Performance and Mechanisms of Sulfidated Nanoscale Zero-Valent Iron Materials for Toxic TCE Removal from the Groundwater
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Preparation of nZVI
2.3. One-Step Preparation of S-nZVI
2.4. TCE Degradation and H2 Evolution
2.5. Characterization
2.6. Kinetic Modelings
2.7. Analytical Methods
3. Results and Discussion
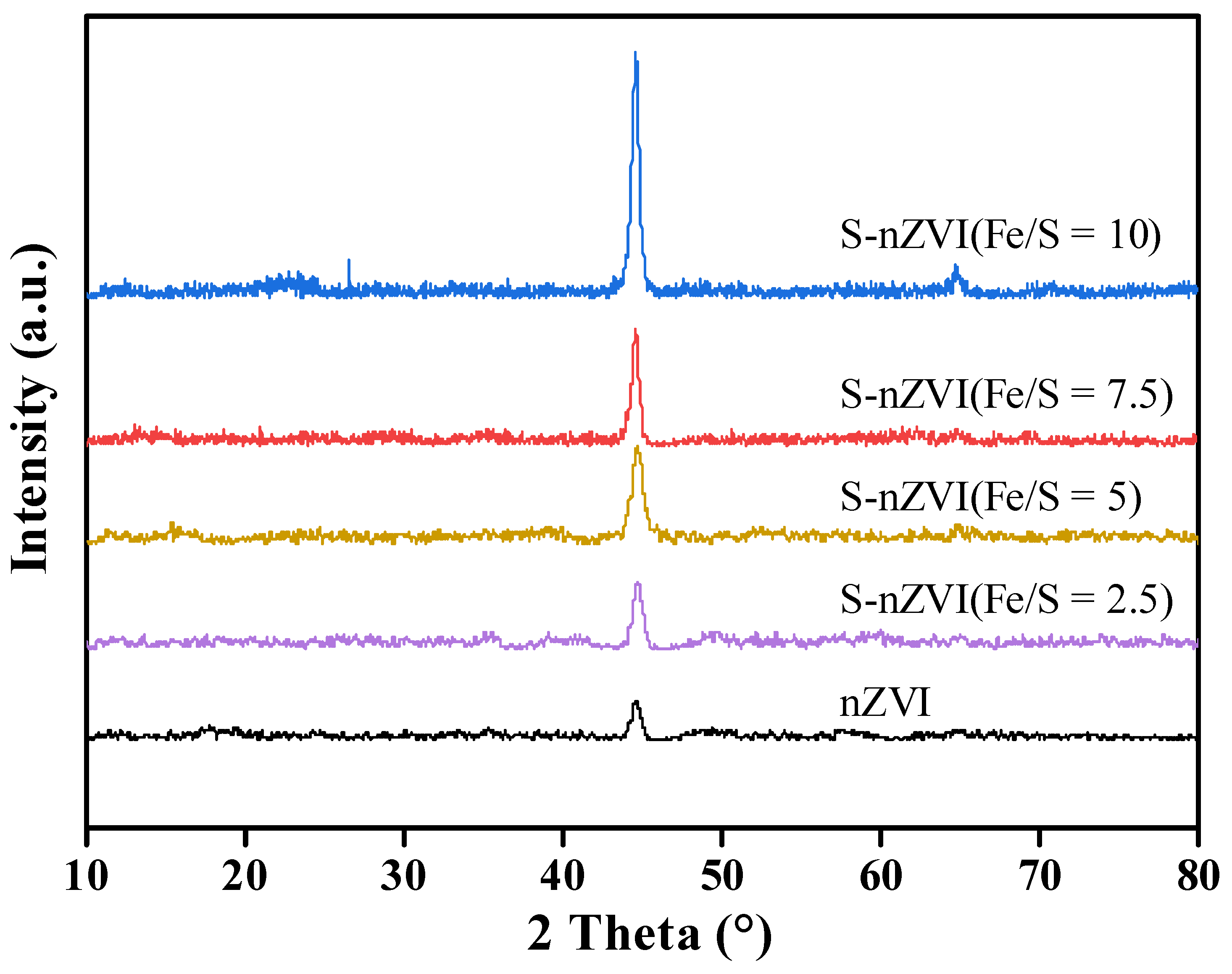
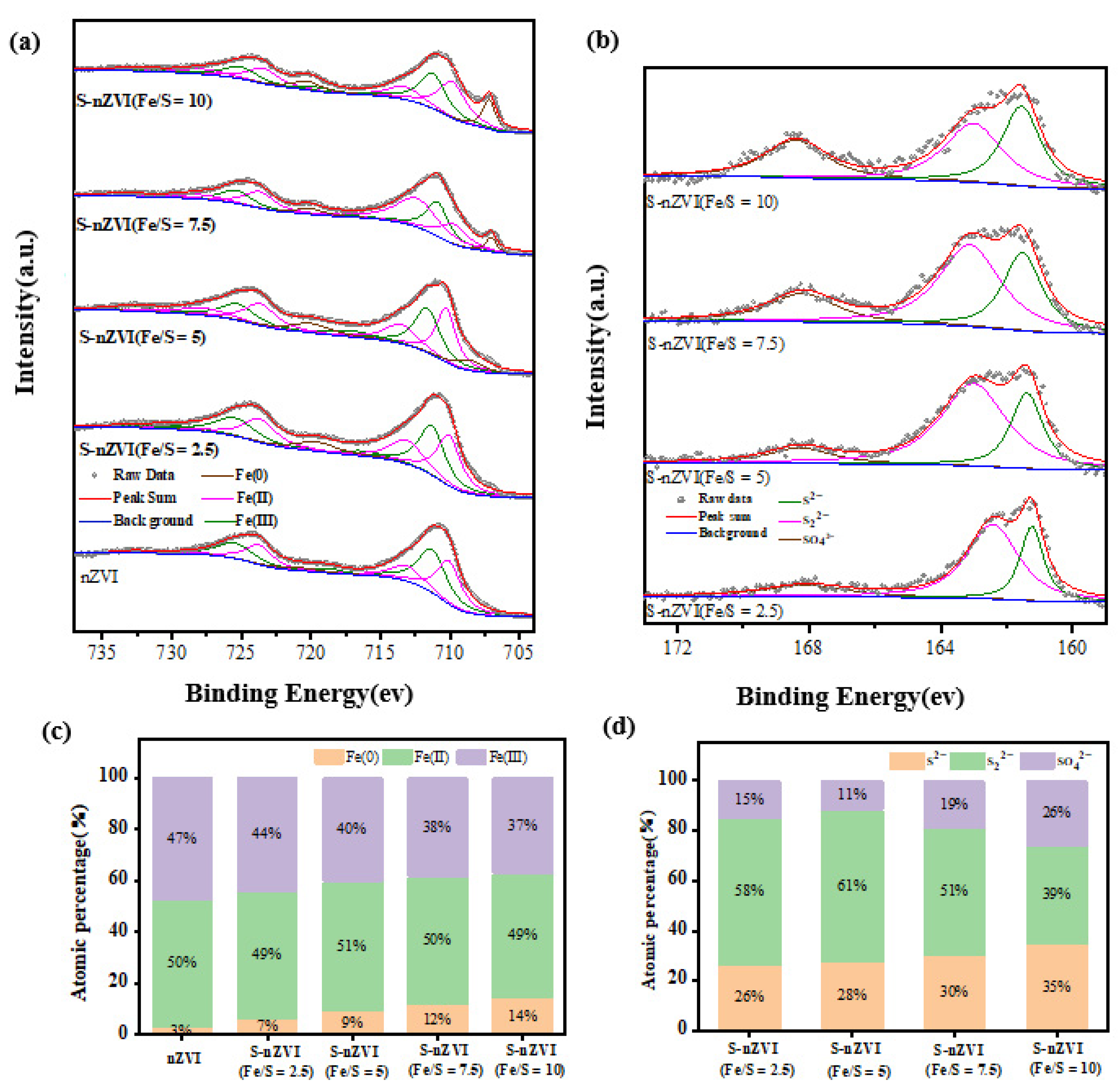
3.1. Materials Characterization
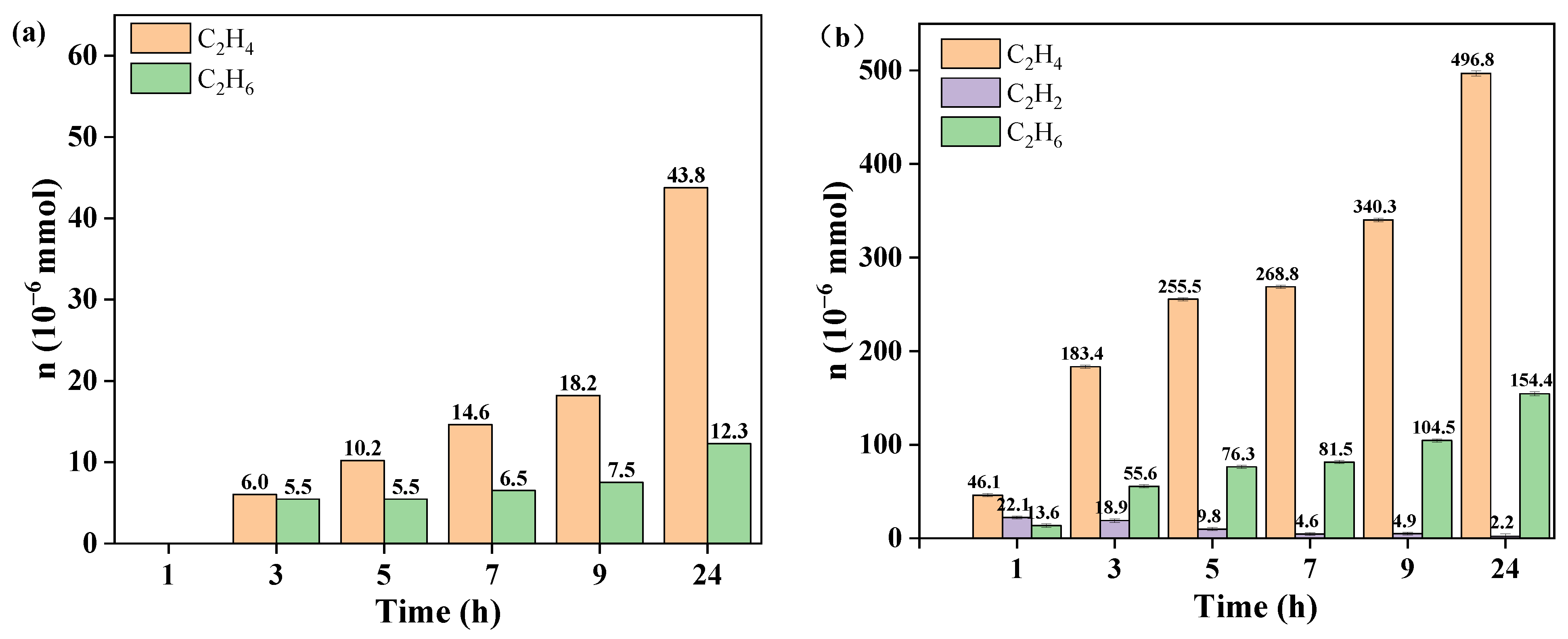
3.2. Performance of TCE Degradation
3.3. Kinetics of TCE Degradation
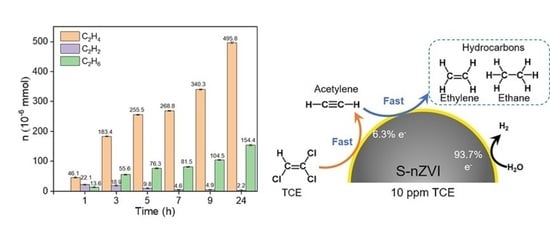
3.4. Mechanism of TCE Degradation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Seyama, T.; Adachi, K.; Yamazaki, S. Kinetics of photocatalytic degradation of trichloroethylene in aqueous colloidal solutions of TiO2 and WO3 nanoparticles. J. Photochem. Photobiol. A 2012, 249, 15–20. [Google Scholar] [CrossRef]
- Wei, Z.S.; Seo, Y. Trichloroethylene (TCE) adsorption using sustainable organic mulch. J. Hazard. Mater. 2010, 181, 147–153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dong, H.R.; He, Q.; Zeng, G.M.; Tang, L.; Zhang, L.H.; Xie, Y.K.; Zeng, Y.L.; Zhao, F. Degradation of trichloroethene by nanoscale zero-valent iron (nZVI) and nZVI activated persulfate in the absence and presence of EDTA. Chem. Eng. J. 2017, 316, 410–418. [Google Scholar] [CrossRef]
- Huang, B.B.; Lei, C.; Wei, C.H.; Zeng, G.M. Chlorinated volatile organic compounds (Cl-VOCs) in environment-sources, potential human health impacts, and current remediation technologies. Environ. Int. 2014, 71, 118–138. [Google Scholar] [CrossRef] [PubMed]
