Pulse Wave Velocity and Sarcopenia in Older Persons—A Systematic Review and Meta-Analysis
Abstract
:1. Introduction
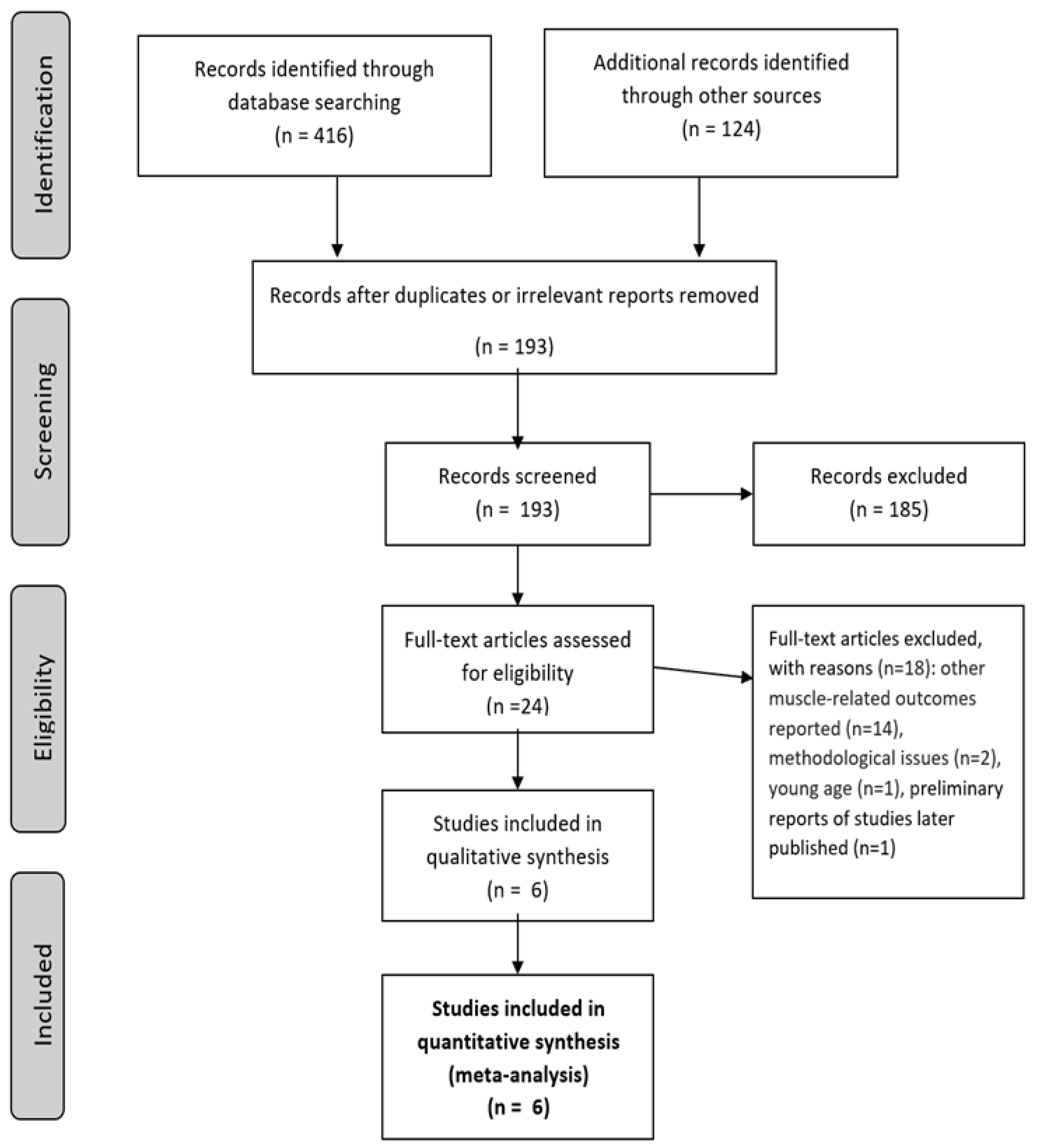
2. Materials and Methods
(sarcopenia [Title/Abstract]) AND (arterial stiffness [Title/Abstract]); (sarcopenia [Title/Abstract]) AND (cardio-ankle vascular index [Title/Abstract]); (sarcopenia [Title/Abstract]) AND (cavi [Title/Abstract]); (sarcopenia [Title/Abstract]) AND (pulse wave velocity [Title/Abstract]); (sarcopenia [Title/Abstract]) AND (pwv [Title/Abstract]); (sarcopenia [Title/Abstract]) AND (augmentation index [Title/Abstract]); (sarcopenia [Title/Abstract]) AND (aIx [Title/Abstract])
3. Results
3.1. The Characteristics of the Included Studies
3.2. The Measures of Sarcopenia
3.3. Pulse Wave Velocity
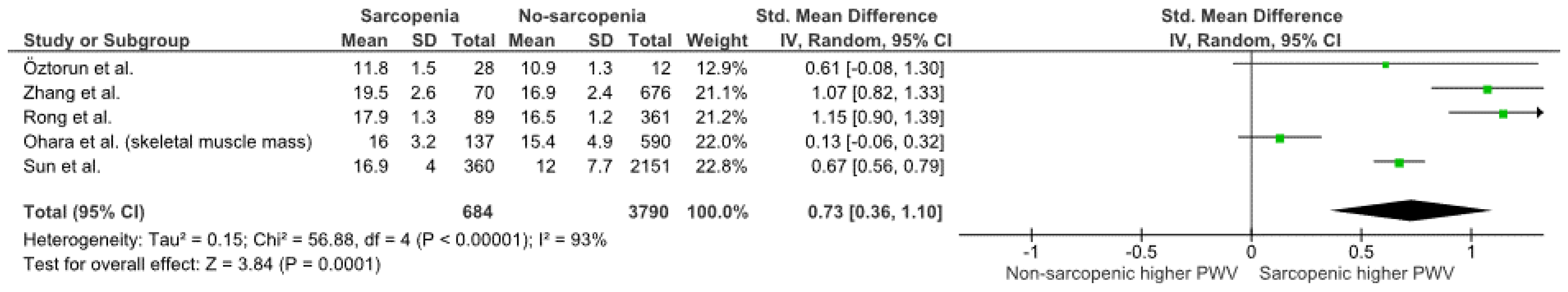
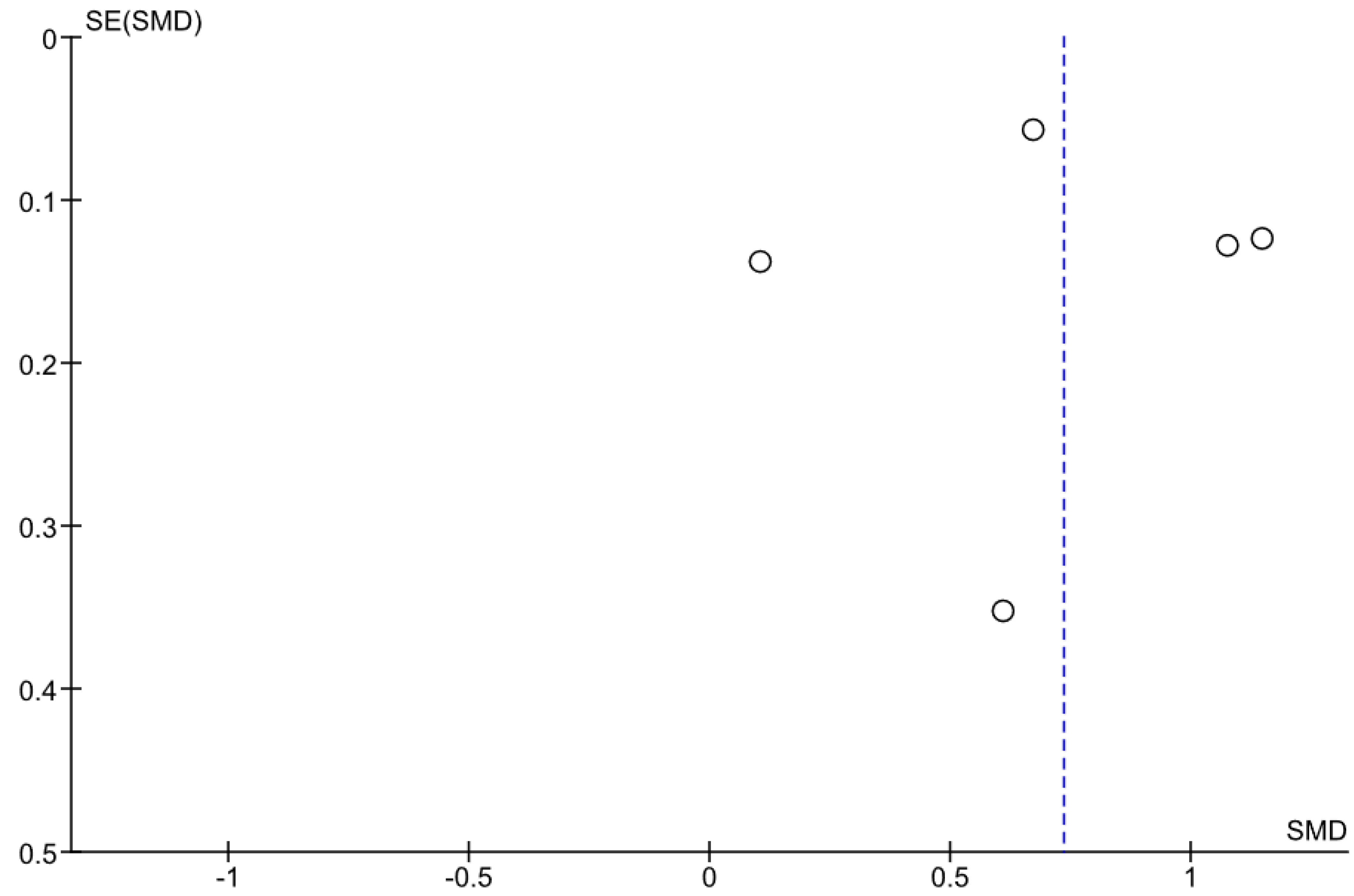
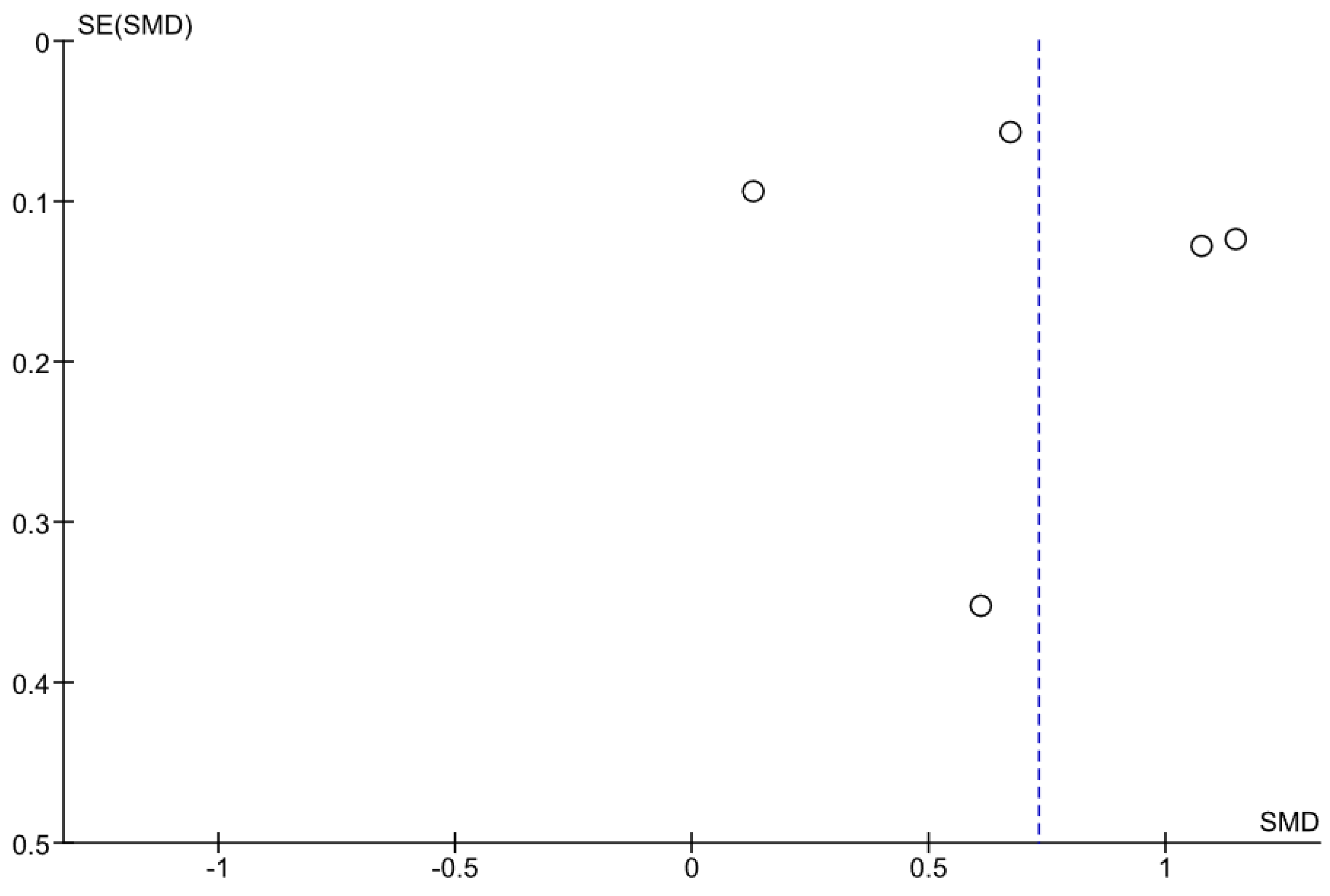
3.4. Relation between PWV and Sarcopenia
4. Discussion
4.1. Sarcopenia and Cardiovascular Disease
4.2. Aging of Arterial System
4.3. Putative Pathophysiologic Link between Arterial Stiffness and Sarcopenia
4.4. The Impact of Methodology of PWV Assessment on the Meta-Analysis Results
4.5. The Impact of Methodology of Muscular Assessment on the Meta-Analysis Results
4.6. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Petermann-Rocha, F.; Balntzi, V.; Gray, S.R.; Lara, J.; Ho, F.K.; Pell, J.P.; Celis-Morales, C. Global Prevalence of Sarcopenia and Severe Sarcopenia: A Systematic Review and Meta-Analysis. J. Cachexia Sarcopenia Muscle 2022, 13, 86–99. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Sayer, A.A. Sarcopenia. Lancet Lond. Engl. 2019, 393, 2636–2646. [Google Scholar] [CrossRef]
- He, N.; Zhang, Y.; Zhang, L.; Zhang, S.; Ye, H. Relationship Between Sarcopenia and Cardiovascular Diseases in the Elderly: An Overview. Front. Cardiovasc. Med. 2021, 8, 743710. [Google Scholar] [CrossRef]
- Ligthart-Melis, G.C.; Luiking, Y.C.; Kakourou, A.; Cederholm, T.; Maier, A.B.; de van der Schueren, M.A.E. Frailty, Sarcopenia, and Malnutrition Frequently (Co-)Occur in Hospitalized Older Adults: A Systematic Review and Meta-Analysis. J. Am. Med. Dir. Assoc. 2020, 21, 1216–1228. [Google Scholar] [CrossRef]
- Chirinos, J.A.; Segers, P.; Hughes, T.; Townsend, R. Large-Artery Stiffness in Health and Disease: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2019, 74, 1237–1263. [Google Scholar] [CrossRef]
- Staessen, J.A.; Gasowski, J.; Wang, J.G.; Thijs, L.; Hond, E.D.; Boissel, J.-P.; Coope, J.; Ekbom, T.; Gueyffier, F.; Liu, L.; et al. Risks of Untreated and Treated Isolated Systolic Hypertension in the Elderly: Meta-Analysis of Outcome Trials. Lancet 2000, 355, 865–872. [Google Scholar] [CrossRef]
