Natural Cyanobacteria Removers Obtained from Bio-Waste Date-Palm Leaf Stalks and Black Alder Cone-Like Flowers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Materials
2.2.1. Carbonization Process
2.2.2. Activation Process
2.3. Characterization of Materials
2.3.1. Proximate/Ultimate Analysis and Lignocellulose Composition of Raw Samples
2.3.2. X-ray Powder Diffraction (XRD)
2.3.3. Fourier-Transform Infrared Spectroscopy (FTIR)
2.3.4. Nitrogen Physisorption at −196 °C
2.3.5. Field Emission Scanning Electron Microscopy (FESEM)
2.4. Qualitative and Quantitative Phytoplankton Analysis
2.5. Analysis of Adsorption Properties of Material
3. Results and Discussion
3.1. Results of Physical and Chemical Characterization of Materials
3.1.1. Lignocellulose Composition
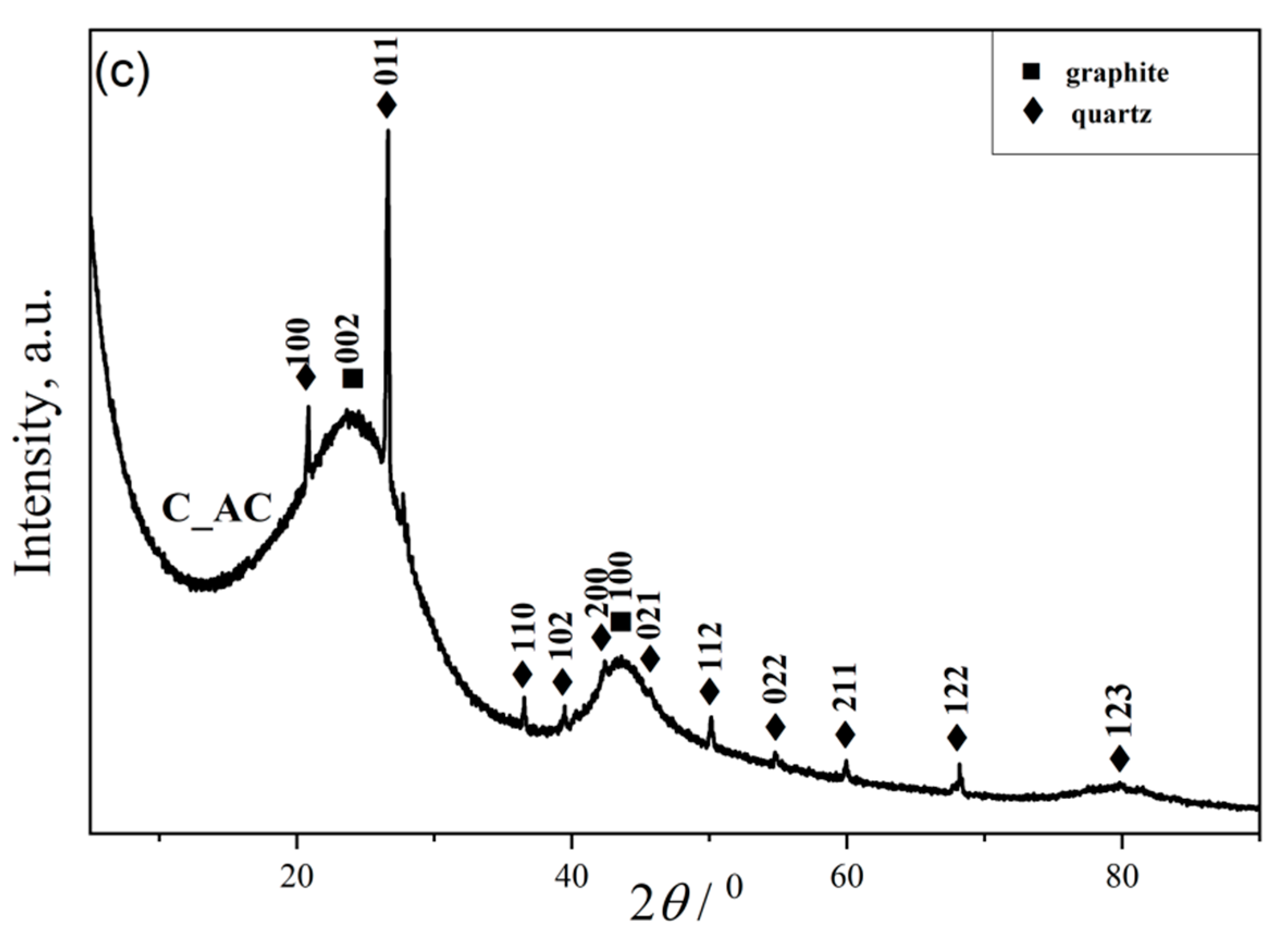
3.1.2. Results of X-ray Powder Diffraction
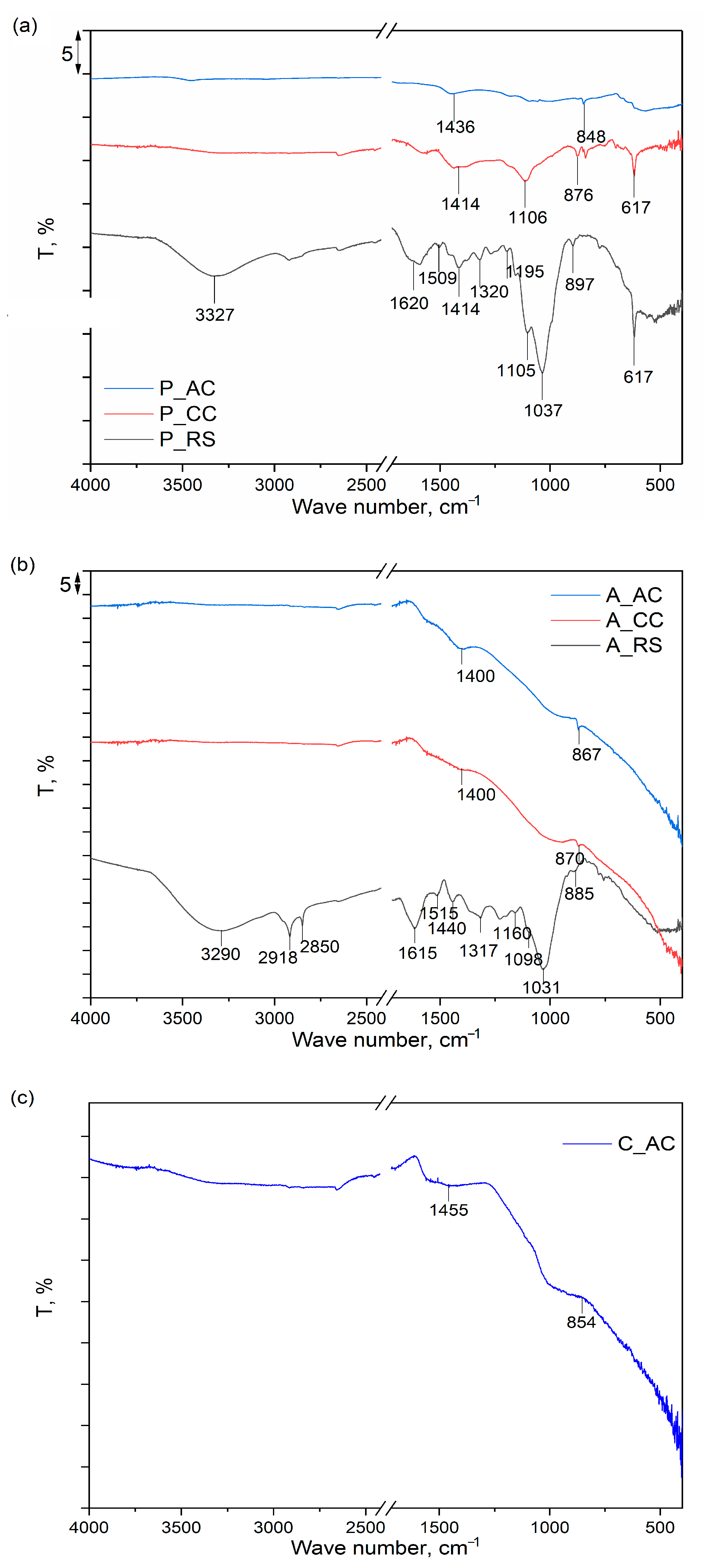
3.1.3. Results of Fourier-Transform Infrared Spectroscopy
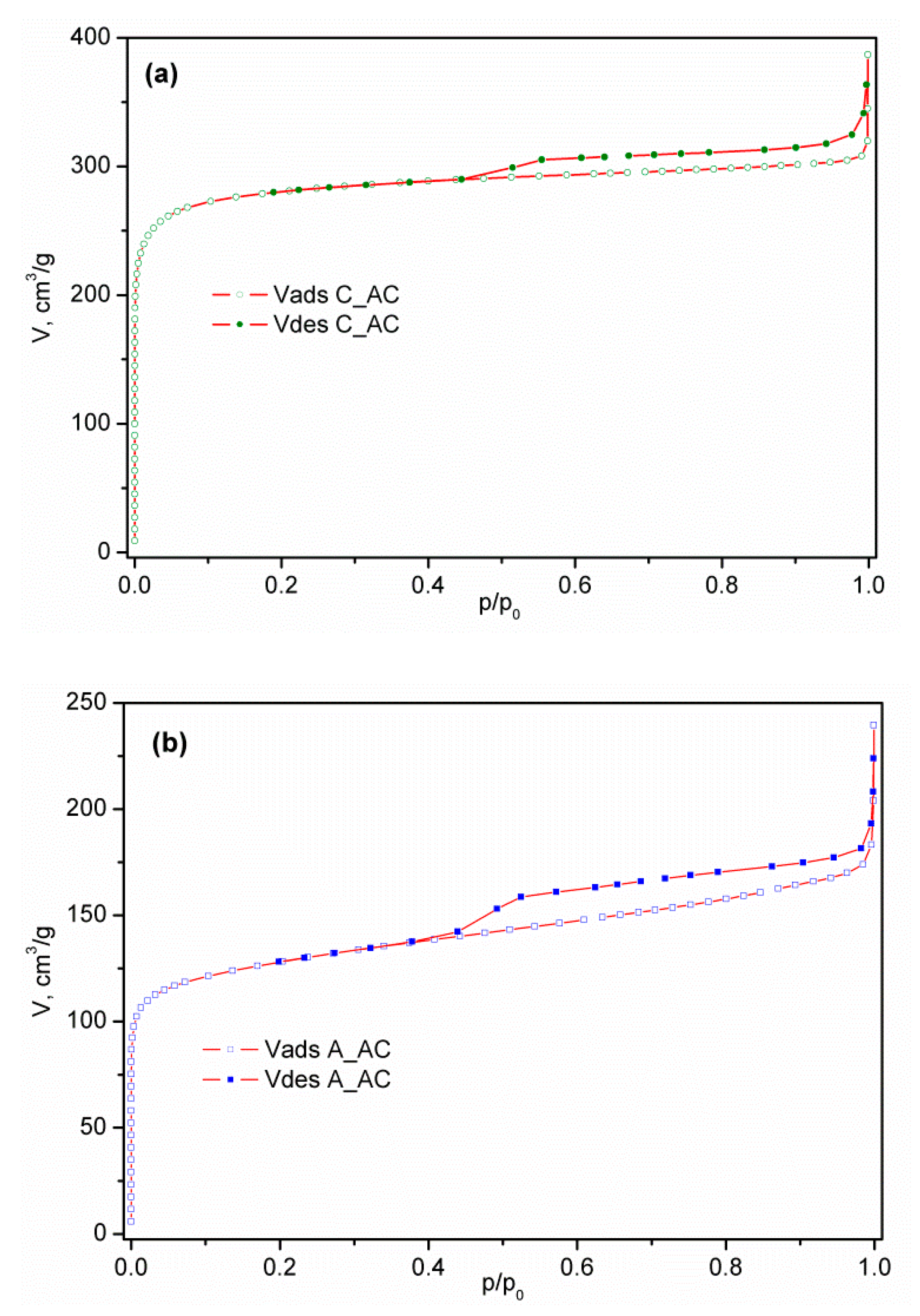
3.1.4. Results of Low Temperature N2 Physisorption
3.1.5. Results Field Emission Scanning Electron Microscopy
3.2. Results of Qualitative and Quantitative Phytoplankton Analysis
3.2.1. Results of Qualitative Phytoplankton Analysis
3.2.2. Results of Quantitative Phytoplankton Analysis
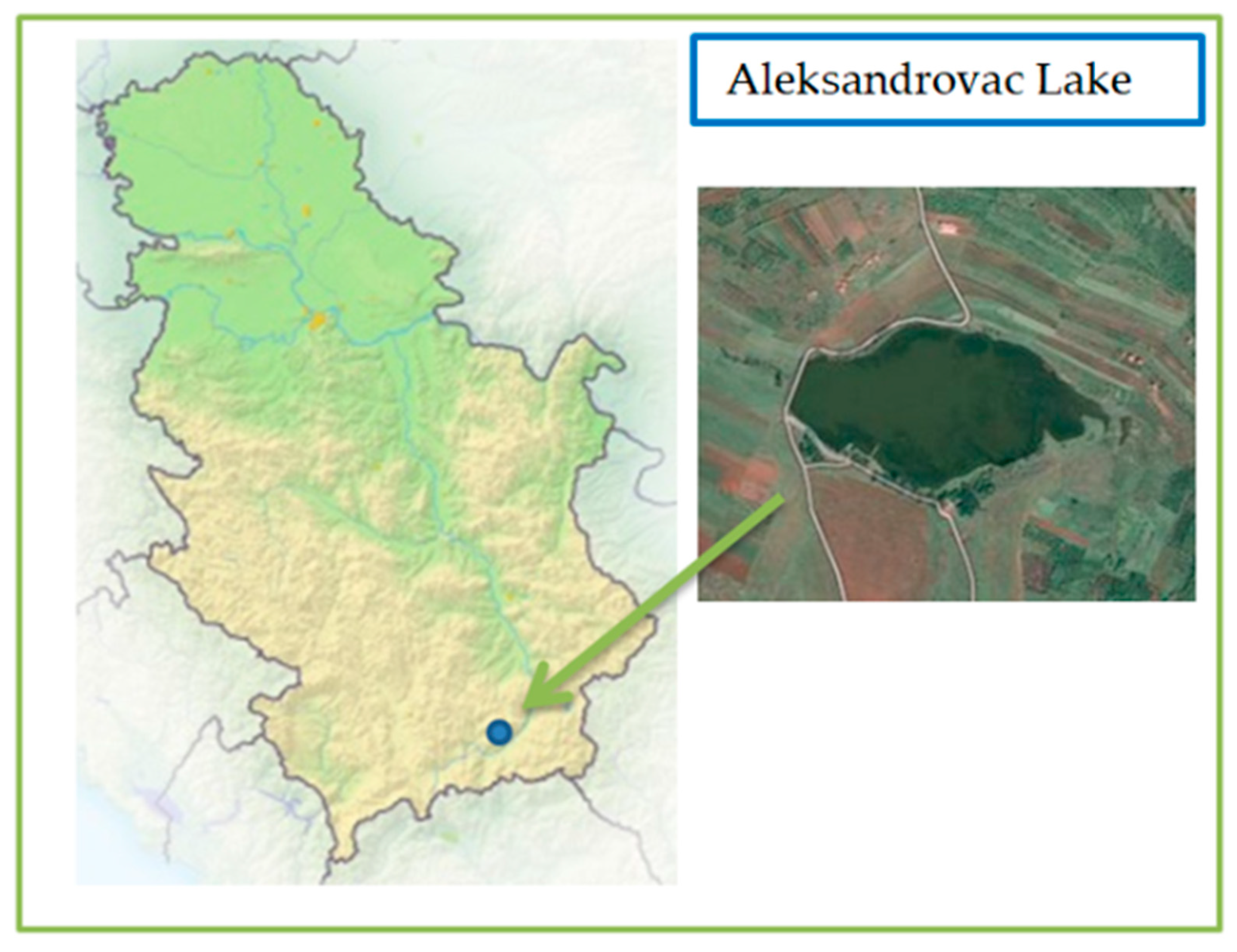
3.2.3. Qualitative and Quantitative Analysis of Cyanobacteria from Aleksandrovac Lake before and after the Water Treatment
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Merel, S.; Walker, D.; Chicana, R.; Snyder, S.; Baurès, E.; Thomas, O. State of knowledge and concerns on cyanobacterial blooms and cyanotoxins. Environ. Int. 2013, 59, 303–327. [Google Scholar] [CrossRef] [PubMed]
- Paerl, H.W.; Huisman, J. Climate change: A catalyst for global expansion of harmful cyanobacterial blooms. Environ. Microbiol. Rep. 2009, 1, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Walker, H.W. Harmful Algae Blooms in Drinking Water: Removal of Cyanobacterial Cells and Toxins; CRC Press: Boca Raton, FL, USA, 2014. [Google Scholar]
- Huang, W.J.; Cheng, B.L.; Cheng, Y.L. Adsorption of microcystin-LR by three types of activated carbon. J. Hazard. Mater. 2007, 141, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Ho, L.; Lambling, P.; Bustamante, H.; Duker, P.; Newcombe, G. Application of powdered activated carbon for the adsorption of cylindrospermopsin and microcystin toxins from drinking water supplies. Water Res. 2011, 45, 2954–2964. [Google Scholar] [CrossRef] [PubMed]
- Dodevski, V.; Janković, B.Ž.; Mirković, M.M.; Kragović, M.M.; Radović, I.; Veljković, F.M.; Stojmenović, M. Carbon dioxide activation of the plane tree seeds derived bio-char: Kinetic properties and application. Therm. Sci. 2020, 24 Pt A, 3807–3821. [Google Scholar] [CrossRef]
- Pallarés, J.; González-Cencerrado, A.; Arauzo, I. Production and characterization of activated carbon from barley straw by physical activation with carbon dioxide and steam. Biomass Bioenergy 2018, 115, 64–73. [Google Scholar] [CrossRef] [Green Version]
- Ukanwa, K.S.; Patchigolla, K.; Sakrabani, R.; Anthony, E.; Mandavgane, S. A review of chemicals to produce activated carbon from agricultural waste biomass. Sustainability 2019, 11, 6204. [Google Scholar] [CrossRef] [Green Version]
- Tay, T.; Ucar, S.; Karagöz, S. Preparation and characterization of activated carbon from waste biomass. J. Hazard. Mater. 2009, 165, 481–485. [Google Scholar] [CrossRef]
- Çeçen, F.; Aktas, Ö. Activated Carbon for Water and Wastewater Treatment: Integration of Adsorption and Biological Treatment; John Wiley & Sons: Hoboken, NJ, USA, 2011. [Google Scholar]