- Valentín, L.; Nousiainen, A.; Mikkonen, A. Introduction to Organic Contaminants in Soil: Concepts and Risks. In Emerging Organic Contaminants in Sludges: Analysis, Fate and Biological Treatment; Springer: Berlin/Heidelberg, Germany, 2013; pp. 1–29. [Google Scholar] [CrossRef]
- Yan, J.C.; Han, L.; Gao, W.G.; Xue, S.; Chen, M.F. Biochar supported nanoscale zerovalent iron composite used as persulfate activator for removing trichloroethylene. Bioresour. Technol. 2015, 175, 269–274. [Google Scholar] [CrossRef]
- Dong, H.R.; Zhang, C.; Deng, J.M.; Jiang, Z.; Zhang, L.H.; Cheng, Y.J.; Hou, K.J.; Tang, L.; Zeng, G.M. Factors influencing degradation of trichloroethylene by sulfide-modified nanoscale zero-valent iron in aqueous solution. Water Res. 2018, 135, 1–10. [Google Scholar] [CrossRef]
- Dong, H.R.; Zhang, C.; Hou, K.J.; Cheng, Y.J.; Deng, J.M.; Jiang, Z.; Tang, L.; Zeng, G.M. Removal of trichloroethylene by biochar supported nanoscale zero-valent iron in aqueous solution. Sep. Purif. Technol. 2017, 188, 188–196. [Google Scholar] [CrossRef]
- Starr, R.C.; Orr, B.R.; Lee, M.H.; Delwiche, M. Final Project Report-Coupled Biogeochemical Process Evaluation for Con-Ceptualizing Trichloriethylene Co-Metabolism: Co-Metabolic Enzyme Activity Probes and Modeling Co-Metabolism and Attenuation; North Wind Inc.: Idaho Falls, ID, USA, 2010. [Google Scholar]
- Slater, G.F.; Dempster, H.S.; Sherwood Lollar, B.; Ahad, J. Headspace analysis: A new application for isotopic characterization of dissolved organic contaminants. Environ. Sci. Technol. 1999, 33, 190–194. [Google Scholar] [CrossRef]
- Meng, Q.J.; Li, P.F.; Qu, J.H.; Liu, Y.; Wang, Y.F.; Chen, Z.B.; Zhang, Y. Study on the community structure and function of anaerobic granular sludge under trichloroethylene stress. Ecotoxicology 2021, 30, 1408–1418. [Google Scholar] [CrossRef]
