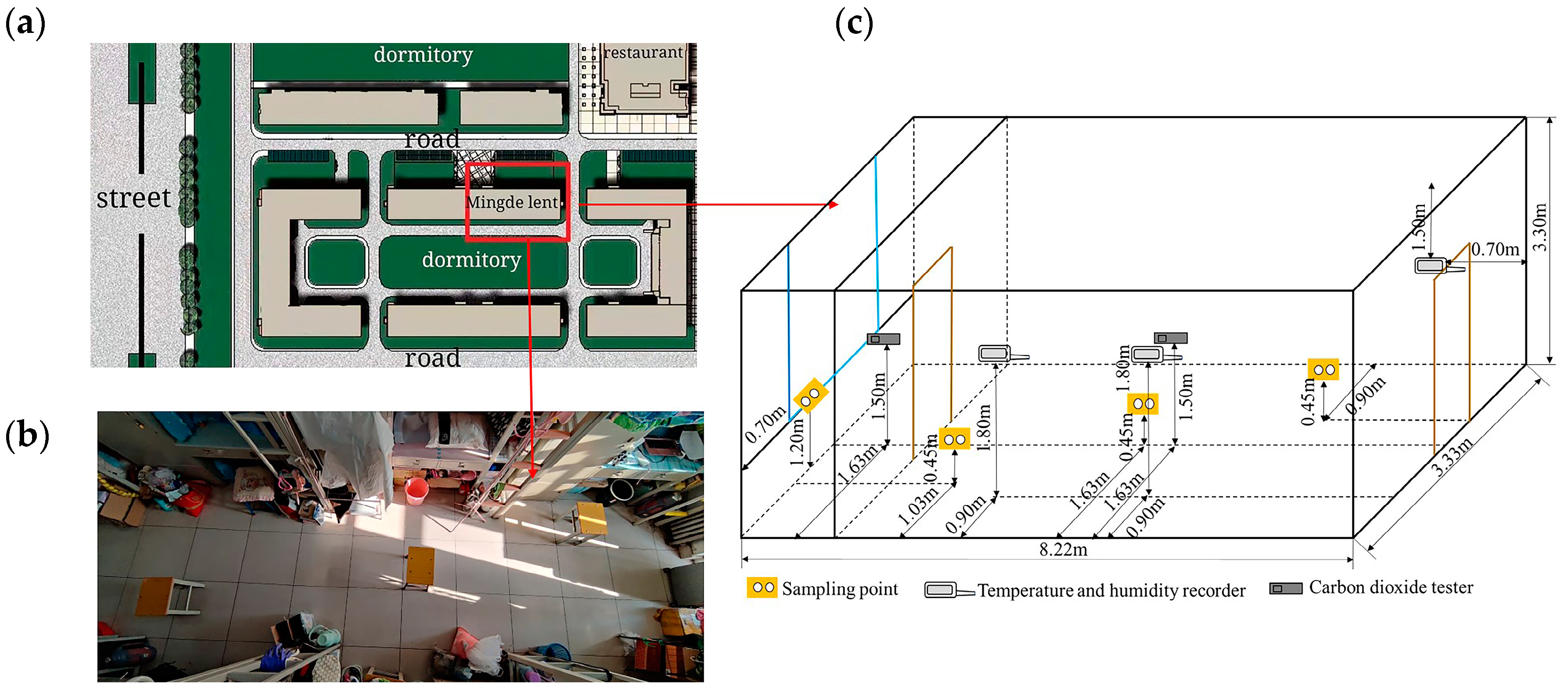
2.1. Sampling Positions
2.2. Sampling Time and Instruments
2.3. Dose Rate Assessment
2.4. Species Identification Method
2.5. Data Analysis
3. Results and Discussion
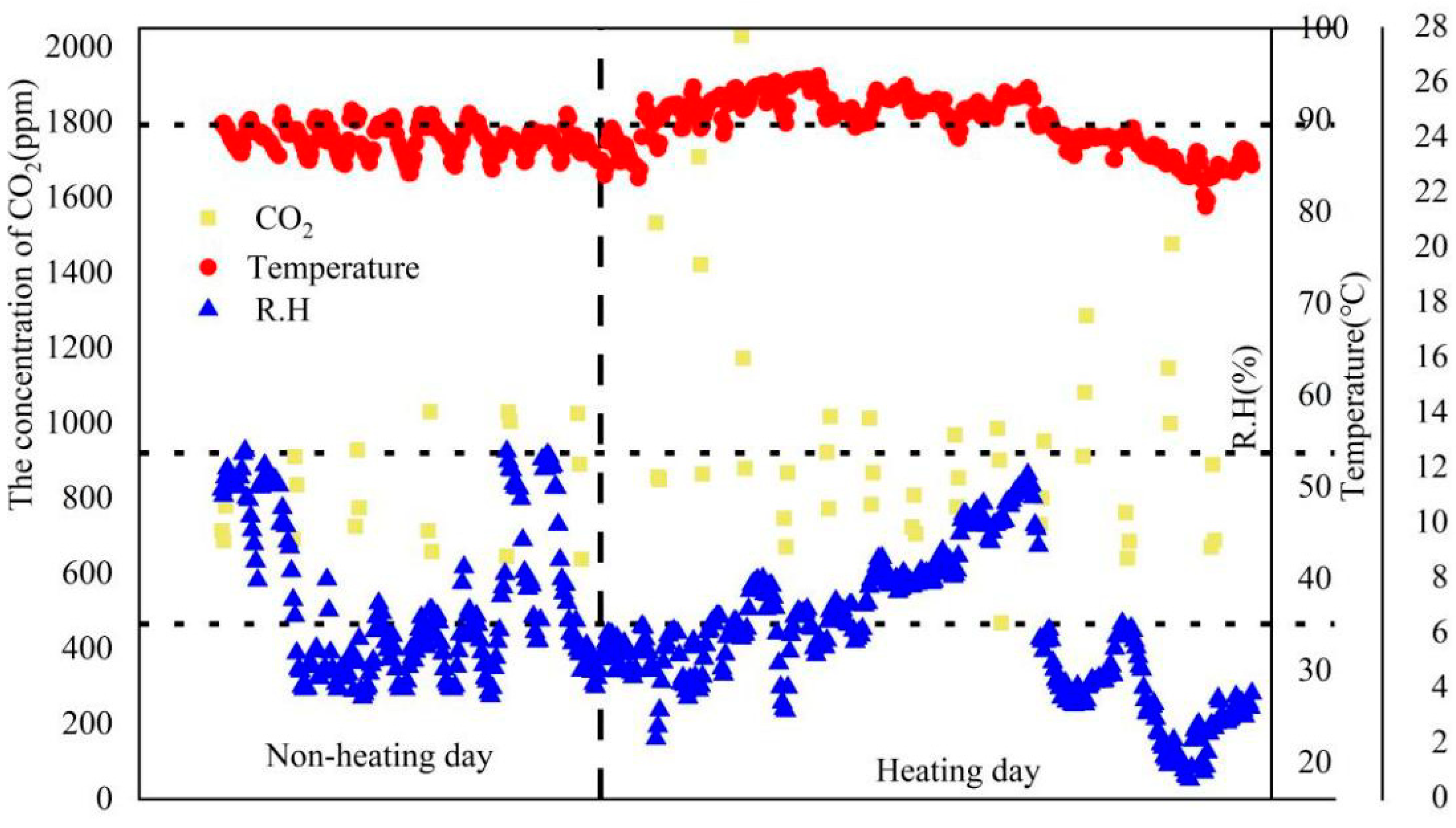
3.1. Temperature, RH and CO2 of the Tested Female Dorm
3.2. Total Bacterial and Fungal Counts in the Female Dormitory
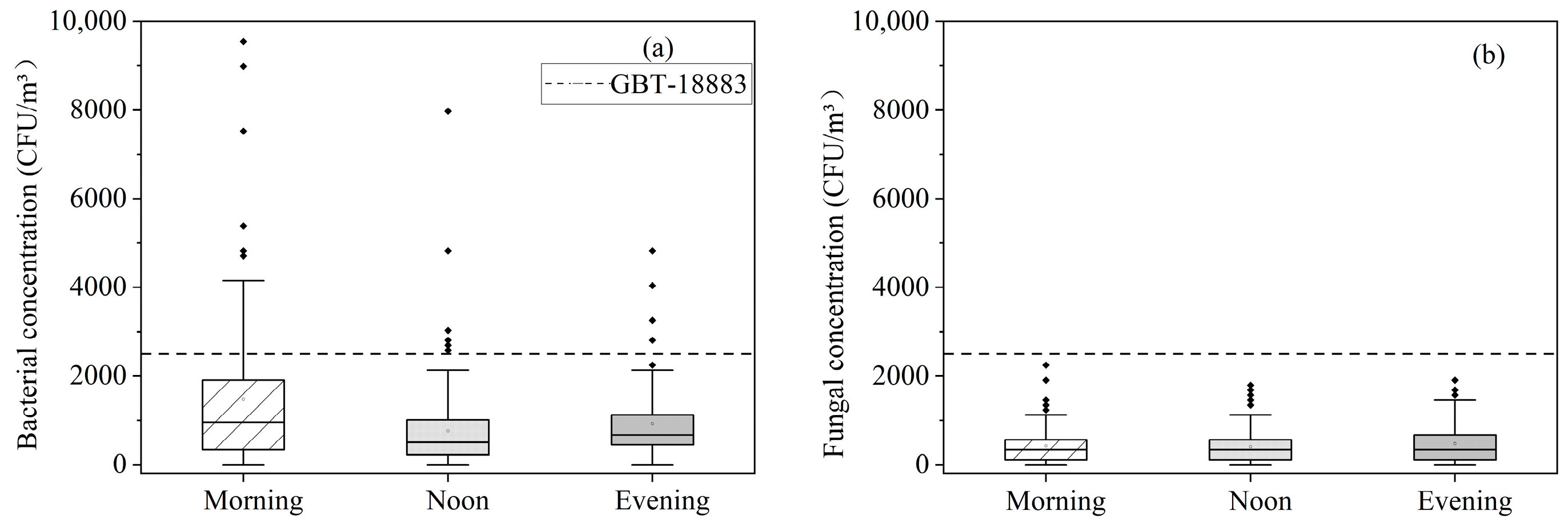
3.2.1. Concentration of Microbes Detected during the Sampling Period
3.2.2. Species of Microbe Tested during the Sampling Period
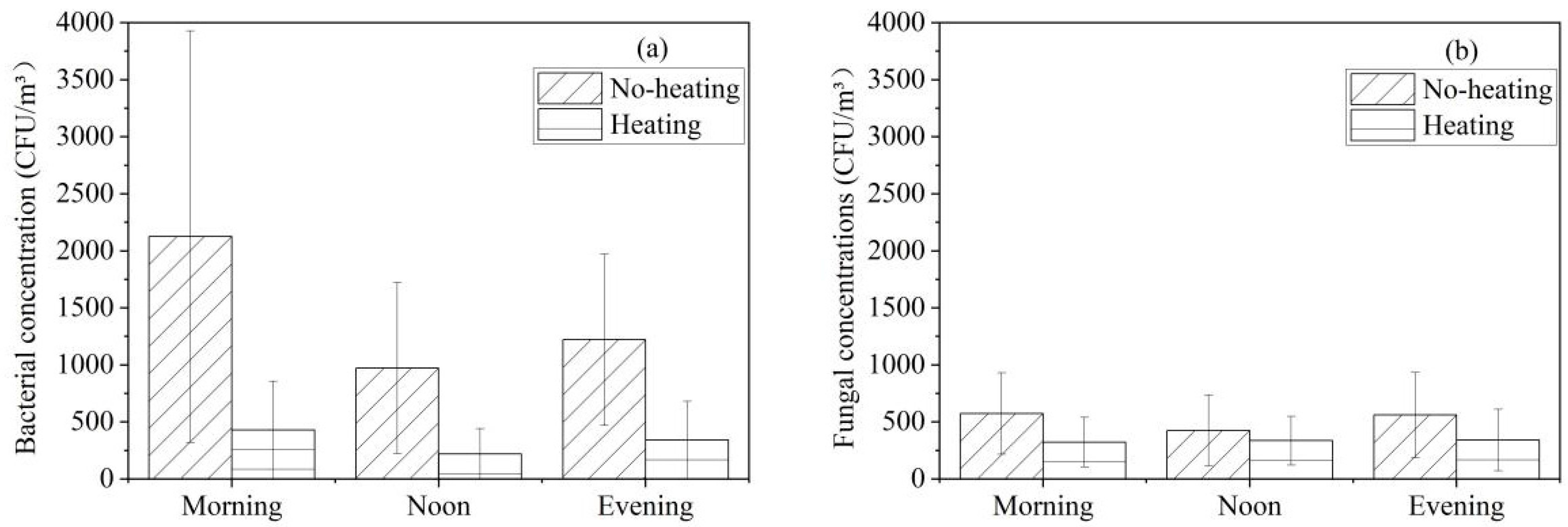
3.3. Effect of Heating on Culturable Airborne Bacterial and Fungal Concentrations
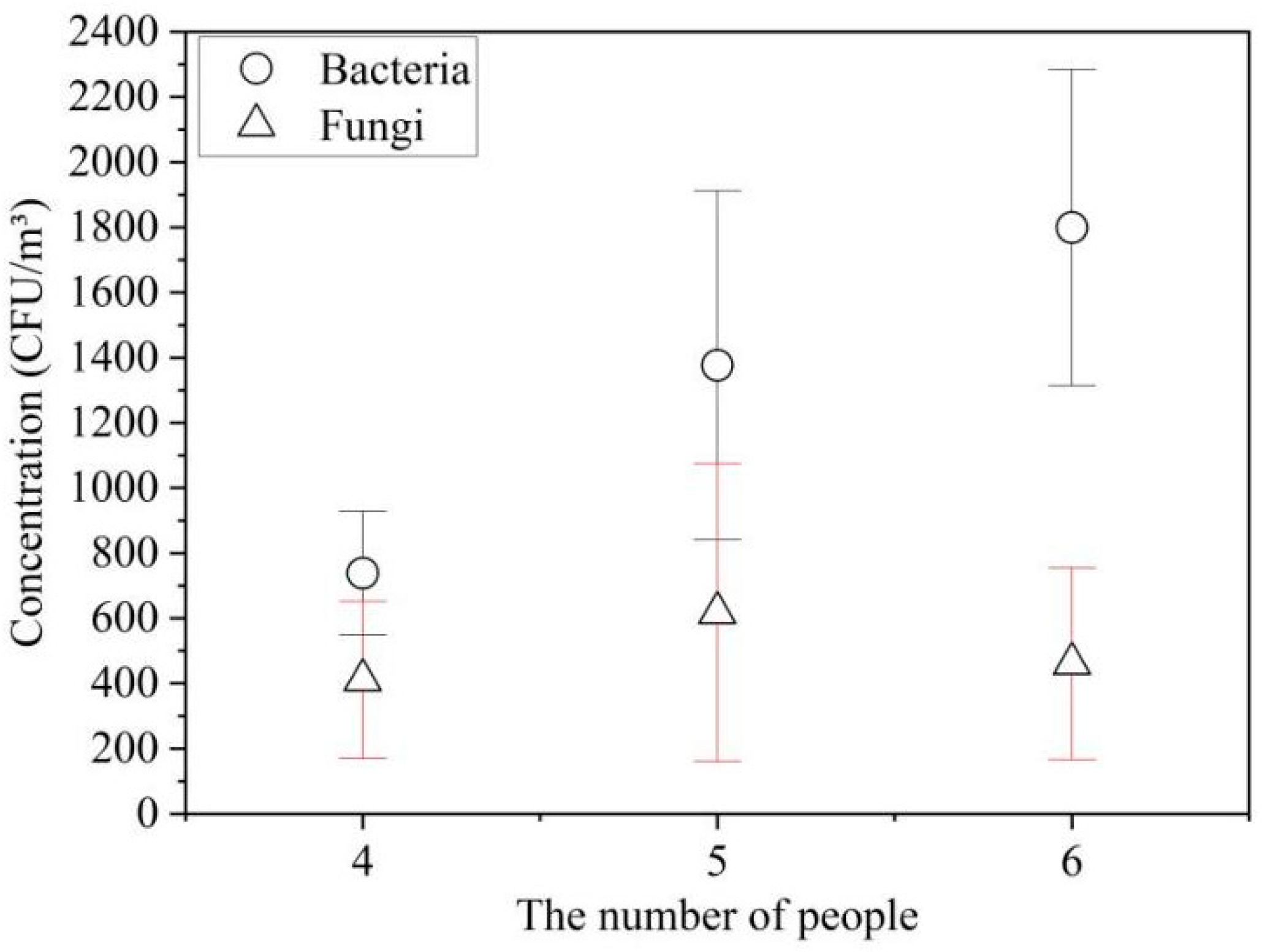
3.4. Effect of the Number of Female Occupants on Bacteria and Fungi
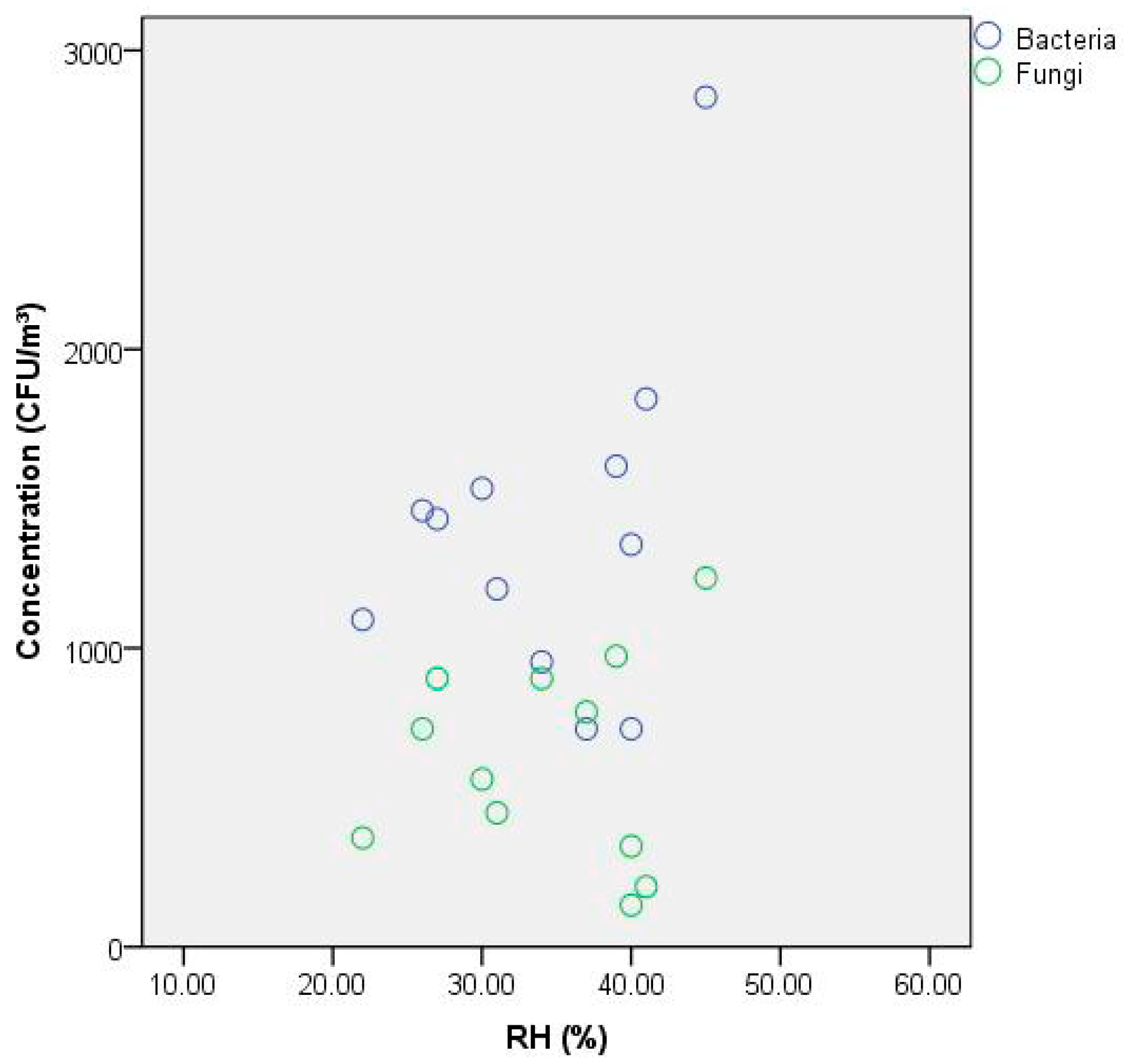
3.5. Effect of RH on Bacterial and Fungal Concentration
3.6. Dose Rate of Bacteria and Fungi
3.7. Limitations
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Klepeis, N.E.; Nelson, W.C.; Ott, W.R.; Robinson, J.P.; Tsang, A.M.; Switzer, P.; Behar, J.V.; Hern, S.C.; Engelmann, W.H. The National Human Activity Pattern Survey (NHAPS): A resource for assessing exposure to environmental pollutants. J. Expo Anal. Environ. Epidemiol. 2001, 11, 231–252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jenkins, P.L.; Phillips, T.J.; Mulberg, E.J.; Hui, S.P. Activity patterns of Californians: Use of and proximity to indoor pollutant sources. Atmos. Environ. Part A Gen. Top. 1992, 26, 2141–2148. [Google Scholar] [CrossRef]
- Burrell, R. Microbiological agents as health risks in indoor air. Environ. Health Perspect. 1991, 95, 29–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Douwes, J.; Thorne, P.; Pearce, N.; Heederik, D. Bioaerosol health effects and exposure assessment: Progress and prospects. Ann. Occup. Hyg. 2003, 47, 187–200. [Google Scholar] [CrossRef] [Green Version]