- Bendahou, A.; Dufresne, A.; Kaddami, H.; Habibi, Y. Isolation and structural characterization of hemicelluloses from palm of Phoenix dactylifera L. Carbohydr. Polym. 2007, 68, 601–608. [Google Scholar] [CrossRef]
- Alotaibi, M.D.; Alshammari, B.A.; Saba, N.; Alothman, O.Y.; Sanjay, M.R.; Almutairi, Z.; Jawaid, M. Characterization of natural fiber obtained from different parts of date palm tree (Phoenix dactylifera L.). Int. J. Biol. Macromol. 2019, 135, 69–76. [Google Scholar] [CrossRef]
- Mokrzycki, J.; Gazińska, M.; Fedyna, M.; Karcz, R.; Lorenc-Grabowska, E.; Rutkowski, P. Pyrolysis and torrefaction of waste wood chips and cone-like flowers derived from black alder (Alnus glutinosa L. Gaertn.) for sustainable solid fuel production. Biomass Bioenergy 2020, 143, 105842. [Google Scholar] [CrossRef]
- Svirčev, Z.; Obradović, V.; Codd, G.A.; Marjanović, P.; Spoof, L.; Drobac, D.; Meriluoto, J. Massive fish mortality and Cylindrospermopsis raciborskii bloom in Aleksandrovac Lake. Ecotoxicology 2016, 25, 1353–1363. [Google Scholar] [CrossRef] [PubMed]
- Ðorđević, N.B.; Simić, S.B. Cyanobacterial Blooms in Oligosaline and Alkaline Microaccumulation Before and After Rehabilitation. Pol. J. Environ. Stud. 2014, 23, 1975–1982. [Google Scholar]
- Prokić, S.; Popović, N.; Trebješanin, B.; Simić, Z.; Babić, P. Rehabilitation of Lake Aleksandrovac—Main Projekt; Jaroslav Černi Institute for the Development of Water Resources: Belgrade, Serbia, 2008. (In Serbian) [Google Scholar]
- Codd, G.A.; Metcalf, J.S. Toxic and non-toxic cyanobacteria: Evolving concepts. Perspect. Phycol. 2014, 1, 3–5. [Google Scholar] [CrossRef] [PubMed]
- Stüken, A.; Rücker, J.; Endrulat, T.; Preussel, K.; Hemm, M.; Nixdorf, B.; Wiedner, C. Distribution of three alien cyanobacterial species (Nostocales) in northeast Germany: Cylindrospermopsis raciborskii, Anabaena bergii and Aphanizomenon aphanizomenoides. Phycologia 2006, 45, 696–703. [Google Scholar] [CrossRef]
- Cvijan, M.; Fužinato, S. The first finding of Cylindrospermopsis raciborskii (Woloszińska) Seenayya et Subba raju, 1972 (cyanoprokaryota) in Serbia. Arch. Biol. Sci. 2011, 63, 507–510. [Google Scholar] [CrossRef]
- Karadžić, V.; Simić, G.S.; Natić, D.; Ržaničanin, A.; Ćirić, M.; Gačić, Z. Changes in the phytoplankton community and dominance of Cylindrospermopsis raciborskii (Wolosz.) Subba Raju in a temperate lowland river (Ponjavica, Serbia). Hydrobiologia 2013, 711, 43–60. [Google Scholar] [CrossRef]
- Antunes, J.T.; Leão, P.N.; Vasconcelos, V.M. Cylindrospermopsis raciborskii: Review of the distribution, phylogeography, and ecophysiology of a global invasive species. Front. Microbiol. 2015, 6, 473. [Google Scholar] [CrossRef] [Green Version]
- Browning, B.L. Methods of Wood Chemistry; John Wiley & Sons: Hoboken, NJ, USA, 1967; Volumes 1 and 2. [Google Scholar]
- Kraus, W.; Nolze, G. POWDER CELL—A program for the representation and manipulation of crystal structures and calculation of the resulting X-ray powder patterns. J. Appl. Cryst. 1996, 29, 301–303. [Google Scholar] [CrossRef]
- Available online: http://powdercell-for-windows.software.informer.com/2.4/ (accessed on 12 November 2021).
- American Mineralogist Crystal Data Structure Base (AMCDSB). Available online: http://rruff.geo.arizona.edu/AMS/amcsd.phpS (accessed on 13 May 2022).
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC technical report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef] [Green Version]