- Chiu, W.A.; Jinot, J.; Scott, C.S.; Makris, S.L.; Cooper, G.S.; Dzubow, R.C.; Bale, A.S.; Evans, M.V.; Guyton, K.Z.; Keshava, N.; et al. Human health effects of trichloroethylene: Key findings and scientific issues. Environ. Health Persp. 2013, 121, 303–311. [Google Scholar] [CrossRef]
- Luo, Y.-S.; Furuya, S.; Soldatov, V.Y.; Kosyk, O.; Yoo, H.S.; Fukushima, H.; Lewis, L.; Iwata, Y.; Rusyn, I. Metabolism and Toxicity of Trichloroethylene and Tetrachloroethylene in Cytochrome P450 2E1 Knockout and Humanized Transgenic Mice. Toxicol. Sci. 2018, 164, 489–500. [Google Scholar] [CrossRef]
- Guha, N.; Loomis, D.; Grosse, Y.; Lauby-Secretan, B.; El Ghissassi, F.E.; Bouvard, V.; Benbrahim-Tallaa, L.; Baan, R.; Mattock, H.; Straif, K. Carcinogenicity of trichloroethylene, tetrachloroethylene, some other chlorinated solvents, and their metabolites. Lancet Oncol. 2012, 13, 1192–1193. [Google Scholar] [CrossRef]
- Ahmad, M.; Lee, S.S.; Dou, X.M.; Mohan, D.; Sung, J.-K.; Yang, J.E.; Ok, Y.S. Effects of pyrolysis temperature on soybean stover- and peanut shell-derived biochar properties and TCE adsorption in water. Bioresour. Technol. 2012, 118, 536–544. [Google Scholar] [CrossRef] [PubMed]
- Shao, H.B.; Butler, E.C. The Relative Importance of Abiotic and Biotic Transformation of Carbon Tetrachloride in Anaerobic Soils and Sediments. Soil Sediment. Contam. Int. J. 2009, 18, 455–469. [Google Scholar] [CrossRef]
- Pasinszki, T.; Krebsz, M. Synthesis and Application of Zero-Valent Iron Nanoparticles in Water Treatment, Environmental Remediation, Catalysis, and Their Biological Effects. Nanomaterials 2020, 10, 917. [Google Scholar] [CrossRef]
- Mu, Y.; Jia, F.L.; Ai, Z.H.; Zhang, L.Z. Iron oxide shell mediated environmental remediation properties of nano zero-valent iron. Environ. Sci. Nano 2017, 4, 27–45. [Google Scholar] [CrossRef]