- Vlachopoulos, C.; Aznaouridis, K.; Stefanadis, C. Prediction of Cardiovascular Events and All-Cause Mortality with Arterial Stiffness: A Systematic Review and Meta-Analysis. J. Am. Coll. Cardiol. 2010, 55, 1318–1327. [Google Scholar] [CrossRef] [Green Version]
- Sequí-Domínguez, I.; Cavero-Redondo, I.; Álvarez-Bueno, C.; Pozuelo-Carrascosa, D.P.; De Arenas-Arroyo, S.N.; Martínez-Vizcaíno, V. Accuracy of Pulse Wave Velocity Predicting Cardiovascular and All-Cause Mortality. A Systematic Review and Meta-Analysis. J. Clin. Med. 2020, 9, 2080. [Google Scholar] [CrossRef]
- Tap, L.; Kirkham, F.A.; Mattace-Raso, F.; Joly, L.; Rajkumar, C.; Benetos, A. Unraveling the Links Underlying Arterial Stiffness, Bone Demineralization, and Muscle Loss. Hypertension 2020, 76, 629–639. [Google Scholar] [CrossRef]
- Banks, N.F.; Rogers, E.M.; Church, D.D.; Ferrando, A.A.; Jenkins, N.D.M. The Contributory Role of Vascular Health in Age-Related Anabolic Resistance. J. Cachexia Sarcopenia Muscle 2022, 13, 114–127. [Google Scholar] [CrossRef]
- Piotrowicz, K.; Gąsowski, J. Risk Factors for Frailty and Cardiovascular Diseases: Are They the Same? Adv. Exp. Med. Biol. 2020, 1216, 39–50. [Google Scholar] [CrossRef]
- Migliavacca, E.; Tay, S.K.H.; Patel, H.P.; Sonntag, T.; Civiletto, G.; McFarlane, C.; Forrester, T.; Barton, S.J.; Leow, M.K.; Antoun, E.; et al. Mitochondrial Oxidative Capacity and NAD+ Biosynthesis Are Reduced in Human Sarcopenia across Ethnicities. Nat. Commun. 2019, 10, 5808. [Google Scholar] [CrossRef] [PubMed]
- Daussin, F.N.; Boulanger, E.; Lancel, S. From Mitochondria to Sarcopenia: Role of Inflammaging and RAGE-Ligand Axis Implication. Exp. Gerontol. 2021, 146, 111247. [Google Scholar] [CrossRef] [PubMed]
- Piotrowicz, K.; Gryglewska, B.; Grodzicki, T.; Gąsowski, J. Arterial Stiffness and Frailty—A Systematic Review and Metaanalysis. Exp. Gerontol. 2021, 153, 111480. [Google Scholar] [CrossRef] [PubMed]
- Amarasekera, A.T.; Chang, D.; Schwarz, P.; Tan, T.C. Vascular Endothelial Dysfunction May Be an Early Predictor of Physical Frailty and Sarcopenia: A Meta-Analysis of Available Data from Observational Studies. Exp. Gerontol. 2021, 148, 111260. [Google Scholar] [CrossRef] [PubMed]
- Amarasekera, A.T.; Chang, D.; Schwarz, P.; Tan, T.C. Does Vascular Endothelial Dysfunction Play a Role in Physical Frailty and Sarcopenia? A Systematic Review. Age Ageing 2021, 50, 725–732. [Google Scholar] [CrossRef]