- Dubinin, M.M. Physical Adsorption of Gases and Vapors in Micropores. In Progress in Surface and Membrane Science; Cadenhead, D.A., Ed.; Academic Press: New York, NY, USA, 1975; Volume 9, pp. 1–70. [Google Scholar]
- Horvath, G.; Kawazoe, K. Method for the Calculation of Effective Pore Size Distribution in Molecular Sieve Carbon. J. Chem. Eng. Jpn. 1983, 16, 470–475. [Google Scholar] [CrossRef] [Green Version]
- Huber-Pestalozzi, G.; Komárek, J.; Fott, B. Das Phytoplankton des Süβwasser. Band XVI, 7. Teil, 1. Hälfte—Chlorophyceae (Grünalgen), Ordnung Chlorococcales; Elster, H.J., Ohle, W., Eds.; Die Binnengawässer. E. Schweizerbartsche Verlagsbuchhandung: Stuttgart, Germany, 1983; 914p. [Google Scholar]
- Komárek, J.; Anagnostidis, K. Cyanoprokaryota 1. Chroococcales. In Süsswasserflora von Mitteleuropa; Ettl, H., Gärtner, G., Heynig, H., Mollenhauer, D., Eds.; Gustav Fischer Verlag: Jena, Germany, 1998; 548p. [Google Scholar]
- Komárek, J.; Anagnostidis, K. Cyanoprokaryota 2. Teil Oscillatoriales. In Süsswasserflora von Mitteleuropa; Büdel, B., Gärtner, G., Krienitz, L., Schagerl, M., Eds.; Gustav Fisher Verlag: Jena, Germany, 2005; 900p. [Google Scholar]
- Komárek, J. Cyanoprokaryota 3. Teil: Heterocytous Genera. In Süßwasserflora von Mitteleuropa; Büdel, B., Gärtner, G., Krienitz, L., Schagerl, M., Eds.; Springer: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Krammer, K.; Lange-Bertalot, H. Bacillariophyceae 1. Teil: Naviculaceae. U. In Süßwasserflora von Mitteleuropa; Ettl, H., Gerloff, J., Heynig, H., Mollenhauer, D., Eds.; Gustav Fischer Verlag: Jena, Germany, 1986; 876p. [Google Scholar]
- Krammer, K.; Lange-Bertalot, H. Bacillariophyceae. 2. Teil: Bacillariaceae, Epithemiaceae, Surirellaceae. U. In Süßwasserflora von Mitteleuropa; Ettl, H., Gerloff, J., Heynig, H., Mollenhauer, D., Eds.; Gustav Fischer Verlag: Jena, Germany, 1988; 596p. [Google Scholar]
- Krammer, K.; Lange-Bertalot, H. Bacillariophyceae. 3. Teil: Centrales, Fragilariaceae, Eunotiaceae. U. In Süßwasserflora von Mitteleuropa; Ettl, H., Gerloff, J., Heynig, H., Mollenhauer, D., Eds.; Elsevier GmbH: Munchen, Germany, 1991; 576p. [Google Scholar]
- Starmach, K. Euglenophyta. Tom 3. U; Starmach, K., Sieminska, J., Eds.; Flora Slodkowodna Polski, 3; Państwowe Wydawnictwo Naukowe: Warsaw, Poland, 1983; 593p. [Google Scholar]
- John, M.D.; Whitton, A.B.; Brook, J.A. The Freshwater Algal Flora of the British Isles: An Identification Guide to Freshwater and Terrestrial Algae; The Nautaral History Museum: Cambridge, UK, 2002; 702p. [Google Scholar]
- Reynolds, C.S. The Ecology of Phytoplankton; Cambridge University Press: Cambridge, UK, 2006. [Google Scholar]
- Cagnon, B.; Py, X.; Guillot, A.; Stoeckli, F.; Chambat, G. Contributions of hemicellulose, cellulose and lignin to the mass and the porous properties of chars and steam activated carbons from various lignocellulosic precursors. Bioresour. Technol. 2009, 100, 292–298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suhas, V.K.; Gupta, P.J.M.; Carrott, P.J.; Singh, R.; Chaudhary, M.; Kushwaha, S. Cellulose: A review as natural, modified and activated carbon adsorbent. Bioresour. Technol. 2016, 216, 1066–1076. [Google Scholar] [CrossRef] [PubMed]
- Yaciuk, P.A.; Colombo, F.; Lecomte, K.L.; De Micco, G.; Bohé, A.E. Cadmium sources, mobility, and natural attenuation in contrasting environments (carbonate-rich and carbonate-poor) in the Capillitas polymetallic mineral deposit, NW Argentina. Appl. Geochem. 2021, 136, 105152. [Google Scholar] [CrossRef]
- Available online: https://www.atsdr.cdc.gov/csem/cadmium/Where-Cadmium-Found.html (accessed on 11 September 2021).
