Characterization of Microbial Communities in Wastewater Treatment Plants Containing Heavy Metals Located in Chemical Industrial Zones
Abstract
:1. Introduction
2. Materials and Methods
2.1. Basic Description of the Studied WWTPs
2.2. DNA Extraction, PCR Amplification, and High-Throughput Sequencing
2.3. Characterization of the Microbial Community and Gene Functional Prediction
2.4. Chemical Analysis
3. Results and Discussion
3.1. Sample Characteristics
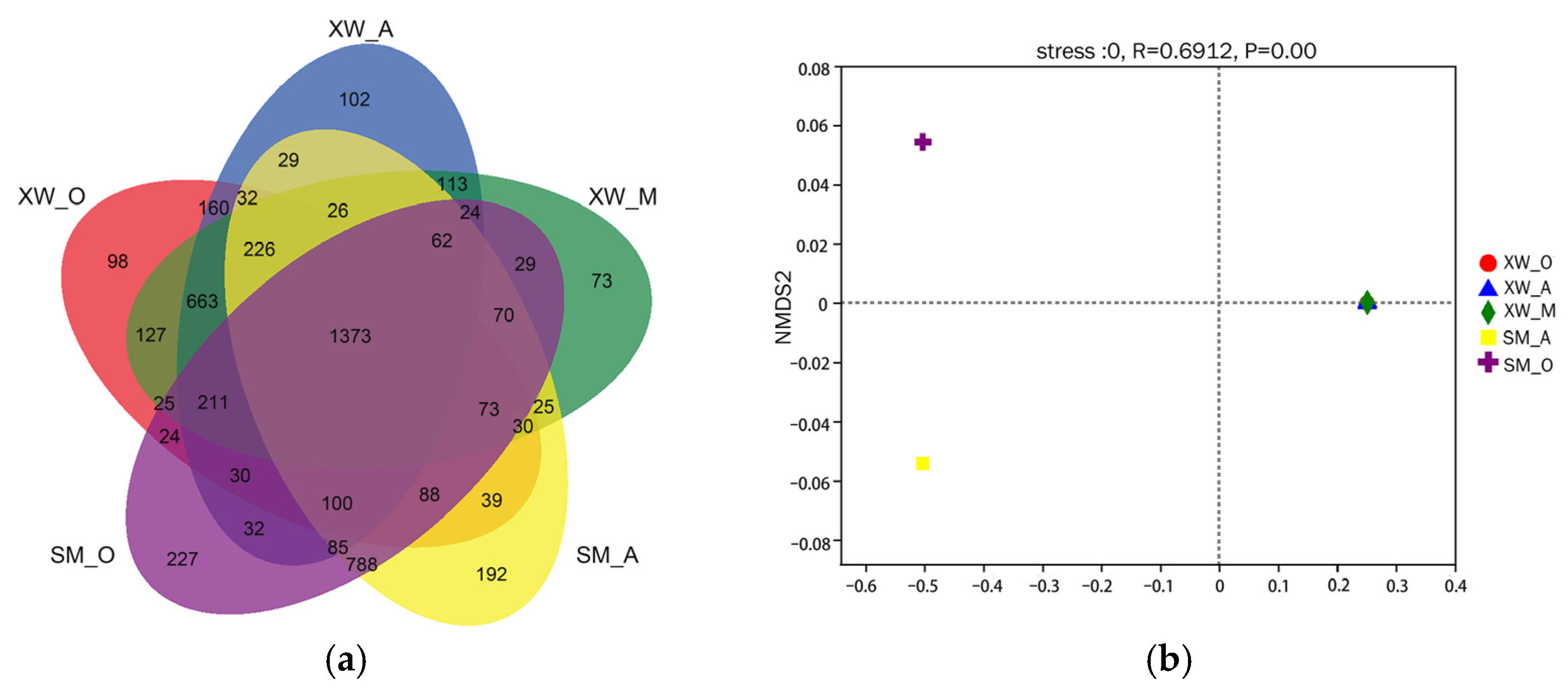
3.2. Microbial Community Diversity
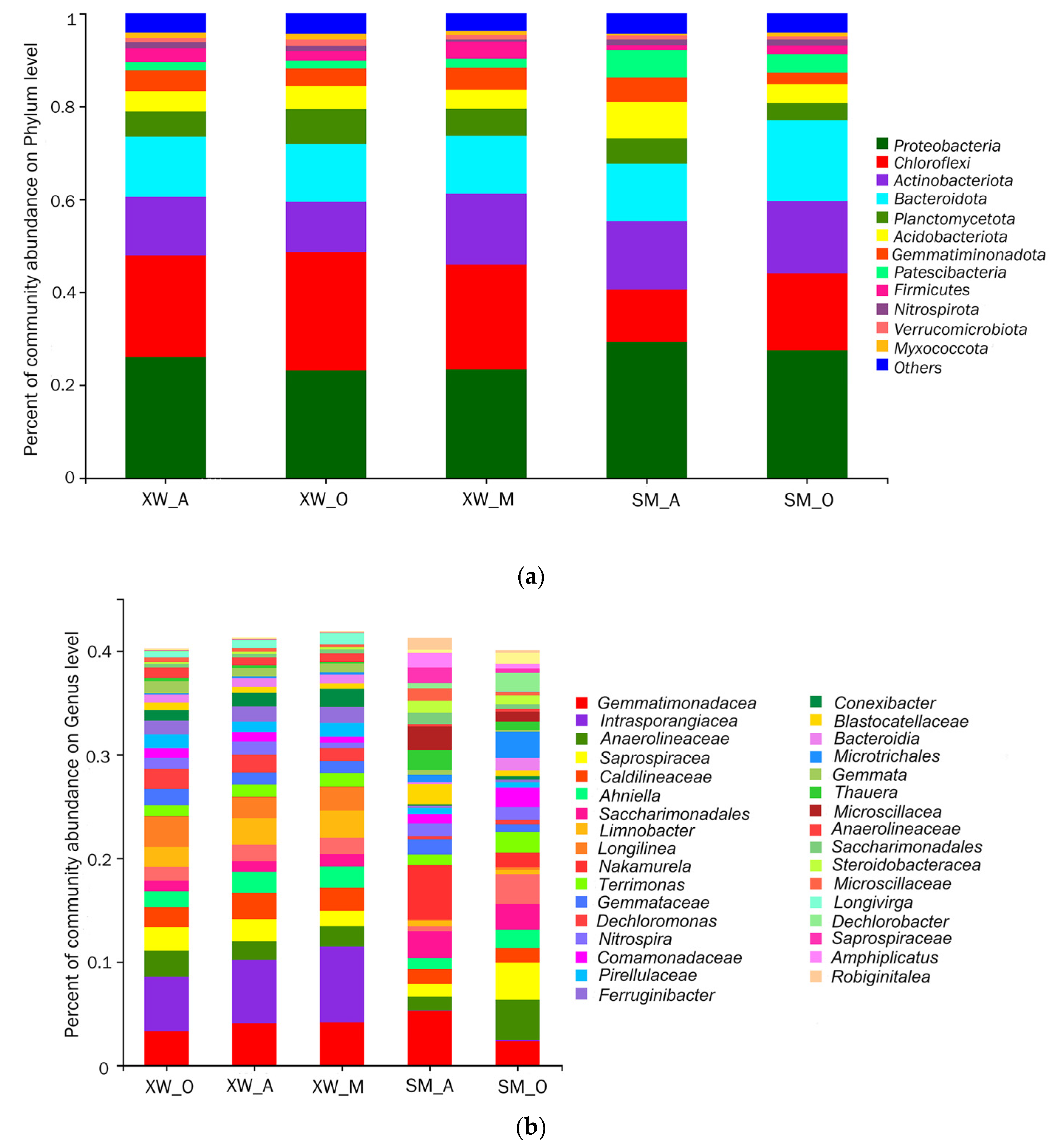
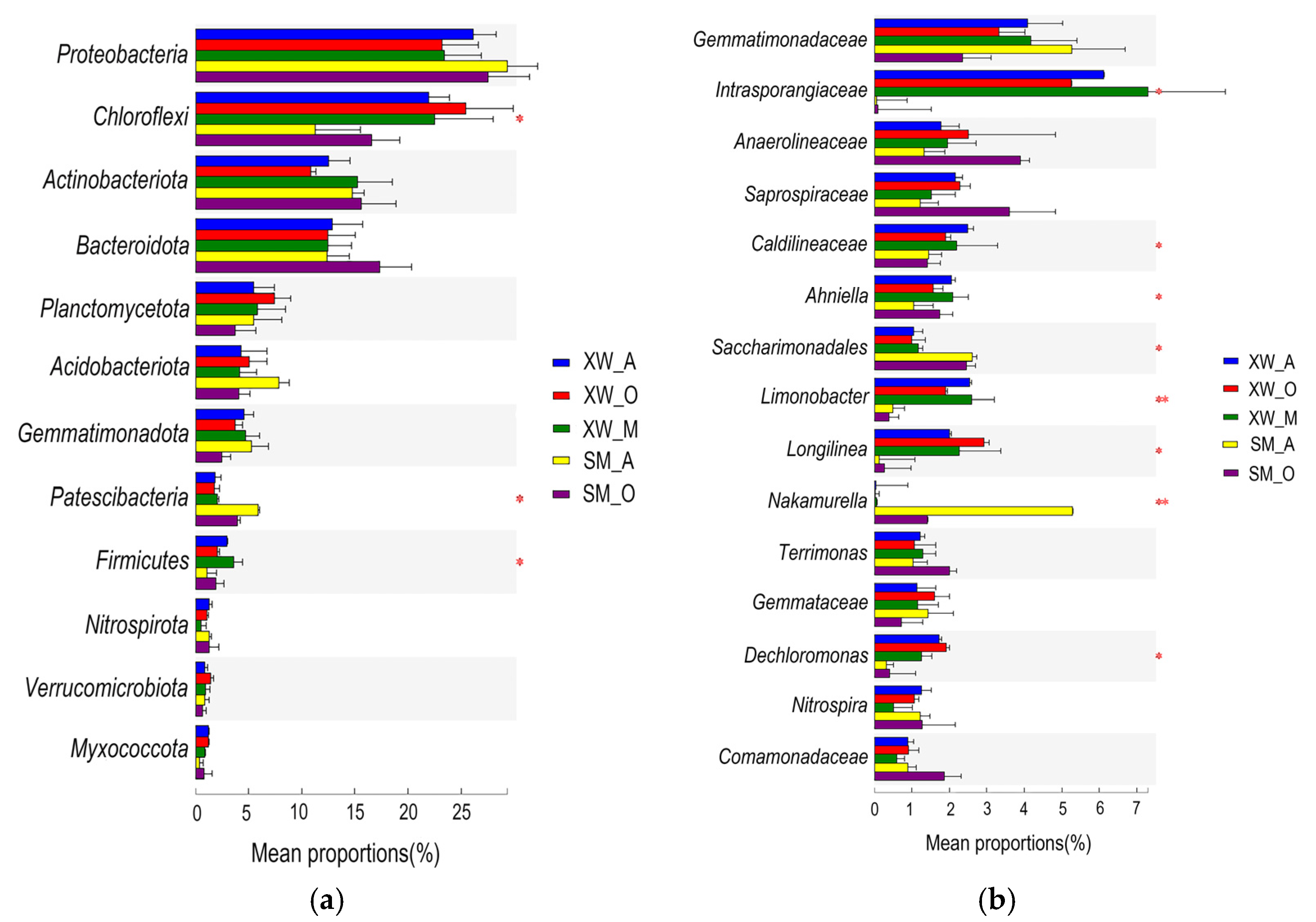
3.3. Microbial Community Composition
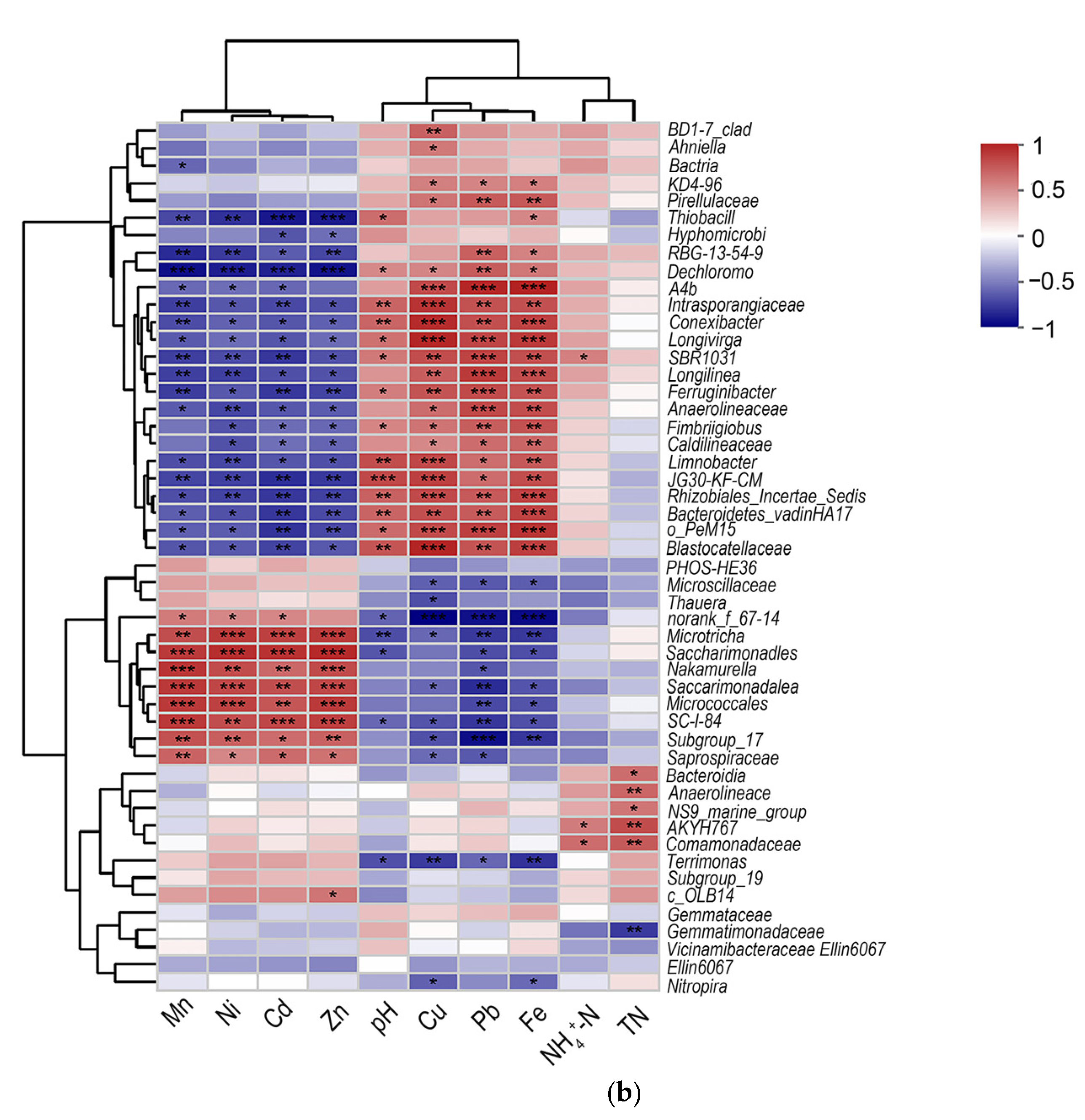
3.4. Effect of Environmental Factors on Microbial Community Structure
3.5. Differentiation of the Microbial Communities
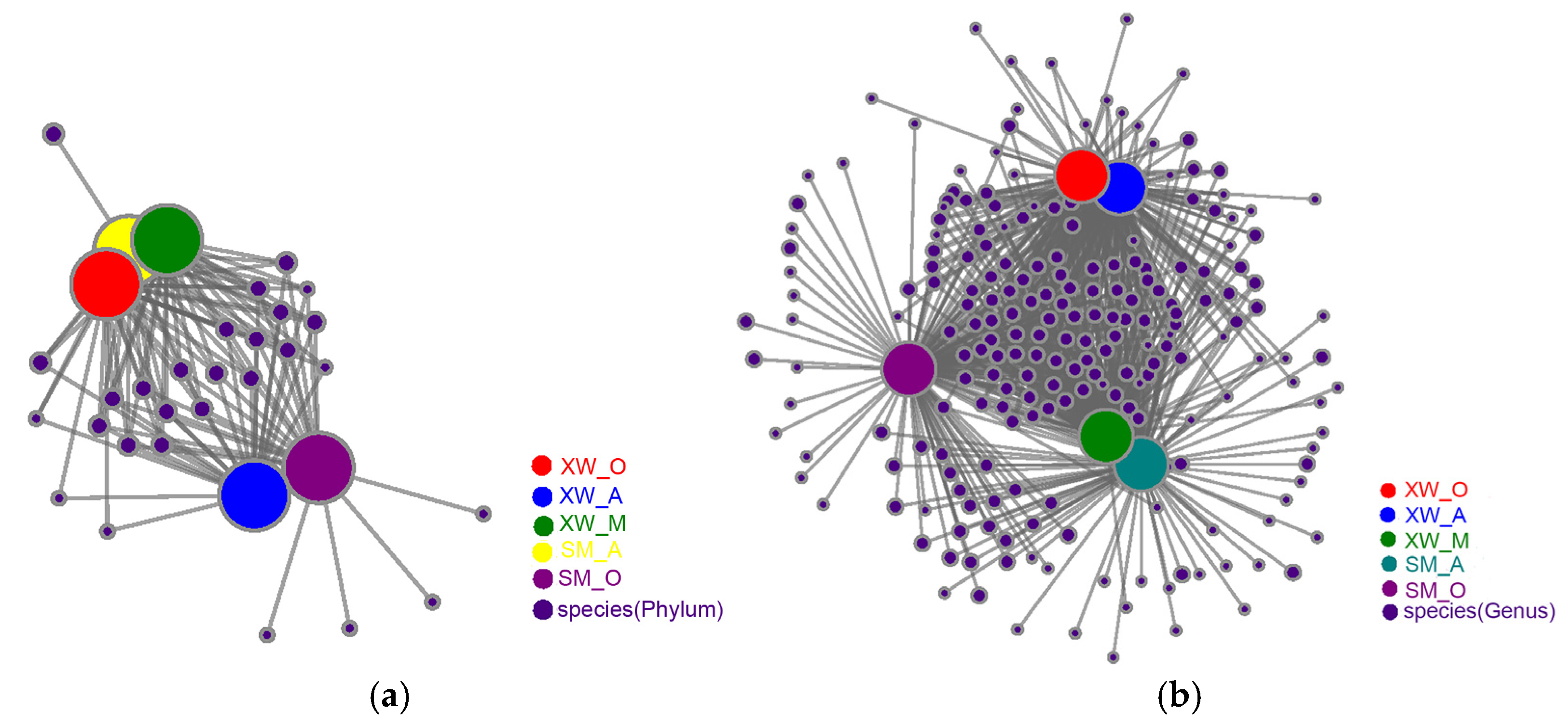
3.6. Microbial Ecological Network Analysis
3.7. Microbial Functional Prediction
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| WWTP | Wastewater treatment plant |
| HM | Heavy metal |
| AS | Activated sludge |
| SS | Suspended solid |
| TP | Total phosphorus |
| NH3-N | Nitrate nitrogen |
| TN | Total nitrogen |
| COD | Chemical oxygen demand |
| BOD5 | Biochemical oxygen demand |
| MBR | Membrane bioreactor |
| SRT | Sludge retention time |
References
- Li, H.X.; Xu, L.; Feng, N.N.; Lu, A.X.; Chen, W.; Wang, Y.P. Occurrence, risk assessment, and source of heavy metals in Liaohe River Protected Area from the watershed of Bohai Sea, China. Mar. Pollut. Bull. 2021, 169, 112489. [Google Scholar] [CrossRef] [PubMed]
- Bafana, A.; Kumar, G.; Kashyap, S.M.; Kanade, G.S.; Shinde, V.M. Dynamics of effluent treatment plant during commissioning of activated sludge process unit. Environ. Sci. Pollut. Res. Int. 2015, 22, 3538–3546. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Zhang, Z.Z.; Xing, X.L.; Hu, T.P.; Qu, C.K.; Chen, W.; Zhang, J.Q. Potentially toxic metals in soil and dominant plants from Tonglushan Cu-Fe deposit, central China. Bull. Environ. Contam. Toxicol. 2019, 102, 92–97. [Google Scholar] [CrossRef]
- Lu, A.; Li, B.; Li, J.; Chen, W.; Xu, L. Heavy metals in paddy soil-rice systems of industrial and township areas from subtropical China: Levels, transfer and health risks. J. Gemochem. Explor. 2018, 194, 210–217. [Google Scholar] [CrossRef]
- Sall, M.L.; Diaw, A.K.D.; Gningue-Sall, D.; Efremova Aaron, S.; Aaron, J.J. Toxic heavy metals: Impact on the environment and human health, and treatment with conducting organic polymers, a review. Environ. Sci. Pollut. Res. Int. 2020, 27, 29927–29942. [Google Scholar] [CrossRef] [PubMed]