- Diba, K.; Alizadeh, Z.; Mokhtari, F.; Yekta, Z. Common fungi and major factors of the contamination of student dormitories indoor. J. Res. Appl. Basic Med. Sci. 2019, 5, 44–49. [Google Scholar]
- Faridi, S.; Hassanvand, M.S.; Naddafi, K.; Yunesian, M.; Nabizadeh, R.; Sowlat, M.H.; Kashani, H.; Gholampour, A.; Nizai, S.; Zare, A.; et al. Indoor/outdoor relationships of bioaerosol concentrations in a retirement home and a school dormitory. Environ. Sci. Pollut. Res. 2015, 22, 8190–8200. [Google Scholar] [CrossRef]
- Chew, G.L.; Rogers, C.; Burge, H.A.; Muilenberg, M.L.; Gold, D.R. Dustborne and airborne fungal propagules represent a different spectrum of fungi with differing relations to home characteristics. Allergy Eur. J. Allergy Clin. Immunol. 2003, 58, 13–20. [Google Scholar] [CrossRef]
- Kalogerakis, N.; Paschali, D.; Lekaditis, V.; Pantidou, A.; Eleftheriadis, K.; Lazaridis, M. Indoor air quality—Bioaerosol measurements in domestic and office premises. J. Aerosol. Sci. 2005, 36, 751–761. [Google Scholar] [CrossRef]
- Degois, J.; Veillette, M.; Poulin, P.; Lévesque, B.; Aubin, D.; Ouazia, B.; Brisson, M.; Maltais, F.; Duchaine, C. Indoor air quality assessment in dwellings with different ventilation strategies in nunavik and impacts on bacterial and fungal microbiota. Indoor Air 2021, 31, 2213–2225. [Google Scholar] [CrossRef]
- Yan, X.; Qiu, D.; Zheng, S.; Yang, J.; Sun, H.Y.; Wei, Y.; Han, J.R.; Sun, J.H.; Su, X.F. Distribution characteristics and noncarcinogenic risk assessment of culturable airborne bacteria and fungi during winter in Xinxiang, China. Environ. Sci. Pollut. Res. 2019, 26, 36698–36709. [Google Scholar] [CrossRef]
- Frankel, M.; Bekö, G.; Timm, M.; Gustavsen, S.; Hansen, W.E.; Madsen, S.M. Seasonal variations of indoor microbial exposures and their relation to temperature, relative humidity, and air exchange rate. Appl. Environ. Microbiol. 