- Nordic Council of Ministers. Cadmium Review; WHO: Geneva, Switzerland, 2003; p. 11. Available online: https://www.who.int/ifcs/documents/forums/forum5/nmr_cadmium- (accessed on 18 November 2020).
- Tejada, C.N.; Almanza, D.; Villabona, A.; Colpas, F.; Granados, C. Characterization of activated carbon synthesized at low temperature from cocoa shell (Theobroma cacao) for adsorbing amoxicillin. Ingeniería Competitividad 2017, 19, 45–54. [Google Scholar]
- Alabadi, A.; Razzaque, S.; Yang, Y.; Chen, S.; Tan, B. Highly porous activated carbon materials from carbonized biomass with high CO2 capturing capacity. Chem. Eng. J. 2015, 281, 606–612. [Google Scholar] [CrossRef]
- Athinarayanan, J.; Vaiyapuri, S.P.; Alshatwi, A.A. Phoenix dactylifera lignocellulosic biomass as precursor for nanostructure fabrication using integrated process. Int. J. Biol. Macromol. 2019, 134, 1179–1186. [Google Scholar] [CrossRef]
- Dodevski, V.; Janković, B.; Stojmenović, M.; Krstić, S.; Popović, J.; Pagnacco, M.C.; Popović, M.; Pašalić, S. Plane tree seed biomass used for preparation of activated carbons (AC) derived from pyrolysis. Modeling the activation process. Colloids Surf. A Physicochem. Eng. Asp. 2017, 522, 83–96. [Google Scholar] [CrossRef]
- Veličković, A.; Avramović, J.; Kostić, M.; Krstić, J.; Stamenković, O.; Veljkovic, V. Modeling the biodiesel production using the wheat straw ash as a catalyst: Original scientific paper. Hemijska Industrija 2021, 75, 257–276. [Google Scholar] [CrossRef]
- Etiégni, L.; Campbell, A. Physical and chemical characteristics of wood ash. Bioresour. Technol. 1991, 37, 173–178. [Google Scholar] [CrossRef]
- Zhou, G.; Taylor, G.; Polle, A. FTIR-ATR-based prediction and modelling of lignin and energy contents reveals independent intra-specific variation of these traits in bioenergy poplars. Plant Methods 2011, 7, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hospodarova, V.; Singovszka, E.; Stevulova, N. Characterization of Cellulosic Fibers by FTIR Spectroscopy for Their Further Implementation to Building Materials. Am. J. Anal. Chem. 2018, 9, 303–310. [Google Scholar] [CrossRef] [Green Version]
- Cheng, S.; Huang, A.; Wang, S.; Zhang, Q. Effect of Different Heat Treatment Temperatures on the Chemical Composition and Structure of Chinese Fir Wood. BioResources 2016, 11, 4006–4016. [Google Scholar] [CrossRef] [Green Version]
- Galletti, A.M.R.; D’Alessio, A.; Licursi, D.; Antonetti, C.; Valentini, G.; Galia, A.; O Di Nasso, N.N. Midinfrared FT-IR as a Tool for Monitoring Herbaceous Biomass Composition and Its Conversion to Furfural. J. Spectrosc. 2015, 2015, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Available online: https://webbook.nist.gov/cgi/cbook.cgi?ID=C7647145&Contrib=IARPA-IR-S&Type=IR-SPEC&Index=0 (accessed on 11 October 2021).
- Available online: https://rruff.info/Sylvite (accessed on 11 October 2021).
- Bera, M.; Gupta, P.; Maji, P.K. Facile One-Pot Synthesis of Graphene Oxide by Sonication Assisted Mechanochemical Approach and Its Surface Chemistry. J. Nanosci. Nanotechnol. 2018, 18, 902–912. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Tu, L.Y.; Liang, Y.; Chen, Q.; Li, Z.S.; Li, C.H.; Li, W. Coconut-based activated carbon fibers for efficient adsorption of various organic dyes. RSC Adv. 2018, 8, 42280–42291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chafidz, A.; Astuti, W.; Augustia, V.; Novira, D.T.; Rofiah, N. Removal of methyl violet dye via adsorption using activated carbon prepared from Randu sawdust (Ceiba pentandra). IOP Conf. Ser. Earth Environ. Sci. 2018, 167, 012013. [Google Scholar] [CrossRef]
- Ma, Z.; Kyotani, T.; Tomita, A. Synthesis Methods for Preparing Microporous Carbons with a Structural Regularity of Zeolite Y. Carbon 2002, 40, 2367–2374. [Google Scholar] [CrossRef]
- Kojima, Y.; Miyaoka, H.; Ichikawa, T. Hydrogen storage materials. In New and Future Developments in Catalysis: Batteries, Hydrogen Storage and Fuel Cells; Suib, S.L., Ed.; Elsevier: Amsterdam, The Netherlands, 2013; Chapter 5; p. 131. [Google Scholar]
- Kyotani, T.; Ma, Z.X.; Tomita, A. Template synthesis of novel porous carbons using various types of zeolites. Carbon 2003, 41, 1451–1459. [Google Scholar] [CrossRef]
- Su, F.B.; Zeng, H.J.; Yu, Y.J.; Lv, L.; Lee, J.Y.; Zhao, X.S. Template synthesis of microporous carbon for direct methanol fuel cell application. Carbon 2005, 43, 2368–2373. [Google Scholar] [CrossRef]
- Yang, Z.X.; Xia, Y.D.; Mokaya, R. Enhanced hydrogen storage capacity of high surface area zeolite-like carbon materials. J. Am. Chem. Soc. 2007, 129, 1673–1679. [Google Scholar] [CrossRef] [PubMed]
- Fasake, V.; Dashora, K. Characterization and Morphology of Natural Dung Polymer for Potential Industrial Application as Bio-Based Fillers. Polymers 2020, 12, 3030. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, X.; Yao, S.; Chang, H.; Wang, Y.; Zhang, Z. UV-blocking, transparent and hazy cellulose nanopaper with superior strength based on varied components of poplar mechanical pulp. Cellulose 2020, 27, 6563–6576. [Google Scholar] [CrossRef]
- Meng, L.-Y.; Ma, M.-G.; Ji, X.-X. Preparation of Lignin-Based Carbon Materials and Its Application as a Sorbent. Materials 2019, 12, 1111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gan, Y.X. Activated Carbon from Biomass Sustainable Sources. C J. Carbon Res. 2021, 7, 39. [Google Scholar] [CrossRef]
- Sun, Y.; Iris, K.M.; Tsang, D.C.; Fan, J.; Clark, J.H.; Luo, G.; Graham, N.J.D. Tailored design of graphitic biochar for high-efficiency and chemical-free microwave-assisted removal of refractory organic contaminants. Chem. Eng. J. 2020, 398, 125505. [Google Scholar] [CrossRef]
- Pui, W.K.; Yusoff, R.; Aroua, M.K. A review on activated carbon adsorption for volatile organic compounds (VOCs). Rev. Chem. Eng. 2019, 35, 649–668. [Google Scholar] [CrossRef]
- Beasley, V.R. Harmful Algal Blooms (Phycotoxins). Earth Syst. Environ. Sci. 2020. [Google Scholar]
- He, X.; Liu, Y.-L.; Conklin, A.; Westrick, J.; Weavers, L.K.; Dionysiou, D.D.; Lenhart, J.J.; Mouser, P.J.; Szlag, D.; Walker, H.W. Toxic cyanobacteria and drinking water: Impacts, detection, and treatment. Harmful Algae 2016, 54, 174–193. [Google Scholar] [CrossRef]
- Humbert, J.F. Toxins of cyanobacteria. In Handbook of Toxicology of Chemical Warfare Agents; Academic Press: Cambridge, MA, USA, 2009; pp. 371–379. [Google Scholar]
- Codd, G.A.; Meriluoto, J.; Metcalf, J.S. Introduction: Cyanobacteria, cyanotoxins, their human impact, and risk management. In Handbook of Cyanobacterial Monitoring and Cyanotoxin Analysis; John Wiley & Sons: Hoboken, NJ, USA, 2016; pp. 3–8. [Google Scholar]
- Đorđević, N.B.; Simić, S.B.; Ćirić, A.R. First identification of the cylindrospermopsin (cyn)-producing cyanobacterium Cylindrospermopsis raciborskii (Woloszyńska) Seenayya & Subba Raju in Serbia. Fresenius Environ. Bull. 2015, 24, 3736–3742. [Google Scholar]
- Recknagel, F.; Orr, P.; Cao, H. Inductive reasoning and forecasting of population dynamics of Cylindrospermopsis raciborskii in three sub-tropical reservoirs by evolutionary computation. Harmful Algae 2014, 31, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Soares, M.C.S.; Lürling, M.; Huszar, V.L. Growth and temperature-related phenotypic plasticity in the cyanobacterium Cylindrospermopsis raciborskii. Phycol. Res. 2013, 61, 61–67. [Google Scholar] [CrossRef]
- World Health Organization WHO. Guidelines for Safe Recreational Water Environments: Coastal and Fresh Waters; World Health Organization: Geneva, Switzerland, 2003; Volume 1, p. 219. [Google Scholar]
- Baulina, O.I.; Titel, K.; Gorelova, O.A.; Malai, O.V.; Ehwald, R. Permeability of cyanobacterial mucous surface structures for macromolecules. Microbiology 2008, 77, 198–205. [Google Scholar] [CrossRef]
- Abbas, T.; Kajjumba, G.W.; Ejjada, M.; Ummeh Masrura, S.; Marti, E.J.; Khan, E.; Jones-Lepp, T.L. Recent Advancements in the Removal of Cyanotoxins from Water Using Conventional and Modified Adsorbents—A Contemporary Review. Water 2020, 12, 2756. [Google Scholar] [CrossRef]
- Buarque, N.M.S.; Buarque, H.L.B.; Capelo-Neto, J. Adsorption kinetics and diffusion of Saxitoxins on granular-activated carbon: Influence of pore size distribution. J. Water Supply Res. Technol. AQUA 2015, 64, 344–353. [Google Scholar] [CrossRef]
- Kajjumba, G.W.; Ayd, S.; Gu, S. Adsorption isotherms and kinetics of vanadium by shale and coal waste. Adsorpt. Sci. Technol. 2018, 36, 936–952. [Google Scholar] [CrossRef]
- Olenina, I.; Hajdu, S.; Edler, L.; Andersson, A.; Wasmund, N.; Busch, S.; Göbel, J.; Gromisz, S.; Huseby, S.; Huttunen, M.; et al. Biovolumes and size-classes of phytoplankton in the Baltic Sea. HELCOM Balt. Sea Environ. Proc. 2006, 106, 144. [Google Scholar]
- Available online: http://nordicmicroalgae.org/quick-view (accessed on 11 January 2022).