- Mochizuki, H. Arsenic neurotoxicity in humans. Int. J. Mol. Sci. 2019, 20, 3418. [Google Scholar] [CrossRef] [Green Version]
- Rahimzadeh, M.R.; Rahimzadeh, M.R.; Kazemi, S.; Moghadamnia, A.-A. Cadmium toxicity and treatment: An update. Casp. J. Intern. Med. 2017, 8, 135–145. [Google Scholar]
- AQSIQ. Wastewater Quality Standards for Discharge to Municipal Sewers; China Environmental Science Press: Beijing, China, 2015. (In Chinese) [Google Scholar]
- SEPA. Discharge Standard of Pollutants for Municipal Wastewater Treatment Plant; China Environmental Science Press: Beijing, China, 2002. (In Chinese) [Google Scholar]
- Xia, Y.; Wen, X.H.; Zhang, B.; Yang, Y.F. Diversity and assembly patterns of activated sludge microbial communities: A review. Biotechnol. Adv. 2018, 36, 1038–1047. [Google Scholar] [CrossRef]
- Guo, J.H.; Ni, B.J.; Han, X.Y.; Chen, X.M.; Bond, P.; Peng, Y.Z.; Yuan, Z.G. Unraveling microbial structure and diversity of activated sludge in a full-scale simultaneous nitrogen and phosphorus removal plant using metagenomic sequencing. Enzym. Microb Technol. 2017, 102, 16–25. [Google Scholar] [CrossRef]
- Li, H.; Cai, Y.; Gu, Z.L.; Yang, Y.L.; Zhang, S.; Yang, X.L.; Song, H.L. Accumulation of sulfonamide resistance genes and bacterial community function prediction in microbial fuel cell-constructed wetland treating pharmaceutical wastewater. Chemosphere 2020, 248, 126014. [Google Scholar] [CrossRef]
- Samie, A.; Obi, C.L.; Igumbor, J.O.; Momba, M.N.B. Focus on 14 sewage treatment plants in the Mpumalanga Province, South Africa in order to gauge the efficiency of wastewater treatment. Afr. J. Biotechnol. 2009, 8, 3276–3285. [Google Scholar]
- Ye, D.D.; Liang, H.B.; Zhou, W.; Yan, J.W.; Zhou, S.Q.; Luo, L.X. Total and active microbial communities in a full-scale system treating wastewater from soy sauce production. Int. Biodeterior. Biodegrad. 2017, 123, 206–215. [Google Scholar] [CrossRef]
- Zeng, T.T.; Jiang, X.M.; Han, K.C.; Chen, S.B.; Zhou, Y.H.; Liu, H.Y. Analysis of microbial community constituent composition of some sewage treatment and processing plant. J. Saf. Environ. 2018, 18, 697–703. (In Chinese) [Google Scholar]
- Zhang, Y.; Hua, Z.S.; Lu, H.; Oehmen, A.; Guo, J.H. Elucidating functional microorganisms and metabolic mechanisms in a novel engineered ecosystem integrating C, N, P and S biotransformation by metagenomics. Water Res. 2019, 148, 219–230. [Google Scholar] [CrossRef]