- Dvoretskiy, S.; Lieblein-Boff, J.C.; Jonnalagadda, S.; Atherton, P.J.; Phillips, B.E.; Pereira, S.L. Exploring the Association between Vascular Dysfunction and Skeletal Muscle Mass, Strength and Function in Healthy Adults: A Systematic Review. Nutrients 2020, 12, 715. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodríguez, A.J.; Karim, M.N.; Srikanth, V.; Ebeling, P.R.; Scott, D. Lower Muscle Tissue Is Associated with Higher Pulse Wave Velocity: A Systematic Review and Meta-Analysis of Observational Study Data. Clin. Exp. Pharmacol. Physiol. 2017, 44, 980–992. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. PRISMA Group Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [Green Version]
- Shamseer, L.; Moher, D.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A. PRISMA-P Group Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols (PRISMA-P) 2015: Elaboration and Explanation. BMJ 2015, 350, g7647. [Google Scholar] [CrossRef] [Green Version]
- Munn, Z.; Tufanaru, C.; Aromataris, E. JBI’s Systematic Reviews: Data Extraction and Synthesis. Am. J. Nurs. 2014, 114, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Ohara, M.; Kohara, K.; Tabara, Y.; Igase, M.; Miki, T. Portable Indices for Sarcopenia Are Associated with Pressure Wave Reflection and Central Pulse Pressure: The J-SHIPP Study. J. Hypertens. 2015, 33, 314–322. [Google Scholar] [CrossRef]
- Öztorun, H.S.; Bahsi, R.; Turgut, T.; Surmeli, D.M.; Cosarderelioğlu, C.; Atmis, V.; Yalcin, A.; Aras, S.; Varli, M. The Relationship between Sarcopenia and Central Hemodynamics in Older Adults with Falls: A Cross-Sectional Study. Blood Press. Monit. 2022, 27, 87–97. [Google Scholar] [CrossRef]
- Rong, Y.-D.; Bian, A.-L.; Hu, H.-Y.; Ma, Y.; Zhou, X.-Z. A Cross-Sectional Study of the Relationships between Different Components of Sarcopenia and Brachial Ankle Pulse Wave Velocity in Community-Dwelling Elderly. BMC Geriatr. 2020, 20, 115. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Guo, Q.; Feng, B.-L.; Wang, C.-Y.; Han, P.-P.; Hu, J.; Sun, X.-D.; Zeng, W.-F.; Zheng, Z.-X.; Li, H.-S.; et al. A Cross-Sectional Study of the Association between Arterial Stiffness and Sarcopenia in Chinese Community-Dwelling Elderly Using the Asian Working Group for Sarcopenia Criteria. J. Nutr. Health Aging 2019, 23, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Ma, Z.; Gao, L.; Wang, Y.; Xie, H. Correlation between Sarcopenia and Arteriosclerosis in Elderly Community Dwellers: A Multicenter Study. J. Nutr. Health Aging 2021, 25, 692–697. [Google Scholar] [CrossRef]
- Zhang, Y.; Miyai, N.; Abe, K.; Utsumi, M.; Uematsu, Y.; Terada, K.; Nakatani, T.; Takeshita, T.; Arita, M. Muscle Mass Reduction, Low Muscle Strength, and Their Combination Are Associated with Arterial Stiffness in Community-Dwelling Elderly Population: The Wakayama Study. J. Hum. Hypertens. 2020, 35, 446–454. [Google Scholar] [CrossRef]