2012, 78, 8289–8297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Islam, A.; Ikeguchi, A.; Naide, T. Concentrations of Aerosol Numbers and Airborne Bacteria, and Temperature and Relative Humidity, and Their Interrelationships in a Tie-Stall Dairy Barn. Animals 2019, 9, 1023. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, M.; Liu, J.; Ren, J.; Liu, L.; Chen, R.Q.; Li, Y.J. Bacterial community in commercial airliner cabins in China. Int. J. Environ. Health Res. 2020, 30, 284–295. [Google Scholar] [CrossRef] [PubMed]
- Prussin, A.J.; Garcia, E.B.; Marr, L.C. Total concentrations of virus and bacteria in indoor and outdoor air. Environ. Sci. Technol. Lett. 2015, 2, 84–88. [Google Scholar] [CrossRef] [PubMed]
- Fatemeh, S.; Mohammad, J.; Mehdi, V.; Malek, A.; Elham, R.; Mehdi, F.; Sahar, G.; Aylar, B. Assessment of types of bacterial bio-aerosols and concentrations in the indoor air of gyms. Environ. Geochem. Health 2021, 43, 2165–2173. [Google Scholar] [CrossRef]
- Halloran, S.K.; Wexler, A.S.; Ristenpart, W.D. A comprehensive breath plume model for disease transmission via expiratory aerosols. PLoS ONE 2012, 7, e37088. [Google Scholar] [CrossRef] [Green Version]
- Jo, W.K.; Seo, Y.J. Indoor and outdoor bioaerosol levels at recreation facilities, elementary schools, and homes. Chemosphere 2005, 61, 1570–1579. [Google Scholar] [CrossRef]
- Joana, S. Association between home characteristics and occupant’s behaviours and concentrations of bacteria, fungi and endotoxins. J. Build. Eng. 2022, 45, 103409. [Google Scholar] [CrossRef]
- Heo, K.J.; Lim, C.E.; Kim, H.B.; Lee, B.U. Effects of human activities on concentrations of culturable bioaerosols in indoor air environments. J. Aerosol. Sci. 2017, 104, 58–65. [Google Scholar] [CrossRef]
- Luongo, J.C.; Barberán, A.; Hacker-Cary, R.; Morgan, E.E.; Miller, S.L.; Fierer, N. Microbial analyses of airborne dust collected from dormitory rooms predict the sex of occupants. Indoor Air 2017, 27, 338–344. [Google Scholar] [CrossRef]