- Xu, R.; Huang, D.Y.; Sun, X.X.; Zhang, M.M.; Wang, D.B.; Yang, Z.H.; Jiang, F.; Gao, P.; Li, B.; Sun, W. Diversity and metabolic potentials of As(III)-oxidizing bacteria in activated sludge. Appl. Environ. Microbiol. 2021, 87, e0176921. [Google Scholar] [CrossRef] [PubMed]
- Bhat, S.A.; Cui, G.; Li, W.; Wei, Y.; Li, F. Effect of heavy metals on the performance and bacterial profiles of activated sludge in a semi-continuous reactor. Chemosphere 2020, 241, 125035. [Google Scholar] [CrossRef]
- Zeng, T.T.; Rene, E.R.; Hu, Q.; Lens, P.N.L. Continuous biological removal of selenate in the presence of cadmium and zinc in UASB reactors at psychrophilic and mesophilic conditions. Biochem. Eng. J. 2019, 141, 102–111. [Google Scholar] [CrossRef]
- Rosenfeld, C.E.; James, B.R.; Santelli, C.M. Persistent bacterial and fungal community shifts exhibited in selenium-contaminated reclaimed mine soils. Appl. Environ. Microbiol. 2018, 84, e01394–e01418. [Google Scholar] [CrossRef] [Green Version]
- Zeng, T.T.; Mo, G.H.; Hu, Q.; Wang, G.H.; Liao, W.; Xie, S.B. Microbial characteristic and bacterial community assessment of sediment sludge upon uranium exposure. Environ. Pollut. 2020, 261, 114176. [Google Scholar] [CrossRef]
- Chen, Y.J.; Huang, H.F.; Ding, Y.; Chen, W.W.; Luo, J.; Li, H.; Wu, J.; Chen, W.; Qi, S.H. Trace metals in aquatic environments of a mangrove ecosystem in Nansha, Guangzhou, South China: Pollution status, sources, and ecological risk assessment. Environ. Monit. Assess. 2019, 191, 629. [Google Scholar] [CrossRef]
- Liu, J.l.; Yao, J.; Wang, F.; Min, N.; Gu, J.H.; Li, Z.F.; Sunahara, G.; Duran, R.; Solevic-Knudsen, T.; Hudson-Edwards, K.A.; et al. Bacterial diversity in typical abandoned multi-contaminated nonferrous metal (loid) tailings during natural attenuation. Environ. Pollut. 2019, 247, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Langille, M.G.; Zaneveld, J.; Caporaso, J.G.; McDonald, D.; Knights, D.; Reyes, J.A.; Clemente, J.C.; Burkepile, D.E.; Vega Thurber, R.L.; Knight, R.; et al. Predictive functional profiling of microbial communities using 16S rRNA marker gene sequences. Nat. Biotechnol. 2013, 31, 814–821. [Google Scholar] [CrossRef] [PubMed]
- NEPA. Water and Waste Water Monitor Analysis Method; China Environmental Science Press: Beijing, China, 1989. (In Chinese) [Google Scholar]
- Zeng, T.T.; Li, D.; Jiang, X.M.; Qiu, W.X.; Chen, Q.; Zhang, J. Microbial characteristics of an ANAMMOX biofilter for sewage treatment. J. Water Process Eng. 2016, 12, 105–110. [Google Scholar] [CrossRef]