- Bahat, G.; Tufan, A.; Tufan, F.; Kilic, C.; Akpinar, T.S.; Kose, M.; Erten, N.; Karan, M.A.; Cruz-Jentoft, A.J. Cut-off Points to Identify Sarcopenia According to European Working Group on Sarcopenia in Older People (EWGSOP) Definition. Clin. Nutr. 2016, 35, 1557–1563. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European Consensus on Definition and Diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef] [Green Version]
- Janssen, I.; Heymsfield, S.B.; Baumgartner, R.N.; Ross, R. Estimation of Skeletal Muscle Mass by Bioelectrical Impedance Analysis. J. Appl. Physiol. 2000, 89, 465–471. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.-K.; Woo, J.; Assantachai, P.; Auyeung, T.-W.; Chou, M.-Y.; Iijima, K.; Jang, H.C.; Kang, L.; Kim, M.; Kim, S.; et al. Asian Working Group for Sarcopenia: 2019 Consensus Update on Sarcopenia Diagnosis and Treatment. J. Am. Med. Dir. Assoc. 2020, 21, 300–307.e2. [Google Scholar] [CrossRef]
- Chen, L.-K.; Liu, L.-K.; Woo, J.; Assantachai, P.; Auyeung, T.-W.; Bahyah, K.S.; Chou, M.-Y.; Chen, L.-Y.; Hsu, P.-S.; Krairit, O.; et al. Sarcopenia in Asia: Consensus Report of the Asian Working Group for Sarcopenia. J. Am. Med. Dir. Assoc. 2014, 15, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Buckinx, F.; Reginster, J.-Y.; Dardenne, N.; Croisiser, J.-L.; Kaux, J.-F.; Beaudart, C.; Slomian, J.; Bruyère, O. Concordance between Muscle Mass Assessed by Bioelectrical Impedance Analysis and by Dual Energy X-Ray Absorptiometry: A Cross-Sectional Study. BMC Musculoskelet. Disord. 2015, 16, 60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bai, T.; Fang, F.; Li, F.; Ren, Y.; Hu, J.; Cao, J. Sarcopenia Is Associated with Hypertension in Older Adults: A Systematic Review and Meta-Analysis. BMC Geriatr. 2020, 20, 279. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Y.-S.; Chai, Y.-H.; Gong, H.-J.; Zhuldyz, Z.; Stehouwer, C.D.A.; Zhou, J.-B.; Simó, R. The Association Between Diabetes Mellitus and Risk of Sarcopenia: Accumulated Evidences From Observational Studies. Front. Endocrinol. 2021, 12, 782391. [Google Scholar] [CrossRef] [PubMed]
- Jakubiak, G.K.; Pawlas, N.; Cieślar, G.; Stanek, A. Chronic Lower Extremity Ischemia and Its Association with the Frailty Syndrome in Patients with Diabetes. Int. J. Environ. Res. Public Health. 2020, 17, 9339. [Google Scholar] [CrossRef]
- Townsend, R.R.; Wilkinson, I.B.; Schiffrin, E.L.; Avolio, A.P.; Chirinos, J.A.; Cockcroft, J.R.; Heffernan, K.S.; Lakatta, E.G.; McEniery, C.M.; Mitchell, G.F.; et al. Recommendations for Improving and Standardizing Vascular Research on Arterial Stiffness: A Scientific Statement From the American Heart Association. Hypertension 2015, 66, 698–722. [Google Scholar] [CrossRef] [Green Version]