- Mirhoseini, S.H.; Nikaeen, M.; Satoh, K.; Makimura, K. Assessment of airborne particles in indoor environments: Applicability of particle counting for prediction of bioaerosol concentrations. Aerosol. Air Qual. Res. 2016, 16, 1903–1910. [Google Scholar] [CrossRef]
- Duquenne, P.; Marchand, G.; Duchaine, C. Measurement of endotoxins in bioaerosols at workplace: A critical review of literature and a standardization Issue. Ann. Occup. Hyg. 2013, 57, 137–172. [Google Scholar] [CrossRef] [PubMed]
- Mandal, J.; Brandl, H. Bioaerosols in indoor environment—A review with special reference to residential and occupational locations. Open Environ. Biol. Monit. J. 2011, 4, 83–96. [Google Scholar] [CrossRef] [Green Version]
- Lee, B.G.; Yang, J.I.; Kim, E.; Sun, W.G.; Park, J.; Yeo, M. Investigation of bacterial and fungal communities in indoor and outdoor air of elementary school classrooms by 16S rRNA gene and ITS region sequencing. Indoor Air 2021, 31, 1553–1562. [Google Scholar] [CrossRef]
- Pereira, E.L.; Madacussengua, O.; Baptista, P.; Feliciano, M. Assessment of indoor air quality in geriatric environments of southwestern Europe. Aerobiologia 2021, 37, 139–153. [Google Scholar] [CrossRef]
- Castro, D.; Slezakova, K.; Delerue-Matos, C.; Alvim-Ferraz, M.C.; Morais, S.; Pereira, M.C. Polycyclic aromatic hydrocarbons in gas and particulate phases of indoor environments influenced by tobacco smoke: Levels, phase distributions, and health risks. Atmos. Environ. 2011, 45, 1799–1808. [Google Scholar] [CrossRef] [Green Version]
- Fonseca, J.; Slezakova, K.; Morais, S.; Pereira, M.C. Assessment of ultrafine particles in Portuguese preschools: Levels and exposure doses. Indoor Air 2014, 24, 618–628. [Google Scholar] [CrossRef] [Green Version]