- Shuaib, M.; Azam, N.; Bahadur, S.; Romman, M.; Yu, Q.; Xiu, C.X. Variation and succession of microbial communities under the conditions of persistent heavy metal and their survival mechanism. Microb. Pathog. 2021, 150, 104713. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Pandey, A.K.; Kim, S.H.; Singh, S.P.; Chaturvedi, P.; Varjani, S. Critical review on microbial community during in-situ bioremediation of heavy metals from industrial wastewater. Environ. Technol. Inno. 2021, 24, 101826. [Google Scholar] [CrossRef]
- Yang, Y.K.; Wang, L.F.; Xiang, F.; Zhao, L.; Qiao, Z. Activated sludge microbial community and treatment performance of wastewater treatment plants in industrial and municipal zones. Int. J. Environ. Res. Public Health 2020, 17, 436. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, W.; Staley, C.; Sidhu, J.; Sadowsky, M.; Toze, S. Amplicon-based profiling of bacteria in raw and secondary treated wastewater from treatment plants across Australia. Appl. Microbiol. Biotechnol. 2017, 101, 1253–1266. [Google Scholar] [CrossRef]
- Madigan, M.T.; Martinko, J.M.; Bender, K.S.; Buckley, D.H.; Stahl, D.A. Brock biology of microorganisms. Int. Microbiol. 2008, 11, 65–73. [Google Scholar]
- Wang, M.; Xiong, W.G.; Zou, Y.; Lin, M.X.; Zhou, Q.; Xie, X.Y.; Sun, Y.X. Evaluating the net effect of sulfadimidine on nitrogen removal in an aquatic microcosm environment. Environ. Pollut. 2019, 248, 1010–1019. [Google Scholar] [CrossRef]
- Qin, H.; Ji, B.; Zhang, S.F.; Kong, Z.H. Study on the bacterial and archaeal community structure and diversity of activated sludge from three wastewater treatment plants. Mar. Pollut. Bull. 2018, 135, 801–807. [Google Scholar] [CrossRef]
- Nierychlo, M.; Milobedzka, A.; Petriglieri, F.; McIlroy, B.; Nielsen, P.H.; McIlroy, S.J. The morphology and metabolic potential of the Chloroflexi in full-scale activated sludge wastewater treatment plants. FEMS Microbiol. Ecol. 2019, 95, fiy228. [Google Scholar] [CrossRef] [PubMed]
- Speirs, L.B.M.; Rice, D.T.F.; Petrovski, S.; Seviour, R.J. The phylogeny, biodiversity, and ecology of the Chloroflexi in activated sludge. Front. Microbiol. 2019, 10, 2015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, N.; Zhong, L.; Ouyang, L.; Xu, W.; Zeng, Q.; Wang, K.; Zaynab, M.; Chen, H.; Xu, F.; Li, S. Community composition and function of bacteria in activated sludge of municipal wastewater treatment plants. Water 2021, 13, 852. [Google Scholar] [CrossRef]
- Hu, H.L.; Deng, C.L.; Wang, X.J.; Chen, Z.G.; Zhong, Z.; Wang, R.X. Performance and mechanism of urea hydrolysis in partial nitritation system based on SBR. Chemosphere 2020, 258, 127228. [Google Scholar] [CrossRef] [PubMed]