- Zhang, W.-X. Nanoscale Iron Particles for Environmental Remediation: An Overview. J. Nanoparticle Res. 2003, 5, 323–332. [Google Scholar] [CrossRef]
- Nurmi, J.T.; Tratnyek, P.G.; Sarathy, V.; Baer, D.R. Characterization and properties of metallic iron nanoparticles: Spectroscopy, electrochemistry, and kinetics. Environ. Sci. Technol. 2005, 39, 1221–1230. [Google Scholar] [CrossRef] [Green Version]
- Fan, D.M.; Anitori, R.P.; Tebo, B.M.; Tratnyek, P.G.; Lezama Pacheco, J.S.; Kukkadapu, R.K.; Engelhard, M.H.; Bowden, M.E.; Kovarik, L.; Arey, B.W. Reductive sequestration of pertechnetate (99TcO4–) by nano zerovalent iron (nZVI) trans-formed by abiotic sulfide. Environ. Sci. Technol. 2013, 47, 5302–5310. [Google Scholar] [CrossRef]
- Park, S.W.; Kim, S.K.; Kim, J.B.; Choi, S.W.; Inyang, H.I.; Tokunaga, S. Particle surface hydrophobicity and the dechlo-rination of chloro-compounds by iron sulfides. Water Air Soil Pollut. 2006, 6, 97–110. [Google Scholar] [CrossRef]
- Han, Y.L.; Yan, W.L. Reductive dechlorination of trichloroethene by zero-valent iron nanoparticles: Reactivity enhancement through sulfidation treatment. Environ. Sci. Technol. 2016, 50, 12992–13001. [Google Scholar] [CrossRef] [PubMed]
- Fan, D.; Lan, Y.; Tratnyek, P.G.; Johnson, R.L.; Filip, J.; O’Carroll, D.M.; Garcia, A.N.; Agrawal, A. Sulfidation of Iron-Based Materials: A Review of Processes and Implications for Water Treatment and Remediation. Environ. Sci. Technol. 2017, 51, 13070–13085. [Google Scholar] [CrossRef] [PubMed]
- Li, J.X.; Zhang, X.Y.; Sun, Y.K.; Liang, L.P.; Pan, B.C.; Zhang, W.M.; Guan, X.H. Advances in Sulfidation of Zerovalent Iron for Water Decontamination. Environ. Sci. Technol. 2017, 51, 13533–13544. [Google Scholar] [CrossRef] [PubMed]