- Madureira, J.; Paciência, I.; Rufo, J.C.; Pereira, C.; Teixeira, J.P.; Fernandes, E.O. Assessment and determinants of airborne bacterial and fungal concentrations in different indoor environments: Homes, child day-care centres, primary schools and elderly care centres. Atmos. Environ. 2015, 109, 139–146. [Google Scholar] [CrossRef] [Green Version]
- Wu, Z.; Lyu, H.; Liang, W.; Jing, X.; Wang, Y.; Ma, X. Microbial community in indoor dusts from university dormitories: Characteristics, potential pathogens and influence factors. Atmos. Pollut. Res. 2021, 12, 321–333. [Google Scholar] [CrossRef]
- Wu, Z.; Lyu, H.; Ma, X.; Ren, G.; Song, J.; Jing, X.; Liu, Y. Comparative effects of environmental factors on bacterial communities in two types of indoor dust: Potential risks to university students. Environ. Res. 2022, 203, 111869. [Google Scholar] [CrossRef]
- Andualem, Z.; Ayenew, Y.; Ababu, T.; Hailu, B. Assessment of Airborne Culturable Fungal Load in an Indoor Environment of Dormitory Rooms:The Case of University of Gondar Student’s Dormitory Rooms, Northwest Ethiopia. Air Soil Water Res. 2020, 13, 1–7. [Google Scholar] [CrossRef]
- Fu, X.; Li, Y.; Meng, Y.; Yuan, Q.; Zhang, Z.; Norbäck, D.; Deng, Y.; Zhang, X.; Sun, Y. Associations between respiratory infections and bacterial microbiome in student dormitories in Northern China. Indoor Air 2020, 30, 816–826. [Google Scholar] [CrossRef] [PubMed]
- Mota, L.C.; Gibbs, S.G.; Green, C.F.; Payan, F.; Ortiz, M. Characterization of seasonal indoor and outdoor bioaerosols in the arid environment of El Paso, Texas. J. Environ. Health 2008, 70, 48–53. [Google Scholar]
- Basilico, M.D.Z.; Chiericatti, C.; Aringoli, E.E.; Althaus, R.L.; Basilico, J.C. Influence of environmental factors on airborne fungi in houses of Santa Fe City, Argentina. Sci. Total. Environ. 2007, 376, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Ge, Y.; Wu, C.; Guan, D.; Liu, J.; Wang, F. Assessment of culturable airborne bacteria of indoor environments in classrooms, dormitories and dining hall at university: A case study in China. Aerobiologia 2020, 36, 313–324. [Google Scholar] [CrossRef]