- Su, C.Y.; Deng, Q.J.; Lu, Y.X.; Qin, R.H.; Chen, S.L.; Wei, J.W.; Chen, M.L.; Huang, Z. Effects of hydraulic retention time on the performance and microbial community of an anaerobic baffled reactor-bioelectricity Fenton coupling reactor for treatment of traditional Chinese medicine wastewater. Bioresour. Technol. 2019, 288, 121508. [Google Scholar] [CrossRef]
- Xia, T.X.; Zhang, X.L.; Wang, H.M.; Zhang, Y.C.; Gao, Y.; Bian, C.C.; Wang, X.; Xu, P. Power generation and microbial community analysis in microbial fuel cells: A promising system to treat organic acid fermentation wastewater. Bioresour. Technol. 2019, 284, 72–79. [Google Scholar] [CrossRef]
- Huang, X.F.; Wu, X.L.; Tang, X.C.; Zhang, Z.M.; Ma, J.R.; Zhang, J.C.; Liu, H.J. Distribution characteristics and risk of heavy metals and microbial community composition around the Wanshan mercury mine in Southwest China. Ecotoxicol. Environ. Saf. 2021, 227, 112897. [Google Scholar] [CrossRef]
- Yan, W.Z.; Wang, N.; Wei, D.; Liang, C.Y.; Chen, X.M.; Liu, L.; Shi, J.P. Bacterial community compositions and nitrogen metabolism function in a cattle farm wastewater treatment plant revealed by Illumina high-throughput sequencing. Environ. Sci. Pollut. Res. Int. 2021, 28, 40895–40907. [Google Scholar] [CrossRef]
- Ogwugwa, V.H.; Oyetibo, G.O.; Amund, O.O. Taxonomic profiling of bacteria and fungi in freshwater sewer receiving hospital wastewater. Environ. Res. 2021, 192, 110319. [Google Scholar] [CrossRef]
- Guo, Q.W.; Li, N.N.; Xie, S.G. Heavy metal spill influences bacterial communities in freshwater sediments. Arch. Microbiol. 2019, 201, 847–854. [Google Scholar] [CrossRef]
- Liu, Y.; Gao, T.P.; Wang, X.Y.; Fu, J.W.; Zuo, M.B.; Yang, Y.L.; Yin, Z.X.; Wang, Z.Z.; Tai, X.S.; Chang, G.H. Effects of heavy metals on bacterial community surrounding Bijiashan mining area located in northwest China. Open Life Sci. 2022, 17, 40–54. [Google Scholar] [CrossRef] [PubMed]
- Betiku, O.C.; Sarjeant, K.C.; Ngatia, L.W.; Aghimien, M.O.; Odewumi, C.O.; Latinwo, L.M. Evaluation of microbial diversity of three recreational water bodies using 16S rRNA metagenomic approach. Sci. Total Environ. 2021, 771, 144773. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.C.; Yao, X.Y.; Jin, H.Y.; Sun, Q.; Zou, Z.S.; Yang, W.J.; He, Z.W.; Zhou, A.J.; Chen, F.; Ren, Y.X.; et al. Stepwise freezing-thawing treatment promotes short-chain fatty acids production from waste activated sludge. Sci. Total Environ. 2021, 818, 151694. [Google Scholar] [CrossRef] [PubMed]