- Salvi, P.; Scalise, F.; Rovina, M.; Moretti, F.; Salvi, L.; Grillo, A.; Gao, L.; Baldi, C.; Faini, A.; Furlanis, G.; et al. Noninvasive Estimation of Aortic Stiffness Through Different Approaches. Hypertension 2019, 74, 117–129. [Google Scholar] [CrossRef] [Green Version]
- Ben-Shlomo, Y.; Spears, M.; Boustred, C.; May, M.; Anderson, S.G.; Benjamin, E.J.; Boutouyrie, P.; Cameron, J.; Chen, C.-H.; Cruickshank, J.K.; et al. Aortic Pulse Wave Velocity Improves Cardiovascular Event Prediction: An Individual Participant Meta-Analysis of Prospective Observational Data from 17,635 Subjects. J. Am. Coll. Cardiol. 2014, 63, 636–646. [Google Scholar] [CrossRef]
- Laurent, S.; Boutouyrie, P. Arterial Stiffness and Hypertension in the Elderly. Front. Cardiovasc. Med. 2020, 7, 544302. [Google Scholar] [CrossRef]
- Karras, A.; Haymann, J.-P.; Bozec, E.; Metzger, M.; Jacquot, C.; Maruani, G.; Houillier, P.; Froissart, M.; Stengel, B.; Guardiola, P.; et al. Large Artery Stiffening and Remodeling Are Independently Associated with All-Cause Mortality and Cardiovascular Events in Chronic Kidney Disease. Hypertension 2012, 60, 1451–1457. [Google Scholar] [CrossRef] [Green Version]
- Li, C.-W.; Yu, K.; Shyh-Chang, N.; Jiang, Z.; Liu, T.; Ma, S.; Luo, L.; Guang, L.; Liang, K.; Ma, W.; et al. Pathogenesis of Sarcopenia and the Relationship with Fat Mass: Descriptive Review. J. Cachexia Sarcopenia Muscle 2022, 13, 781–794. [Google Scholar] [CrossRef] [PubMed]
- Liberale, L.; Badimon, L.; Montecucco, F.; Lüscher, T.F.; Libby, P.; Camici, G.G. Inflammation, Aging, and Cardiovascular Disease: JACC Review Topic of the Week. J. Am. Coll. Cardiol. 2022, 79, 837–847. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, K.-I.; Fukumoto, Y. Sarcopenia as a Comorbidity of Cardiovascular Disease. J. Cardiol. 2021, 79, 596–604. [Google Scholar] [CrossRef] [PubMed]
- Hurrle, S.; Hsu, W.H. The etiology of oxidative stress in insulin resistance. Biomed. J. 2017, 40, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Laurent, S.; Cockcroft, J.; Van Bortel, L.; Boutouyrie, P.; Giannattasio, C.; Hayoz, D.; Pannier, B.; Vlachopoulos, C.; Wilkinson, I.; Struijker-Boudier, H. Expert Consensus Document on Arterial Stiffness: Methodological Issues and Clinical Applications. Eur. Heart J. 2006, 27, 2588–2605. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Milan, A.; Zocaro, G.; Leone, D.; Tosello, F.; Buraioli, I.; Schiavone, D.; Veglio, F. Current Assessment of Pulse Wave Velocity: Comprehensive Review of Validation Studies. J. Hypertens. 2019, 37, 1547–1557. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Zhu, M.; Bai, B.; Chi, C.; Yu, S.; Teliewubai, J.; Xu, H.; Wang, K.; Xiong, J.; Zhou, Y.; et al. Comparison of Carotid-Femoral and Brachial-Ankle Pulse-Wave Velocity in Association with Target Organ Damage in the Community-Dwelling Elderly Chinese: The Northern Shanghai Study. J. Am. Heart Assoc. 2017, 6, e004168. [Google Scholar] [CrossRef] [Green Version]