- GB/T 18883; Standardization Administration of the People’s Republic of China (SAC) Indoor Air Quality Standard. Standards Press of China: Beijing, China, 2003.
- Menteşe, S.; Arisoy, M.; Rad, A.Y.; Güllü, G. Bacteria and fungi levels in various indoor and outdoor environments in Ankara, Turkey. Clean-Soil Air Water 2009, 37, 487–493. [Google Scholar] [CrossRef]
- Hayleeyesus, S.F.; Ejeso, A.; Derseh, F.A. Quantitative assessment of bio-aerosols contamination in indoor air of university dormitory rooms. Int. J. Health Sci. 2015, 9, 249–256. [Google Scholar] [CrossRef]
- Ministry of Environment Protection of the People’s Republic of China (MEPC). Exposure Factors Handbook of Chinese Population (Adults); China Environmental Press: Beijing, China, 2013. [Google Scholar]
- Adams, R.I.; Miletto, M.; Lindow, S.E.; Taylor, J.W.; Bruns, T.D. Airborne bacterial communities in residences: Similarities and differences with fungi. PLoS ONE 2014, 9, e91283. [Google Scholar] [CrossRef]
- Castellanos-Arévalo, A.P.; Camarena-Pozos, D.A.; Castellanos-Arévalo, D.C.; Rangel-Córdova, A.A.; Peña-Cabriales, J.J.; Arévalo-Rivas, B.; Peña, D.G.; Maldonado-Vega, M. Microbial contamination in the indoor environment of tanneries in Leon, Mexico. Indoor Built. Environ. 2016, 25, 524–540. [Google Scholar] [CrossRef]
- Hayleeyesus, S.F.; Manaye, A.M. Microbiological quality of indoor air in University libraries. Asian Pac. J. Trop. Biomed. 2014, 4, S312–S317. [Google Scholar] [CrossRef] [Green Version]
- Qian, J.; Hospodsky, D.; Yamamoto, N.; Nazaroff, W.W.; Peccia, J. Size-resolved emission rates of airborne bacteria and fungi in an occupied classroom. Indoor Air 2012, 22, 339–351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soto, T.; Murcia, G.; Franco, A.; Vicente-Soler, J.; Cansado, J.; Gacto, M. Indoor Airborne Microbial Load in a Spanish University (University of Murcia, Spain). Ann. Biol. 2009, 31, 109–115. [Google Scholar]