- Xing, W.; Wang, Y.; Hao, T.Y.; He, Z.G.; Jia, F.X.; Yao, H. pH control and microbial community analysis with HCl or CO2 addition in H2-based autotrophic denitrification. Water Res. 2020, 168, 115200. [Google Scholar] [CrossRef] [PubMed]
- Gao, T.P.; Li, H.J.; He, Y.Q.; Shen, Y.Y.; Li, G.W.; Li, X.K.; Chen, Y.L.; Liu, Y.B.; Li, C.M.; Ji, J.; et al. The variations of bacterial community structures in tailing soils suffering from heavy metal contaminations. Water Air Soil Pollut. 2021, 232, 392. [Google Scholar] [CrossRef]
- Liang, X.; Yao, X.Y.; Li, L. Development and application of AAOA high-standard phosphorus and nitrogen removal technology for urban sewage. Chin. J. Environ. Eng. 2022, 16, 612–620. (In Chinese) [Google Scholar]
- Miao, L.; Wang, S.Y.; Li, B.K.; Cao, T.H.; Zhang, F.Z.; Wang, Z.; Peng, Y.Z. Effect of carbon source type on intracellular stored polymers during endogenous denitritation (ED) treating landfill leachate. Water Res. 2016, 100, 405–412. [Google Scholar] [CrossRef]
- Cao, J.S.; Zhang, T.; Wu, Y.; Sun, Y.Q.; Zhang, Y.L.; Huang, B.; Fu, B.M.; Yang, E.; Zhang, Q.; Luo, J.Y. Correlations of nitrogen removal and core functional genera in full-scale wastewater treatment plants: Influences of different treatment processes and influent characteristics. Bioresour. Technol. 2020, 297, 122455. [Google Scholar] [CrossRef]
- Cao, J.H.; Zhu, Q.R.; Zhang, T.; Wu, Y.; Zhang, Q.; Fu, B.M.; Fang, F.; Feng, Q.; Luo, J.Y. Distribution patterns of microbial community and functional characteristics in full-scale wastewater treatment plants: Focusing on the influent types. Chemosphere 2021, 281, 130899. [Google Scholar] [CrossRef]
- Wang, C.Y.; Wu, B.D.; Jiang, K.; Wei, M.; Wang, S. Effects of different concentrations and types of Cu and Pb on soil N-fixing bacterial communities in the wheat rhizosphere. Appl. Soil Ecol. 2019, 144, 51–59. [Google Scholar] [CrossRef]
- Zhang, L.; Fan, J.; Nguyen, H.N.; Li, S.; Rodrigues, D.F. Effect of cadmium on the performance of partial nitrification using sequencing batch reactor. Chemosphere 2019, 222, 913–922. [Google Scholar] [CrossRef]
- Qi, Q.X.; Sun, C.; Cristhian, C.; Zhang, T.Y.; Zhang, J.X.; Tian, H.L.; He, Y.L.; Tong, Y.W. Enhancement of methanogenic performance by gasification biochar on anaerobic digestion. Bioresour. Technol. 2021, 330, 124993. [Google Scholar] [CrossRef] [PubMed]
- Tsui, T.H.; Zhang, L.; Lim, E.Y.; Lee, J.T.E.; Tong, Y.W. Timing of biochar dosage for anaerobic digestion treating municipal leachate: Altered conversion pathways of volatile fatty acids. Bioresour. Technol. 2021, 335, 125283. [Google Scholar] [CrossRef] [PubMed]