- Yu, W.-C.; Chuang, S.-Y.; Lin, Y.-P.; Chen, C.-H. Brachial-Ankle vs Carotid-Femoral Pulse Wave Velocity as a Determinant of Cardiovascular Structure and Function. J. Hum. Hypertens. 2008, 22, 24–31. [Google Scholar] [CrossRef]
- VanderLaan, P.A.; Reardon, C.A.; Getz, G.S. Site Specificity of Atherosclerosis. Arter. Thromb. Vasc. Biol. 2004, 24, 12–22. [Google Scholar] [CrossRef] [Green Version]
- Andrade, C. Mean Difference, Standardized Mean Difference (SMD), and Their Use in Meta-Analysis: As Simple as It Gets. J. Clin. Psychiatry 2020, 81, 20f13681. [Google Scholar] [CrossRef] [PubMed]
- Van Bortel, L.M.; Laurent, S.; Boutouyrie, P.; Chowienczyk, P.; Cruickshank, J.K.; De Backer, T.; Filipovsky, J.; Huybrechts, S.; Mattace-Raso, F.U.S.; Protogerou, A.D.; et al. Expert Consensus Document on the Measurement of Aortic Stiffness in Daily Practice Using Carotid-Femoral Pulse Wave Velocity. J. Hypertens. 2012, 30, 445–448. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Study | n | Male Sex (%) | Age (SD), Years | Non-Sarcopenic (%) | Age (SD), Years | SBP(SD)/DBP(SD) mm Hg | BMI (kg/m2) | HT (%) | CAD (%) | DM (%) | Ever Smoking (%) | Sarcopenic (%) | Age (SD), Years | SBP(SD)/DBP(SD) mm Hg | BMI (kg/m2) | HT (%) | CAD (%) | DM (%) | Ever Smoking (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Öztorun et al. 2022 [23] | 40 | 30.0 | 79.2 (6.4) | 30.0 | 75.6 (5.4) (p = 0.03) | 142.4 (21.6)/ 79(65–93) (p = 0.17/…) | 29.4 (5.0) (p = 0.003) | 91.7 (p = 0.02) | 33.3 (p= 0.72) | 66.7 (p = 0.11) | 50.0 (p = 0.02) | 70.0 | 80.7 (6.8) | 131.3 (23.5)/ 70(60–111) | 23.9 (4.8) | 53.6 | 39.3 | 39.3 | 85.7 |
| Sun et al. 2021 [26] | 2511 | 41.3 | 68.6 (5.8) | 85.7 | 68.4 (5.7) (p = 0.006) | … | 24.4 (3.3) (p = 0.60) | 35.5 (p = 0.0004) | 10.5 (p = 0.002) | 12.8 (p = 0.0001) | … | 14.3 | 69.3 (6.0) | … | 24.5 (3.4) | 45.3 | 16.1 | 20.3 | … |
| Rong et al. 2020 [24] | 450 | 59.1 | 71.3 (4.2) | 80.2 | 71.1 (4.2) (p = 0.005) | … | 25.2 (2.3) (p = 0.000) | 29.1 (p = 0.66) | 28.0 (p = 0.29) | 13.6 (p = 0.60) | 13.6 (p = 0.43) | 19.8 | 72.5 (4.7) | … | 24.0 (1.9) | 31.5 | 33.7 | 15.7 | 16.9 |
| Zhang Y. et al. 2020 [27] | 746 | 43.3 | 71.4 (4.3) | 93.3 | 71.1 (4.2) (p < 0.0001) | 131 (17)/ 75(10) (p = 1.0/0.42) | 23.8 (3.0) (p < 0.0001) | … | … | … | 33.6 (p = 0.04) | 6.7 | 74.1 (5.6) | 131 (19)/74 (9) | 19.6 (2.4) | … | … | … | 21.4 |
| Zhang L. et al. 2019 [25] | 1002 | 41.9 | 72.3 (5.2) | 89.3 | … | … | … | … | … | … | … | 10.7 | … | … | … | … | … | … | … |