- Kooken, J.M.; Fox, K.F.; Fox, A. Characterization of micrococcus strains isolated from indoor air. Mol. Cell. Probes. 2012, 26, 1–5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gneiding, K.; Frodl, R.; Funke, G. Identities of Microbacterium spp. encountered in human clinical specimens. J. Clin. Microbiol. 2008, 46, 3646–3652. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krajewska-Kułak, E.; Lukaszuk, C.; Tsokantaridis, C.; Hatzopoulu, A.; Theodosopoyloy, E.; Hatzmanasi, D.; Kosmois, D. Indoor air studies of fungi contamination at the neonatal department and intensive care unit an palliative care in kavala hospital in greece. Adv. Med. Sci. 2007, 52 (Suppl. S1), 11–14. [Google Scholar]
- Dannemiller, K.C.; Weschler, C.J.; Peccia, J. Fungal and bacterial growth in floor dust at elevated relative humidity levels. Indoor Air 2017, 27, 354–363. [Google Scholar] [CrossRef]
- Hospodsky, D.; Qian, J.; Nazaroff, W.W.; Yamamoto, N.; Bibby, K.; Rismani-Yazdi, H.; Peccia, J. Human occupancy as a source of indoor airborne bacteria. PLoS ONE 2012, 7, e34867. [Google Scholar] [CrossRef] [Green Version]
- Heo, K.J.; Lee, B.U. Seasonal variation in the concentrations of culturable bacterial and fungal aerosols in underground subway systems. J. Aerosol. Sci. 2016, 92, 122–129. [Google Scholar] [CrossRef]
- Kettleson, E.M.; Adhikari, A.; Vesper, S.; Coombs, K.; Indugula, R.; Reponen, T. Key Determinants of the Fungal and Bacterial Microbiomes in Homes. Environ. Res. 2015, 138, 130–135. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.H.; Jo, W.K. Exposure to airborne fungi and bacteria while commuting in passenger cars and public buses. Atmos. Environ. 2005, 39, 7342–7350. [Google Scholar] [CrossRef]
- Green, C.F.; Scarpino, P.V.; Gibbs, S.G. Assessment and modeling of indoor fungal and bacterial bioaerosol concentrations. Aerobiologia 2003, 19, 159–169. [Google Scholar] [CrossRef]