- Tsui, T.H.; Zhang, L.; Zhang, J.X.; Dai, Y.J.; Tong, Y.W. Engineering interface between bioenergy recovery and biogas desulfurization: Sustainability interplays of biochar application. Renew. Sustain. Energy Rev. 2022, 157, 112053. [Google Scholar] [CrossRef]
- Hosokawa, S.; Kuroda, K.; Narihiro, T.; Aoi, Y.; Ozaki, N.; Ohashi, A.; Kindaichi, T. Cometabolism of the superphylum patescibacteria with anammox bacteria in a long-term freshwater anammox column reactor. Water 2021, 13, 208. [Google Scholar] [CrossRef]
- Wu, H.T.; Mi, Z.L.; Zhang, J.X.; Chen, C.; Xie, S.G. Bacterial communities associated with an occurrence of colored water in an urban drinking water distribution system. Biomed. Environ. Sci. 2014, 27, 646–650. [Google Scholar]
- Zhang, L.; Shen, Z.; Fang, W.K.; Gao, G. Composition of bacterial communities in municipal wastewater treatment plant. Sci. Total Environ. 2019, 689, 1181–1191. [Google Scholar] [CrossRef]
- Zeng, T.T.; Hu, Q.; Zhang, X.L.; Wang, L.Q.; Cai, P.L.; Zhang, Y.; Liu, Y.J. Effectiveness of anaerobic granular sludge in treating acidic wastewater containing Se and Cd with low carbon source and microbial community characteristics. J. Environ. Eng. 2021, 15, 2080–2090. (In Chinese) [Google Scholar]
- Wang, K.D.; Chen, X.L.; Yan, D.K.; Xu, Z.C.; Hu, P.J.; Li, H.S. Petrochemical and municipal wastewater treatment plants activated sludge each own distinct core bacteria driven by their specific incoming wastewater. Sci. Total Environ. 2022, 826, 153962. [Google Scholar] [CrossRef]
- Delforno, T.P.; Lacerda, G.V., Jr.; Sierra-Garcia, I.N.; Okada, D.Y.; Macedo, T.Z.; Varesche, M.B.; Oliveira, V.M. Metagenomic analysis of the microbiome in three different bioreactor configurations applied to commercial laundry wastewater treatment. Sci. Total Environ. 2017, 587, 389–398. [Google Scholar] [CrossRef]
- Ou, D.; Li, W.; Li, H.; Wu, X.; Li, C.; Zhuge, Y.Y.; Liu, Y.D. Enhancement of the removal and settling performance for aerobic granular sludge under hypersaline stress. Chemosphere 2018, 212, 400–407. [Google Scholar] [CrossRef] [PubMed]


| BOD5 | COD | NH3-N | TN | TP | |
|---|---|---|---|---|---|
| SM-WWTP | 130 | 300 | 25 | 35 | 4 |
| XW-WWTP | 130 | 250 | 20 | 35 | 3 |
| Sample | Shannon | Simpson | Ace | Chao | Coverage |
|---|---|---|---|---|---|
| SM_A | 5.963 ± 0.079 | 0.009 ± 0.001 | 3106.841 ± 44.726 | 3070.979 ± 65.839 | 0.982 ± 0 |