- Ballot, A.; Dadheech, P.K.; Haande, S.; Krienitz, L. Morphological and Phylogenetic Analysis of Anabaenopsis abijatae and Anabaenopsis elenkinii (Nostocales, Cyanobacteria) from Tropical Inland Water Bodies. Microb. Ecol. 2007, 55, 608–618. [Google Scholar] [CrossRef]
- Available online: https://www.montclair.edu/water-science/freshwater-cyanobacteria-of-new-jersey/visual-guide-to-cyanobacteria-in-new-jersey/coccoid/colonial/aphanothece/ (accessed on 11 January 2022).
- Simic, S.B.; Komárek, J.; Đorđević, N.B. The confirmation of the genus Glaucospira (Cyanobacteria) and the occurrence of Glaucospira laxissima (GS West) comb. nova in Serbia. Cryptogamie Algologie 2014, 35, 259–267. [Google Scholar] [CrossRef]
- Available online: https://dornsife.usc.edu/labs/cyanobacteria–guide/jaaginema/ (accessed on 11 January 2022).
- Zhu, M.; Yu, G.; Li, X.; Tan, W.; Li, R. Taxonomic and phylogenetic evaluation of Limnothrix strains (Oscillatoriales, Cyanobacteria) by adding Limnothrix planktonica strains isolated from central China. Hydrobiologia 2012, 698, 367–374. [Google Scholar] [CrossRef]
- Stal, L.J. Poly (hydroxyalkanoate) in cyanobacteria: An overview. FEMS Microbiol. Rev. 1992, 9, 169–180. [Google Scholar] [CrossRef]
- Available online: www.planktonforum.eu (accessed on 11 January 2022).
- NOAA Center of Excellence for Great Lakes and Human Health. Cylindrospermopsis raciborskii Factsheet. Available online: https://en.wikipedia.org/wiki/Cylindrospermopsis_raciborskii (accessed on 13 May 2022).
- Available online: https://www.planktonforum.eu/index.php?id=33&no_cache=1&L=1&tx_pydb_pi1%5Bstart%5D=320&tx_pydb_pi1%5Bdetails%5D=20931&tx_pydb_pi1%5Bcur%5D=5 (accessed on 13 May 2022).

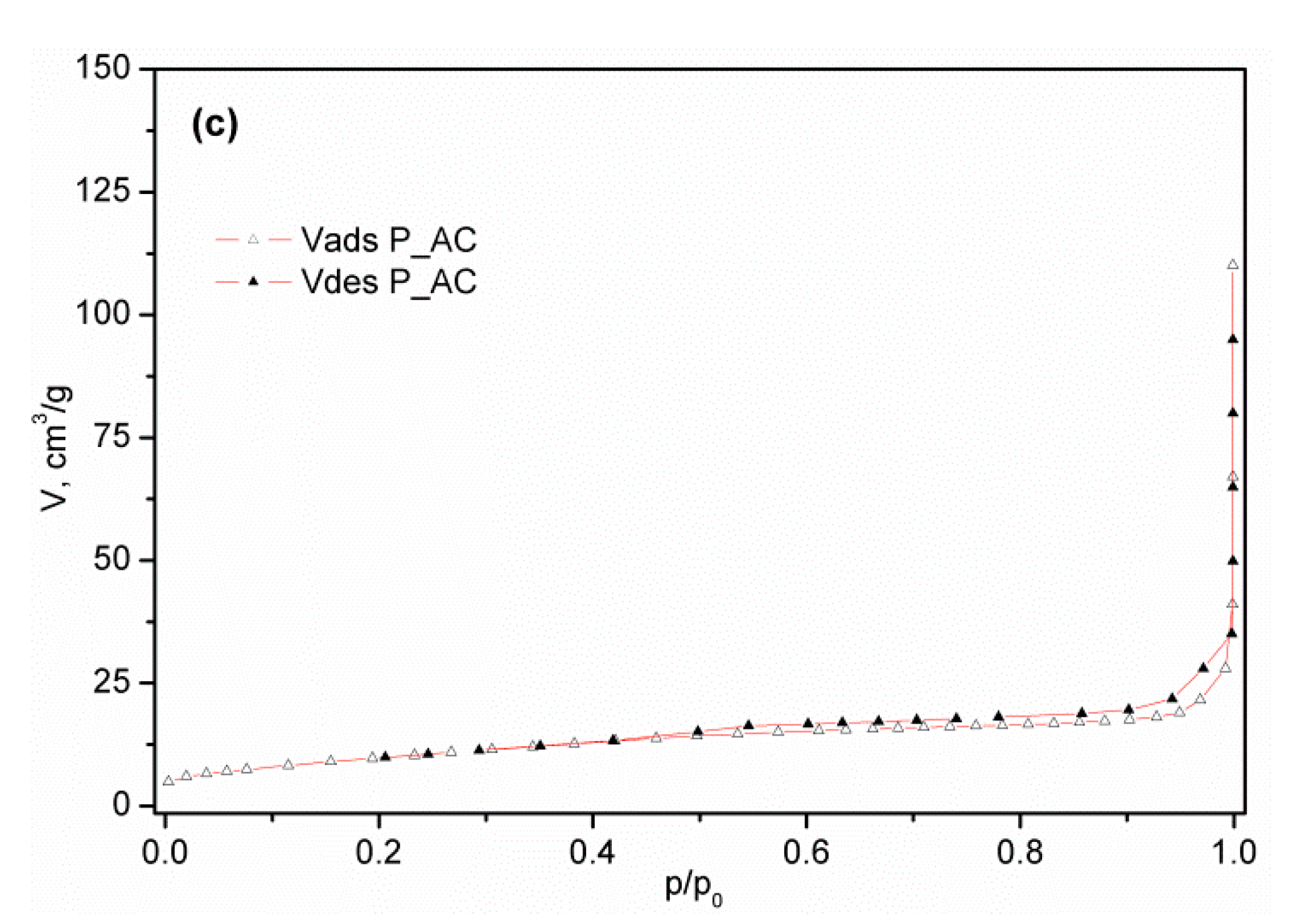



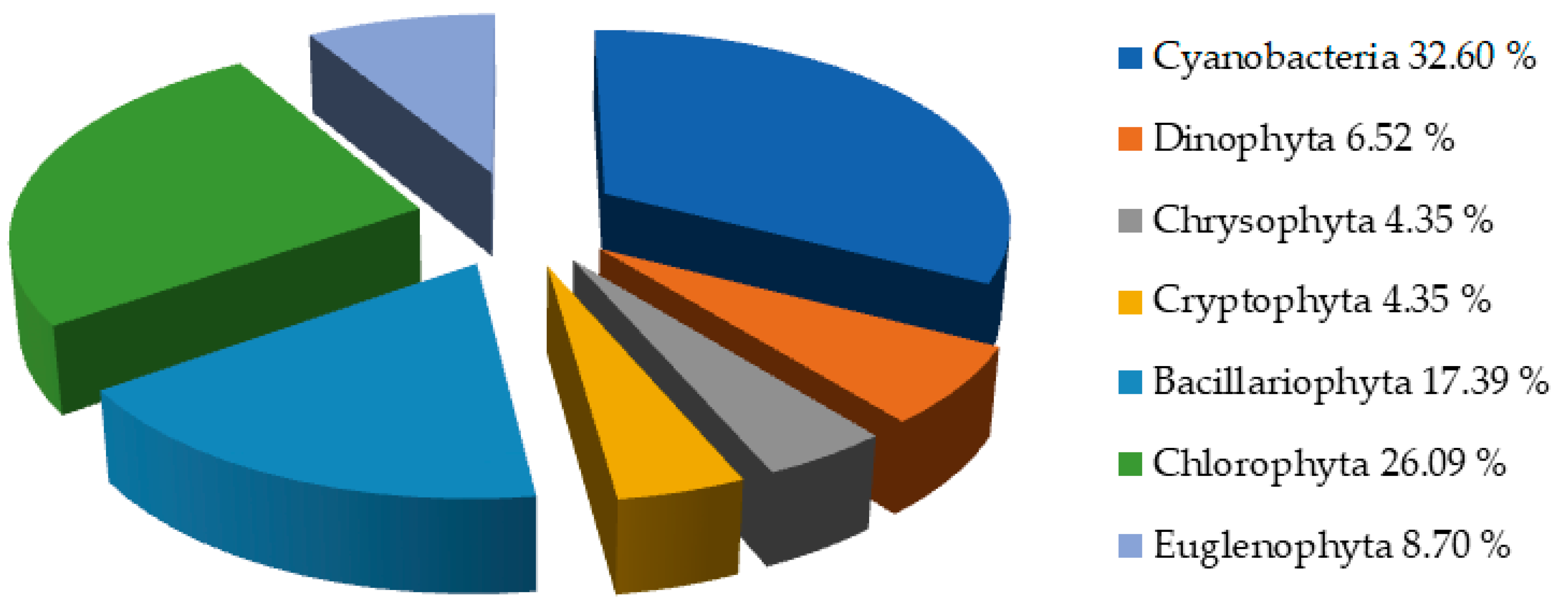
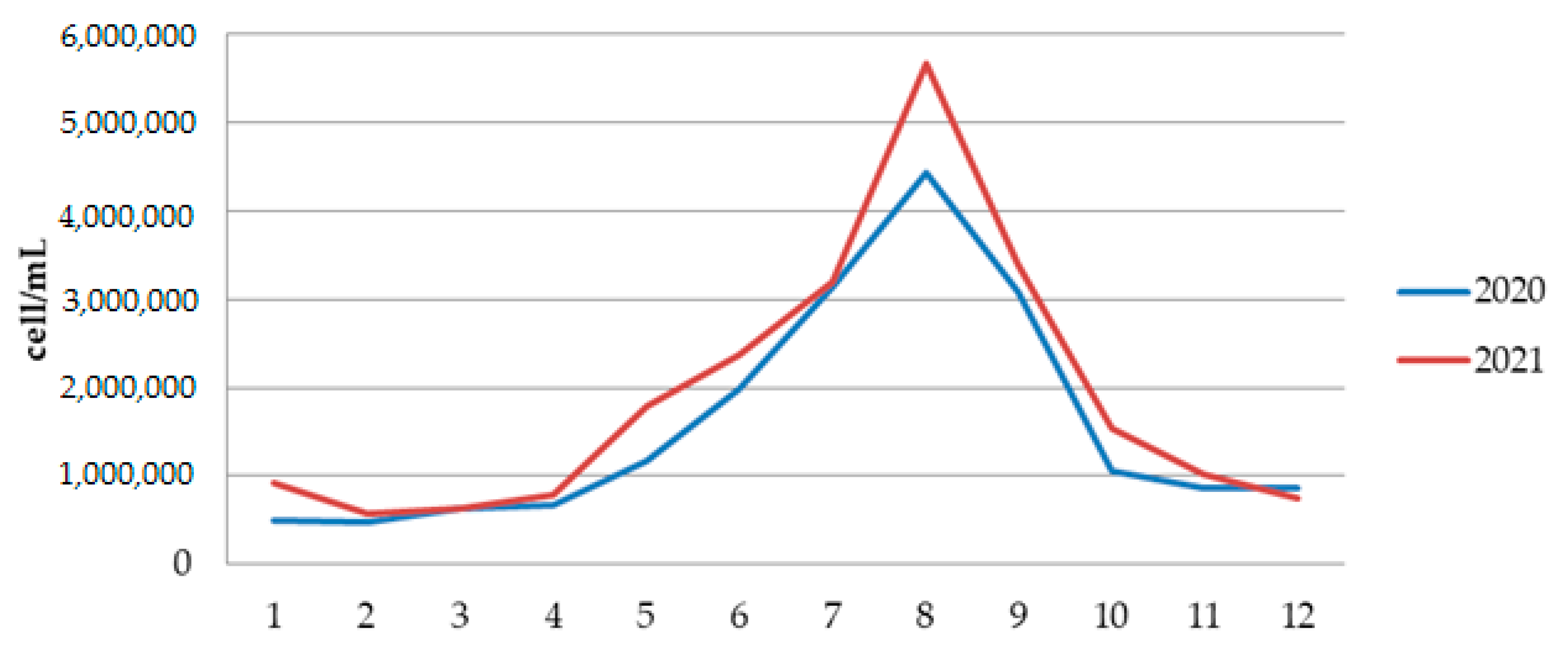
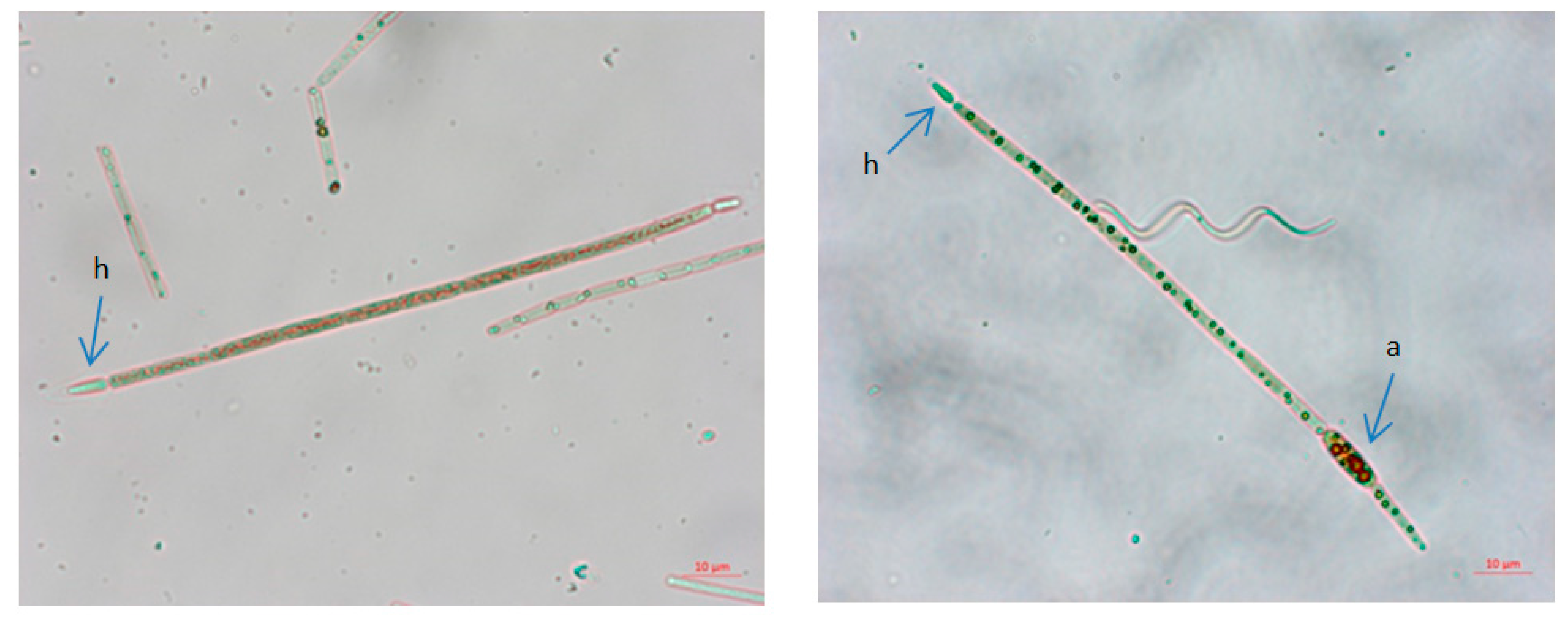
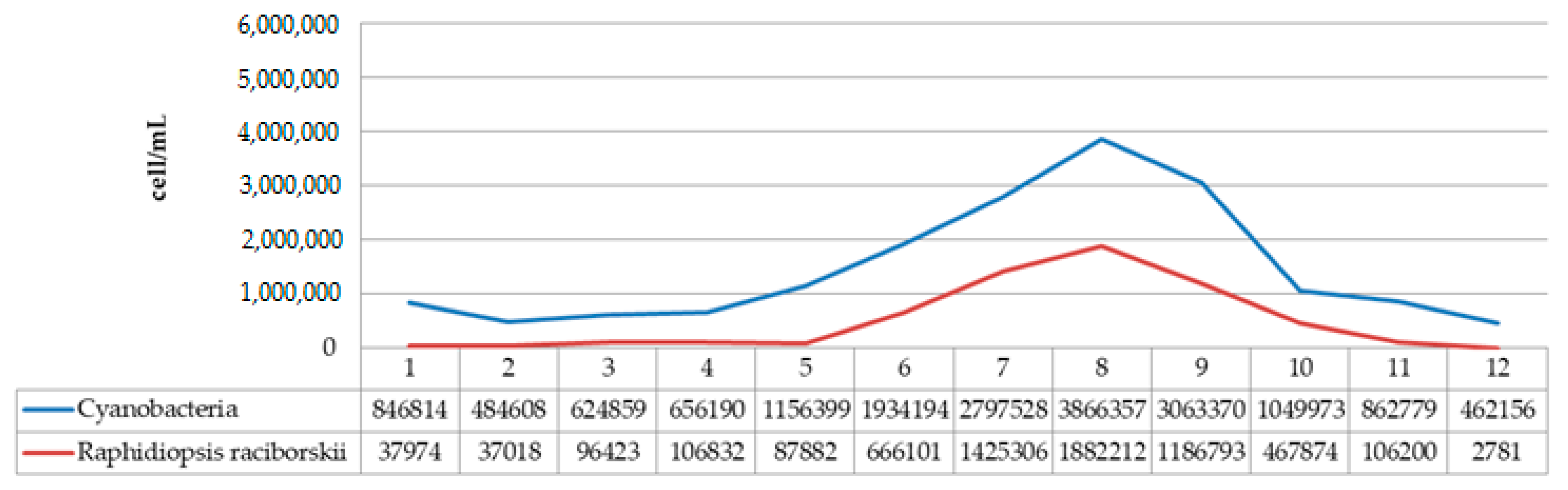
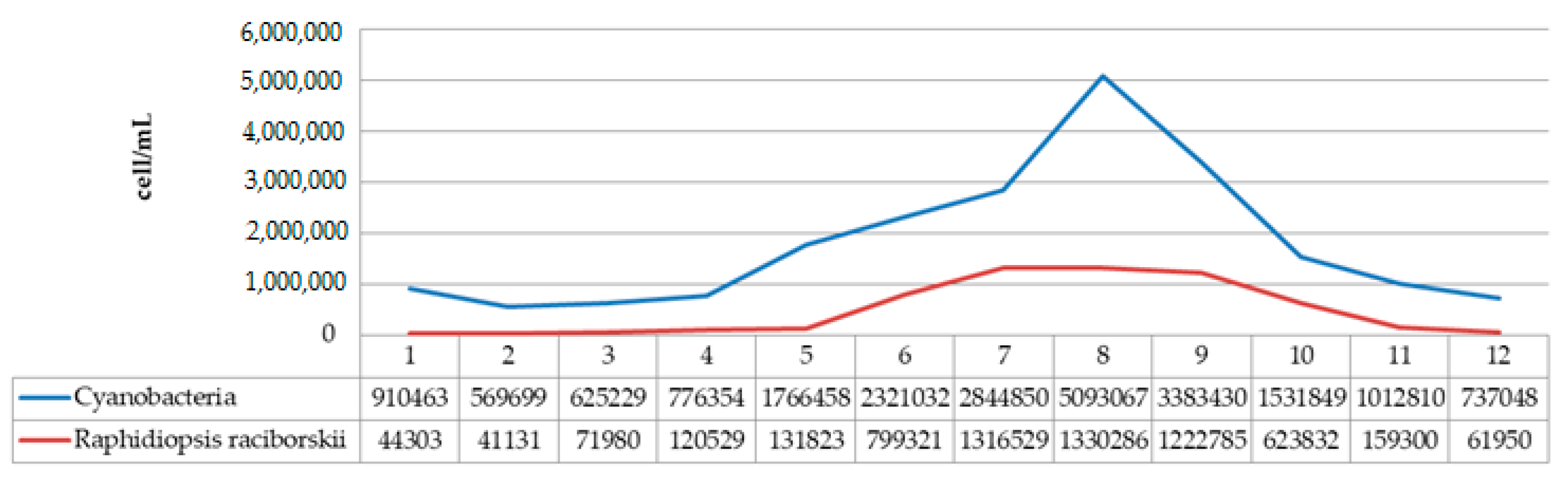

| Cellulose, % | Lignin, % | Hot Water Extractives, % | Hemicelluloses, % | Moisture, % | Ash (900 °C), % | |
|---|---|---|---|---|---|---|
| Date-palm Leaf Stalks | ||||||
| P_RS ** | 25.1 ± 0.7 | 7.9 ± 0.5 | 48.66 ± 0.04 | 1.31 | 7.9 ± 0.2 * | 4.5 ± 0.3 |
| Bendahou et al., 2007 [11] | 44.0 | 14.0 | / | 28.0 | / | 2.5 |
| Alotaibi et al., 2019 [12] | 35.00 | 20.13 | / | 15.40 | 15.6 | 12.6 |
| Black Alder Cone-like Flowers | ||||||
| A_RS ** | 19.1 ± 1.2 | 29.6 ± 0.9 | 28.65 ± 1,14 | 10.67 | 10.5 ± 0.4 * | 2.7 ± 0.1 |
| Mokrzycki et al., 2019 [13] | 20.9 | 29.7 | / | 29.3 | 6.4 | 2.6 |
| C_AC | P_AC | A_AC | ||
|---|---|---|---|---|
| Specific surface area (Brunauer–Emmett–Teller), Journal of the American Chemical Society. 60 (2), 309–319 | C | 1292 | 52 | 1287 |
| S, m2 g−1 | 1100 | 36.6 | 485 | |
| t-Plot (Lippens and de Boer); standard isotherms from literature: A. Lecloux, J.P. Pirard, J. Colloid Interface Sci. 70 (2), 1979 | Total surface area, m2 g−1 | 1105 | 36.1 | 446 |
| Micropore volume, cm3 g−1 | 0.457 | 0.023 | 0.240 | |