- Ye, J.; Qian, H.; Zhang, J.; Sun, F.; Zhuge, Y.; Zheng, X.; Cao, G. Concentrations and size-resolved i/o ratios of household airborne bacteria and fungi in nanjing, southeast china. Sci. Total Environ. 2021, 774, 145559. [Google Scholar] [CrossRef]
| Sampling Time | Temperature (°C) | Wind Speed (m s−1) | RH (%) | Weather |
|---|---|---|---|---|
| No heating | 14 ± 5 °C | 3.7 ± 1.5 | 56 ± 18 | Sunny, calm and cloudy |
| Heating | 9 ± 6 °C | 4.0 ± 1.9 | 59 ± 24 | Sunny, calm, cloudy, rainy, snowy |
| Exposure Pollutants | Dose Rate (CFU/(Kg·d)) | |
|---|---|---|
| Sleeping | Reading | |
| Bacteria | 48 | 27 |
| Fungi | 19 | 11 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Wang, X.; Cao, G.; Wang, Y.; Miao, Q.; He, J. An Assessment of Airborne Bacteria and Fungi in the Female Dormitory Environment: Level, Impact Factors and Dose Rate. Int. J. Environ. Res. Public Health 2022, 19, 6642. https://doi.org/10.3390/ijerph19116642
Li Y, Wang X, Cao G, Wang Y, Miao Q, He J. An Assessment of Airborne Bacteria and Fungi in the Female Dormitory Environment: Level, Impact Factors and Dose Rate. International Journal of Environmental Research and Public Health. 2022; 19(11):6642. https://doi.org/10.3390/ijerph19116642
Chicago/Turabian StyleLi, Yanju, Xinyu Wang, Guoqing Cao, Yu Wang, Qingqing Miao, and Jinlu He. 2022. "An Assessment of Airborne Bacteria and Fungi in the Female Dormitory Environment: Level, Impact Factors and Dose Rate" International Journal of Environmental Research and Public Health 19, no. 11: 6642. https://doi.org/10.3390/ijerph19116642
APA StyleLi, Y., Wang, X., Cao, G., Wang, Y., Miao, Q., & He, J. (2022). An Assessment of Airborne Bacteria and Fungi in the Female Dormitory Environment: Level, Impact Factors and Dose Rate. International Journal of Environmental Research and Public Health, 19(11), 6642. https://doi.org/10.3390/ijerph19116642