- Garcia, A.N.; Zhang, Y.Y.; Ghoshal, S.; He, F.; O’Carroll, D.M. Recent advances in sulfidated zerovalent iron for contaminant transformation. Environ. Sci. Technol. 2021, 55, 8464–8483. [Google Scholar] [CrossRef]
- Kim, E.J.; Kim, J.H.; Azad, A.M.; Chang, Y.S. Facile synthesis and characterization of Fe/FeS nanoparticles for environ-mental applications. ACS Appl. Mater. Interfaces 2011, 3, 1457–1462. [Google Scholar] [CrossRef]
- Rajajayavel, S.R.C.; Ghoshal, S. Enhanced reductive dechlorination of trichloroethylene by sulfidated nanoscale zerovalent iron. Water Res. 2015, 78, 144–153. [Google Scholar] [CrossRef]
- Fang, H.Y.; Huang, T.Z.; Mao, J.F.; Dinesh, M.M.; Sun, Y.; Liang, D.; Qi, L.; Yu, J.M.; Jiang, Z.K. Investigation on the cat-alytic performance of reduced-graphene-oxide-interpolated FeS2 and FeS for oxygen reduction reaction. ChemistrySelect 2018, 3, 10418–10427. [Google Scholar] [CrossRef] [Green Version]
- Mangayayam, M.; Dideriksen, K.; Ceccato, M.; Tobler, D.J. The Structure of Sulfidized Zero-Valent Iron by One-Pot Synthesis: Impact on Contaminant Selectivity and Long-Term Performance. Environ. Sci. Technol. 2019, 53, 4389–4396. [Google Scholar] [CrossRef]
- Cao, Z.; Liu, X.; Xu, J.; Zhang, J.; Yang, Y.; Zhou, J.L.; Xu, X.H.; Lowry, G.V. Removal of Antibiotic Florfenicol by Sulfide-Modified Nanoscale Zero-Valent Iron. Environ. Sci. Technol. 2017, 51, 11269–11277. [Google Scholar] [CrossRef]
- Guo, Y.N.; Park, T.; Yi, J.W.; Henzie, J.; Kim, J.; Wang, Z.L.; Jiang, B.; Bando, Y.; Sugahara, Y.; Tang, J.; et al. Nanoarchitectonics for transition-metal-sulfide-based electrocatalysts for water splitting. Adv. Mater. 2019, 31, 1807134. [Google Scholar] [CrossRef]