| SM_O | 6.247 ± 0.106 | 0.005 ± 0.001 | 3148.173 ± 98.253 | 3170.752 ± 86.135 | 0.982 ± 0 |
| XW_A | 5.906 ± 0.125 | 0.01 ± 0.001 | 2920.2 ± 143.276 | 2945.665 ± 152.135 | 0.983 ± 0.001 |
| XW_M | 5.873 ± 0.054 | 0.01 ± 0.001 | 3026.602 ± 284.754 | 2963.67 ± 71.806 | 0.983 ± 0.001 |
| XW_O | 5.957 ± 0.06 | 0.009 ± 0.002 | 2973.613 ± 126.663 | 3003.2 ± 164.09 | 0.983 ± 0.001 |
| Node Name (The Top) | Degree | Weighted Degree | |
|---|---|---|---|
| Phylum | Proteobacteria | 5 | 52,766 |
| Chloroflexi | 5 | 39,755 | |
| Actinobacteriota | 5 | 28,079 | |
| Bacteroidota | 5 | 27,485 | |
| Planctomycetota | 5 | 11,367 | |
| Genus | Gemmatimonadaceae | 5 | 7810 |
| Intrasporangiaceae | 5 | 7659 | |
| Anaerolineaceae | 5 | 4662 | |
| Saprospiraceae | 5 | 4394 | |
| Caldilineaceae | 5 | 3840 | |
| Ahniella | 5 | 3472 | |
| Saccharimonadales | 5 | 3371 | |
| Limnobacter | 5 | 3218 | |
| Longilinea | 5 | 3092 | |
| Nakamurella | 5 | 2779 |
| Phylum Level | Genus Level | ||
|---|---|---|---|
| Node Name | Degree | Node Name | Degree |
| SM_A | 52 | SM_A | 868 |
| SM_O | 55 | SM_O | 872 |
| XW_A | 56 | XW_A | 904 |
| XW_O | 55 | XW_O | 889 |
| XW_M | 53 | XW_M | 855 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeng, T.; Wang, L.; Zhang, X.; Song, X.; Li, J.; Yang, J.; Chen, S.; Zhang, J. Characterization of Microbial Communities in Wastewater Treatment Plants Containing Heavy Metals Located in Chemical Industrial Zones. Int. J. Environ. Res. Public Health 2022, 19, 6529. https://doi.org/10.3390/ijerph19116529
Zeng T, Wang L, Zhang X, Song X, Li J, Yang J, Chen S, Zhang J. Characterization of Microbial Communities in Wastewater Treatment Plants Containing Heavy Metals Located in Chemical Industrial Zones. International Journal of Environmental Research and Public Health. 2022; 19(11):6529. https://doi.org/10.3390/ijerph19116529
Chicago/Turabian StyleZeng, Taotao, Liangqin Wang, Xiaoling Zhang, Xin Song, Jie Li, Jinhui Yang, Shengbing Chen, and Jie Zhang. 2022. "Characterization of Microbial Communities in Wastewater Treatment Plants Containing Heavy Metals Located in Chemical Industrial Zones" International Journal of Environmental Research and Public Health 19, no. 11: 6529. https://doi.org/10.3390/ijerph19116529
APA StyleZeng, T., Wang, L., Zhang, X., Song, X., Li, J., Yang, J., Chen, S., & Zhang, J. (2022). Characterization of Microbial Communities in Wastewater Treatment Plants Containing Heavy Metals Located in Chemical Industrial Zones. International Journal of Environmental Research and Public Health, 19(11), 6529. https://doi.org/10.3390/ijerph19116529