| Ohara et al. 2015 [22] | 463 * 727 ** | … | … | 86.8 * 81.2 ** | … | … | … | … | … | … | … | 13.2 * 18.8 ** | … | … | … | … | … | … | … |
| Sarcopenia Definition | Muscle Strength | Muscle Mass | Physical Performance | Arterial Stiffness | ||||
|---|---|---|---|---|---|---|---|---|
| Measure | Threshold | Measure | Threshold | Measure | Threshold | |||
| Öztorun et al. 2022 [23] | EWGSOP2 criteria, 2018 [29] | Handgrip strength; electronic manual dynamometer (GRIP-D, Takei, Niigata, Japan) Dominant hand; the mean value of three measurements; done while the arm was in elbow flexion of 90° | Women < 22 kg Men < 32 kg | BIA Estimation of skeletal muscle mass (SMM) with Janssen formula [30] Absolute skeletal muscle mass (ASMM) = SMM/ht2 | For ASMM/ht2: Women < 7.4 kg/m2 Men < 9.2 kg/m2 | Gait speed (4 m) | <0.8 m/s | PWV (aorto-brachial) Mobil-O-Graph Blood Pressure 24 h PWA Monitor (IEM GmbH, Stolberg, Cologne, Germany) |
| Sun et al. 2021 [26] | AWGS criteria, 2019 [31] | Handgrip strength; a spring-type dynamometer (TSN100/WL, Physical Fitness Sports Technology Company, Beijing, China) Dominant hand; the maximum value of three measurements, conducted while standing and extending their arms at their sides | Women < 18 kg Men < 26 kg | BIA Estimation of skeletal muscle mass (SMM) with formula: SMM (kg) = 0.566 × FFM (fat free mass) [28] Appendicular muscle mass index (AMI) = appendicular skeletal muscle mass (ASM)/ht2 | For ASM/ht2: Women < 5.7 kg/m2 Men < 7.0 kg/m2 | … | … | baPWV IIM-AS-100 system (Institute of Intelligent Machines) |
| Rong et al. 2020 [24] | AWGS criteria, 2014 [32] | Handgrip strength; Jamar hand dynamometer 5030 J1 (Lafayette Instrument Company, Lafayette, IN, USA) Both hands; the max value of three measurements for each hand; conducted while sitting comfortably on a standard chair with legs, back support and fixed arms | Women < 18 kg Men < 26 kg | BIA (InBodyS10, InBody Japan Inc., Tokyo, Japan) Estimation of appendicular skeletal muscle mass (ASM) with Buckinx formula [33] Appendicular muscle mass index (ASMI) = appendicular skeletal muscle mass (ASM)/ht2 | For ASM/ht2: Women < 5.7 kg/m2 Men < 7.0 kg/m2 | Gait speed (6 m) | <0.8 m/s | baPWV VP1000 (Colin Company of Japan) |
| Zhang Y. et al. 2020 [27] | Sarcopenia: low HG + low ASM index | Handgrip strength; a Smedley type digital grip dynamometer (T.K.K.5401, TAKEI Scientific Instruments Co., Ltd., Niigata, Japan) Both hands; the max value of two measurements for each hand | Women < 18 kg Men < 26 kg | BIA (Physion MD, Physion Co., Ltd., Kyoto, Japan) The appendicular skeletal muscle mass (ASM) = the sum of skeletal muscle in the arms and legs ASM index = ASM/ht2 | For ASM/ht2: Women < 3.7 kg/m2 Men < 4.4 kg/m2 (threshold modified by the researchers as sex-specific lowest quintile) | … | … | baPWV BP-203RPE II/III, Fukuda Colin Co., Ltd., Tokyo, Japan |