- Gu, Y.W.; Wang, B.B.; He, F.; Bradley, M.J.; Tratnyek, P.G. Mechanochemically Sulfidated Microscale Zero Valent Iron: Pathways, Kinetics, Mechanism, and Efficiency of Trichloroethylene Dechlorination. Environ. Sci. Technol. 2017, 51, 12653–12662. [Google Scholar] [CrossRef] [PubMed]




| Sample | SBET (m2/g) | Daverage (nm) | Vtotal (cm3/g) |
|---|---|---|---|
| nZVI | 8.76 | 3.41 | 0.01 |
| S-nZVI (Fe/S = 10) | 27.11 | 3.82 | 0.05 |
| S-nZVI (Fe/S = 7.5) | 57.60 | 3.41 | 0.12 |
| S-nZVI (Fe/S = 5) | 53.52 | 22.17 | 0.31 |
| S-nZVI (Fe/S = 2.5) | 95.66 | 18.91 | 0.48 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lang, Y.; Yu, Y.; Zou, H.; Ye, J.; Zhang, S. Performance and Mechanisms of Sulfidated Nanoscale Zero-Valent Iron Materials for Toxic TCE Removal from the Groundwater. Int. J. Environ. Res. Public Health 2022, 19, 6299. https://doi.org/10.3390/ijerph19106299
Lang Y, Yu Y, Zou H, Ye J, Zhang S. Performance and Mechanisms of Sulfidated Nanoscale Zero-Valent Iron Materials for Toxic TCE Removal from the Groundwater. International Journal of Environmental Research and Public Health. 2022; 19(10):6299. https://doi.org/10.3390/ijerph19106299
Chicago/Turabian StyleLang, Yue, Yanan Yu, Hongtao Zou, Jiexu Ye, and Shihan Zhang. 2022. "Performance and Mechanisms of Sulfidated Nanoscale Zero-Valent Iron Materials for Toxic TCE Removal from the Groundwater" International Journal of Environmental Research and Public Health 19, no. 10: 6299. https://doi.org/10.3390/ijerph19106299
APA StyleLang, Y., Yu, Y., Zou, H., Ye, J., & Zhang, S. (2022). Performance and Mechanisms of Sulfidated Nanoscale Zero-Valent Iron Materials for Toxic TCE Removal from the Groundwater. International Journal of Environmental Research and Public Health, 19(10), 6299. https://doi.org/10.3390/ijerph19106299