| Mesopores surface m2 g−1 | 6.3 | 3.4 | 10.4 | |
| Micropore volume (Dubinin and Raduskevich), cm3 g−1 | 0.422 | 0.012 | 0.186 | |
| Micropores (Horvath and Kawazoe), with potential function: N2 on Graphite @77.3 K from literature: G. Horvath, K. Kawazoe. J. Chem. Eng. Japan, 16, 6(1983), 470–475 | Maximum pore diameter, nm | 0.48 | - | 0.48 |
| Cumulative pore volume, cm3 g−1 | 0.431 | - | 0.195 | |
| Mesopore volume, cm3 g−1 | Adsorption branch of isotherm | 0.057 | 0.022 | 0.097 |
| Desorption branch of isotherm | 0.099 | 0.029 | 0.125 | |
| Total pore volume (Gurvich), cm3 g−1 | At p/p0 = 0.98 | 0.474 | 0.038 | 0.268 |
| Cyanobacteria |
| Anabaenopsis elenkinii V.Milleer |
| Anathece minutissima (West) Komárek, Kastovsky and Jezberová |
| Aphanocapsa Nägeli sp. |
| Glaucospira G.Lagerheim sp. |
| Jaaginema subtilissimum (Kütz. Ex De Toni) Anagn. et Kom. |
| Limnothrix planctonica (Wolos.) Meffert |
| Merismopedia glauca (Ehr.) Kütz. |
| Microcystyis aeruginosa Kütz. |
| Microcystis flos-aquae (Wittr.) Kirchner |
| Oscillatoria limosa C.Agardh ex Gomont |
| Planktolyngbya limnetica (Lemmermann) Komárková-Legnerová and Cronberg |
| Pseudanabaena limnetica (Lemm.) Komarek |
| Raphidiopsis raciborskii Woloszynska) Aguilera et al. |
| Snowella Elenkin sp. |
| Synechocystis cf. aquatilis Sauvageau |
| Dinophyta |
| Gymnodinium Stein sp. |
| Peridiniopsis Lemm. sp. |
| Peridiniopsis cunningtonii Lemm. |
| Chrysophyta |
| Mallomonas Perty sp. |
| Ochromonas Vysotskii (Wysotzki, Wyssotzki) sp. |
| Cryptophyta |
| Cryptomonas Ehr. spp. |
| Rhodomonas lacustris Pascher and Ruttner |
| Bacillariophyta |
| Achnanthidium Kützing sp. (cf. minutissimum) |
| Amphora lybica Ehrenberg |
| Cyclotella (Kütz.) Bréb.sp. |
| Cymbella C.Agardh. sp. |
| Fragilaria ulna Sippen acus sensu L.-B. |
| Gomphonema Ehr. sp. |
| Navicula Bory sp. |
| Stephanodiscus Ehr. sp. |
| Chlorophyta |
| Chlamydomonas Ehr. sp. |
| Chlorella Beij. sp. |
| Cosmarium Corda sp. |
| Kirchneriella contorta (Schmidle) Bohl. |
| Kirchneriella irregularis (G.M.Smith) Korshikov |
| Monoraphidium contortum (Thur.) Com.-Legn. |
| Mougeotia C.Agardh sp. |
| Scenedesmus quadricauda (Turp.) Bréb. |
| Scenedesmus semprevirens Chod. |
| Sphaerocystis Chodat sp. |
| Staurastrum Meyen ex Ralfs sp. |
| Tetraedron minimum (A.Br.) Hansg |
| Euglenophyta |
| Euglena Ehr. sp. |
| Euglena acus Ehr. |
| Phacus pyrum (Ehr) W.Archer |
| Trachelomonas volvocina Ehr. |
| No | Cyanobacteria | Cyanotoxins | Reference |
|---|---|---|---|
| 1. | Anabaenopsis elenkinii | not determined | / |
| 2. | Anathece minutissima | not determined | / |
| 3. | Aphanocapsa sp. | microcystin | [70] |
| 4. | Glaucospira sp. | not determined | / |
| 5. | Jaaginema subtilissimum | not determined | / |
| 6. | Limnothrix planctonica | not determined | / |
| 7. | Merismopedia glauca | not determined | / |
| 8. | Microcystyis aeruginosa | anatoxin, microcystin | [70] |
| 9. | Microcystis flos-aquae | microcystin | [70] |