| Zhang L. et al. 2019 [25] | AWGS criteria, 2014 [32] | Handgrip strength Dominant hand; the mean value of two measurements | Women < 18 kg Men < 26 kg | BIA (InBody 770; Biospace Co., Ltd., Seoul, Korea) Appendicular skeletal muscle mass (ASM) = the sum of skeletal muscle in the arms and legs Relative skeletal muscle mass index =ASM/ht2 | For ASM/ht2: Women < 5.7 kg/m2 Men < 7.0 kg/m2 | Gait speed (4 m) | <0.8 m/s | baPWV Vascular Profiler-1000 device (Omron, Kyoto, Japan) |
| Ohara et al. 2015 [22] | Sarcopenia: -values of HG or SMM < −1SD of values obtained from patients aged below 50 years OR -values of HG or SMM in the lowest 20% of those obtained from all study participants of the same sex | Handgrip strength; (T.K.K. 5410; Takei Scientific Instruments Co., Ltd., Niigata, Japan) Both hands; the max value of one measurement for each hand | Values of HG < −1SD of values obtained from patients aged below 50 years OR values of HG in the lowest 20% of those obtained from all study participants of the same sex | BIA (HBF-701; Omron Healthcare Co. Ltd., Kyoto, Japan) | Values of SMM < −1SD of values obtained from patients aged below 50 years OR values SMM in the lowest 20% of those obtained from all study participants of the same sex | … | … | baPWV PWV/ABI; Omron Healthcare Co. Ltd. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piotrowicz, K.; Klich-Rączka, A.; Skalska, A.; Gryglewska, B.; Grodzicki, T.; Gąsowski, J. Pulse Wave Velocity and Sarcopenia in Older Persons—A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2022, 19, 6477. https://doi.org/10.3390/ijerph19116477
Piotrowicz K, Klich-Rączka A, Skalska A, Gryglewska B, Grodzicki T, Gąsowski J. Pulse Wave Velocity and Sarcopenia in Older Persons—A Systematic Review and Meta-Analysis. International Journal of Environmental Research and Public Health. 2022; 19(11):6477. https://doi.org/10.3390/ijerph19116477
Chicago/Turabian StylePiotrowicz, Karolina, Alicja Klich-Rączka, Anna Skalska, Barbara Gryglewska, Tomasz Grodzicki, and Jerzy Gąsowski. 2022. "Pulse Wave Velocity and Sarcopenia in Older Persons—A Systematic Review and Meta-Analysis" International Journal of Environmental Research and Public Health 19, no. 11: 6477. https://doi.org/10.3390/ijerph19116477
APA StylePiotrowicz, K., Klich-Rączka, A., Skalska, A., Gryglewska, B., Grodzicki, T., & Gąsowski, J. (2022). Pulse Wave Velocity and Sarcopenia in Older Persons—A Systematic Review and Meta-Analysis. International Journal of Environmental Research and Public Health, 19(11), 6477. https://doi.org/10.3390/ijerph19116477