| 10. | Oscillatoria limosa | microcystin | [70] |
| 11. | Planktolyngbya limnetica | not determined | / |
| 12. | Pseudanabaena limnetica | Anatoxin | [70] |
| 13. | Rhapidiopsis raciborskii | cylindrospermopsin, saxitoxin | [70] |
| 14. | Snowella sp. | not determined | / |
| 15. | Synechocystis cf. aquatilis | not determined | / |
| Cyanotoxin | Chemical Structure | Molecular Weight | Effect |
|---|---|---|---|
| Anatoxin | Bicyclic alkaloid | 252 Da [70], 165 and 179 Da [71] | Neurotoxin, inhibits acetylcholine esterase [72,73] |
| Cylindrospermopsin | Tricyclic guanidine alkaloid | 415 Da [72] | Toxic effect on multiple organs; neurotoxic, genotoxic, protein synthesis inhibitor, hepatotoxin [72,73] |
| Microcystin | Cyclic peptides | from 800 to 1100 Da [72] | Hepatotoxic, tumor-causing, inhibition of eukaryotic phosphatase proteins PP1, PP2A, as well as phosphoprotein phosphatase PPP4, PPP5 [72,73] |
| Saxitoxin | Alkaloids | from 241 to 491 [72] | Neurotoxic, blocks sodium transport channels [72,73] |
| No. | Cyanobacteria | Before Treatment | After Treatment | ||
|---|---|---|---|---|---|
| P_AC | A_AC | C_AC | |||
| [cell/mL] | [cell/mL] | ||||
| 1. | Anabaenopsis elenkinii | 280 | 0 | 0 | 0 |
| 2. | Anathece minutissima | 1280 | 0 | 0 | 0 |
| 3. | Aphanocapsa sp. | 640 | 0 | 0 | 247 |
| 4. | Glaucospira sp. | 49,150 | 143 | 93 | 20,876 |
| 5. | Jaaginema subtilissimum | 1,050,812 | 0 | 193 | 15,825 |
| 6. | Limnothrix planctonica | 1,018,116 | 463 | 194 | 6206 |
| 7. | Merismopedia glauca | 842,052 | 0 | 0 | 362,880 |
| 8. | Microcystyis aeruginosa | 63,193 | 0 | 0 | 0 |
| 9. | Microcystis flos-aquae | 17,554 | 0 | 0 | 0 |
| 10. | Oscillatoria limosa | 14,043 | 0 | 0 | 0 |
| 11. | Planktolyngbya limnetica | 129,898 | 0 | 0 | 0 |
| 12. | Pseudanabaena limnetica | 59,683 | 0 | 28 | 10,584 |
| 13. | Rhapidiopsis raciborskii | 1,330,286 | 104 | 98 | 5916 |
| 14. | Snowella sp. | 484,483 | 0 | 0 | 0 |
| 15. | Synechocystis cf. aquatilis | 31,597 | 0 | 0 | 0 |
| SUM | 5,093,067 | 710 | 606 | 422,534 | |
| The removal efficiency (%) | 99.99 | 99.99 | 89.79 | ||
| No. | Cyanobacteria | Cell Shape | Length (µm) | Diameter/Width (µm) | Talus Type | Trichome Length (µm) | References |
|---|---|---|---|---|---|---|---|
| 1. | Anabaenopsis elenkinii | ellipsoid | 5.5–9.3 (3) 4–9 (12.9) | (2.8) 4–6(8) | trichome | ≤100 | [34,79,80,81] |
| 2. | Anathece minutissima | ellipsoid | 1.5 1–1.5–2 (?2.8) | 0.9 0.8–1 (1.6) | colony | / | [79,80] [32] |
| 3. | Aphanocapsa sp. | sphere | / | 1–1.5 * | colony | / | [32,82] |
| 4. | Glaucospira sp. | cylindrical | 0.5–2 | 2.7–6 | trichome | 36–60 | [83] |
| 5. | Jaaginema subtilissimum | cylindrical | Up to 5 µm | 1–1.5 | trichome | 150–300 | [33,84] |
| 6. | Limnothrix planctonica | cylindrical | 2–4 6–10 | 1.5–2.5 (3) | trichome | / | [33,79,80,85] |
| 7. | Merismopedia glauca | sphere | / | (2.8) 3–6 | colony | / | [32,79,80] |
| 8. | Microcystyis aeruginosa | sphere | / | 4–6 | colony | / | [32,79,80] |
| 9. | Microcystis flos-aquae | sphere | / | (2.5)3.5–4.8 (5.6?) | colony | / | [32,79,80] |
| 10. | Oscillatoria limosa | cylindrical | 1.8–3 1.5–5(6) | 10 (6?–9) 10–20 (22) | trichome | 10–100 | [86,87] [33] |
| 11. | Planktolyngbya limnetica | cylindrical | 1–2–5 (9?) | 0.5–1–1.8 (2.5?) | trichome | / | [33,79,80] |
| 12. | Pseudanabaena limnetica | cylindrical | 5-14 (1.2?) 4–12 (20?) | (1) 1.2–1.5 (2) | trichome | / | [33,79,80,88] |
| 13. | Rhapidiopsis raciborskii | cylindrical | 2.3–6.5 2.5–12 (16) | (1.7) 2–2.4 (−4?) | trichome | 107, 50–300 | [19,89,90,91] |
| 14. | Snowella sp. | sphere or elipsoid | 2.4–4 | 3.2 | colony | / | [32,79,80] |
| 15. | Synechocystis aquatilis | sphere | / | (3) 4.5–7 (7.8?) | colony | / | [32,92] |
| No. | Cyanobacteria | Cell One Length | Average Trichomes Length before Treatment | Average Trichomes Length after Treatment | ||
|---|---|---|---|---|---|---|
| P_AC | A_AC | C_AC | ||||
| 1. | Anabaenopsis elenkinii | 7 µm | 30 µm | / | / | / |
| 2. | Glaucospira sp. | 1.5 µm | 35 µm | 23–30 µm | 21–35 µm | 21–36 µm |
| 3. | Jaaginema subtilissimum | 1.7 µm | 48 µm | 20–41 µm | 18–45 µm | 21–48 µm |
| 4. | Limnothrix planctonica | 5 µm | 59 µm | 16–21 µm | 13–27 µm | 15–29 µm |
| 5. | Oscillatorialimosa | 3 µm | 37 µm | 30–35 µm | / | 32–35 µm |
| 6. | Planktolyngbya limnetica | 2 µm | 17 µm | / | / | / |
| 7. | Pseudanabaena limnetica | 5 µm | 141 µm | 38–63 µm | 32–59 µm | 39–62 µm |
| 8. | Rhapidiopsis raciborskii | 7 µm | 71 µm | 27–41 µm | 15–45 µm | 25–47 µm |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kandić, I.; Kragović, M.; Krstić, J.B.; Gulicovski, J.; Popović, J.; Rosić, M.; Karadžić, V.; Stojmenović, M. Natural Cyanobacteria Removers Obtained from Bio-Waste Date-Palm Leaf Stalks and Black Alder Cone-Like Flowers. Int. J. Environ. Res. Public Health 2022, 19, 6639. https://doi.org/10.3390/ijerph19116639
Kandić I, Kragović M, Krstić JB, Gulicovski J, Popović J, Rosić M, Karadžić V, Stojmenović M. Natural Cyanobacteria Removers Obtained from Bio-Waste Date-Palm Leaf Stalks and Black Alder Cone-Like Flowers. International Journal of Environmental Research and Public Health. 2022; 19(11):6639. https://doi.org/10.3390/ijerph19116639
Chicago/Turabian StyleKandić, Irina, Milan Kragović, Jugoslav B. Krstić, Jelena Gulicovski, Jasmina Popović, Milena Rosić, Vesna Karadžić, and Marija Stojmenović. 2022. "Natural Cyanobacteria Removers Obtained from Bio-Waste Date-Palm Leaf Stalks and Black Alder Cone-Like Flowers" International Journal of Environmental Research and Public Health 19, no. 11: 6639. https://doi.org/10.3390/ijerph19116639
APA StyleKandić, I., Kragović, M., Krstić, J. B., Gulicovski, J., Popović, J., Rosić, M., Karadžić, V., & Stojmenović, M. (2022). Natural Cyanobacteria Removers Obtained from Bio-Waste Date-Palm Leaf Stalks and Black Alder Cone-Like Flowers. International Journal of Environmental Research and Public Health, 19(11), 6639. https://doi.org/10.3390/ijerph19116639








