Aerosolization Behaviour of Fungi and Its Potential Health Effects during the Composting of Animal Manure
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. DdPCR and ITS Sequencing
2.3. Determination of Heavy Metals and Physical and Chemical Parameters
2.4. Data Analysis
3. Results and Discussion
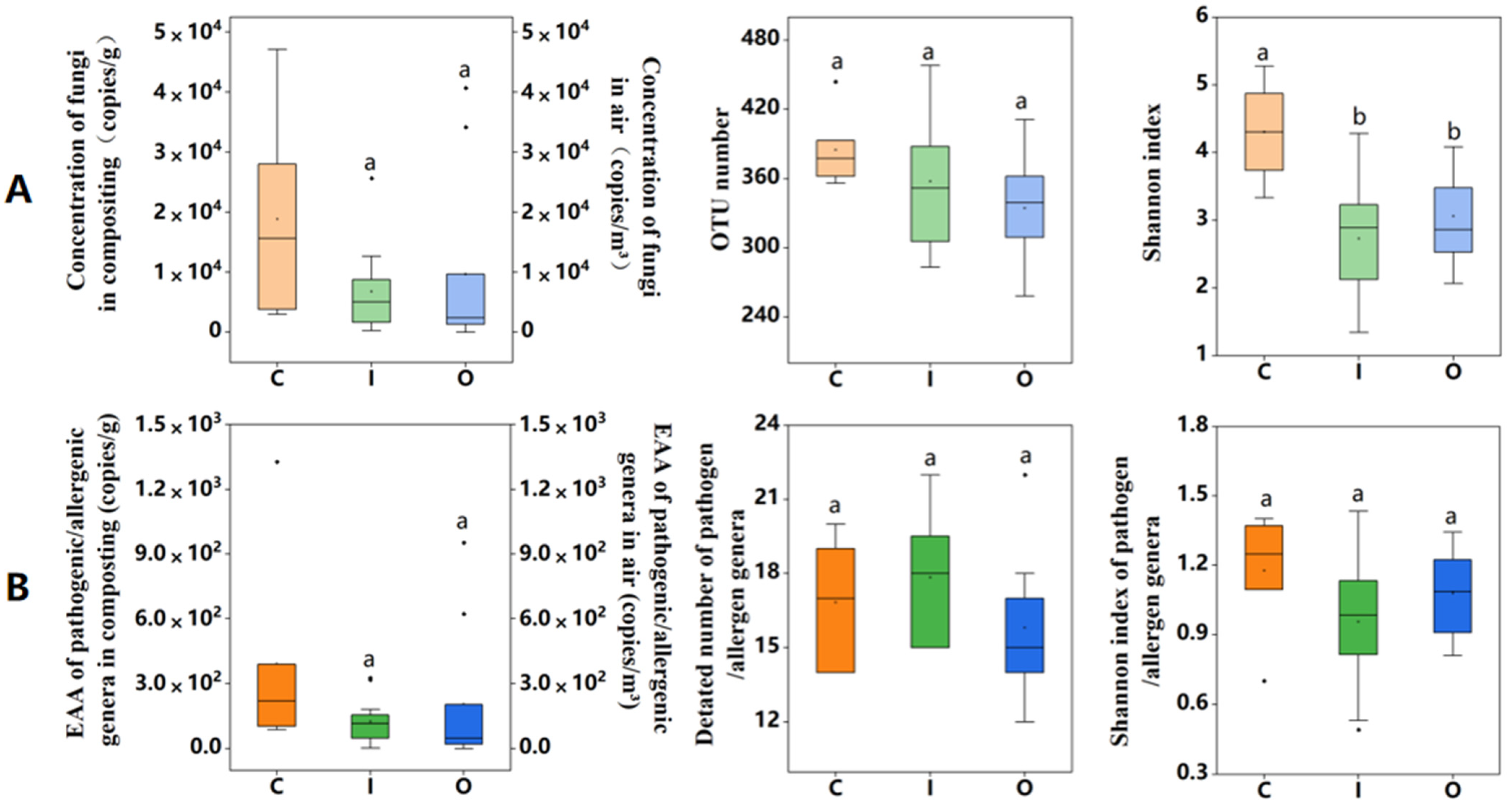
3.1. Fungal Abundance and Diversity in Air and Compost Piles
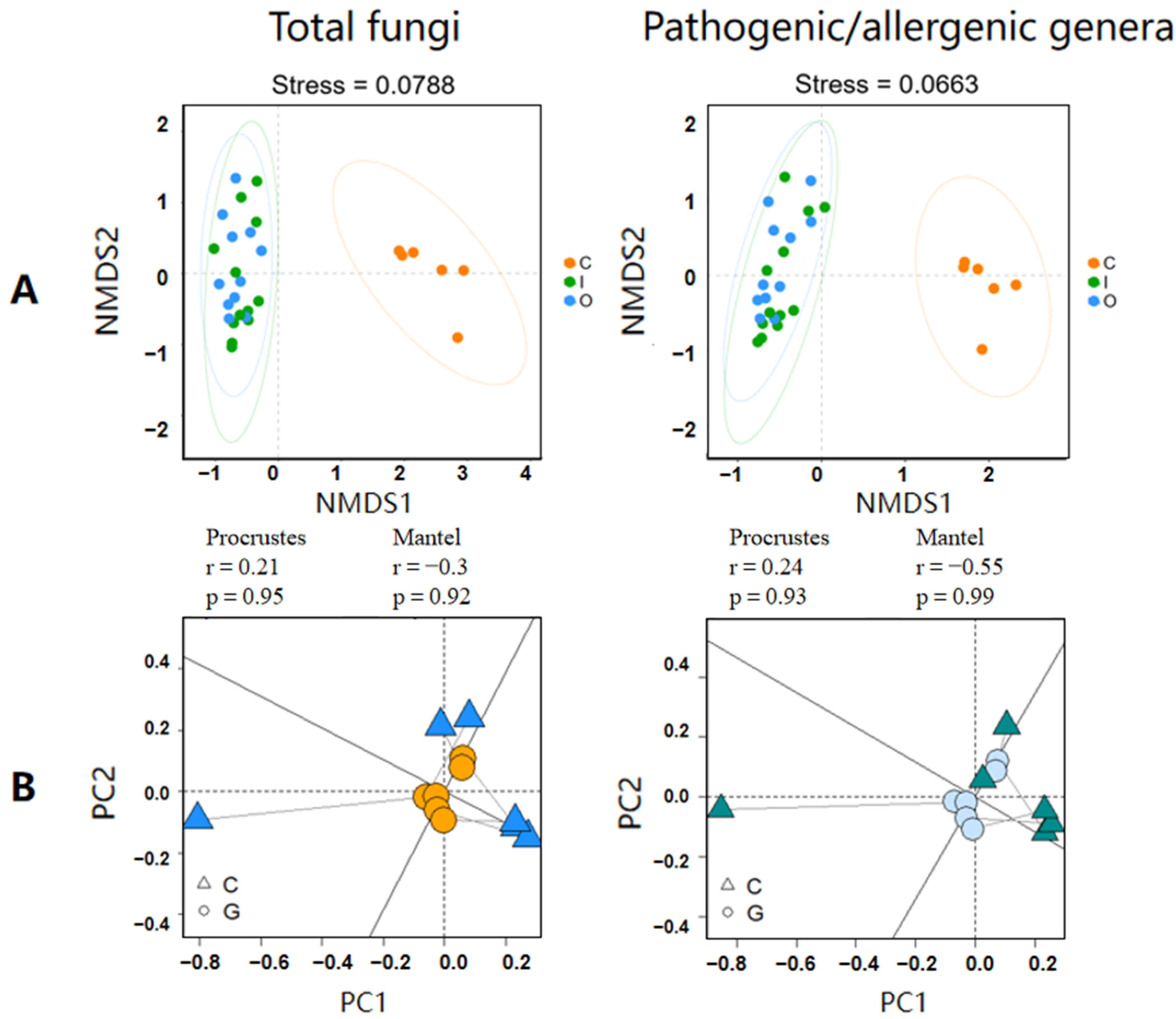
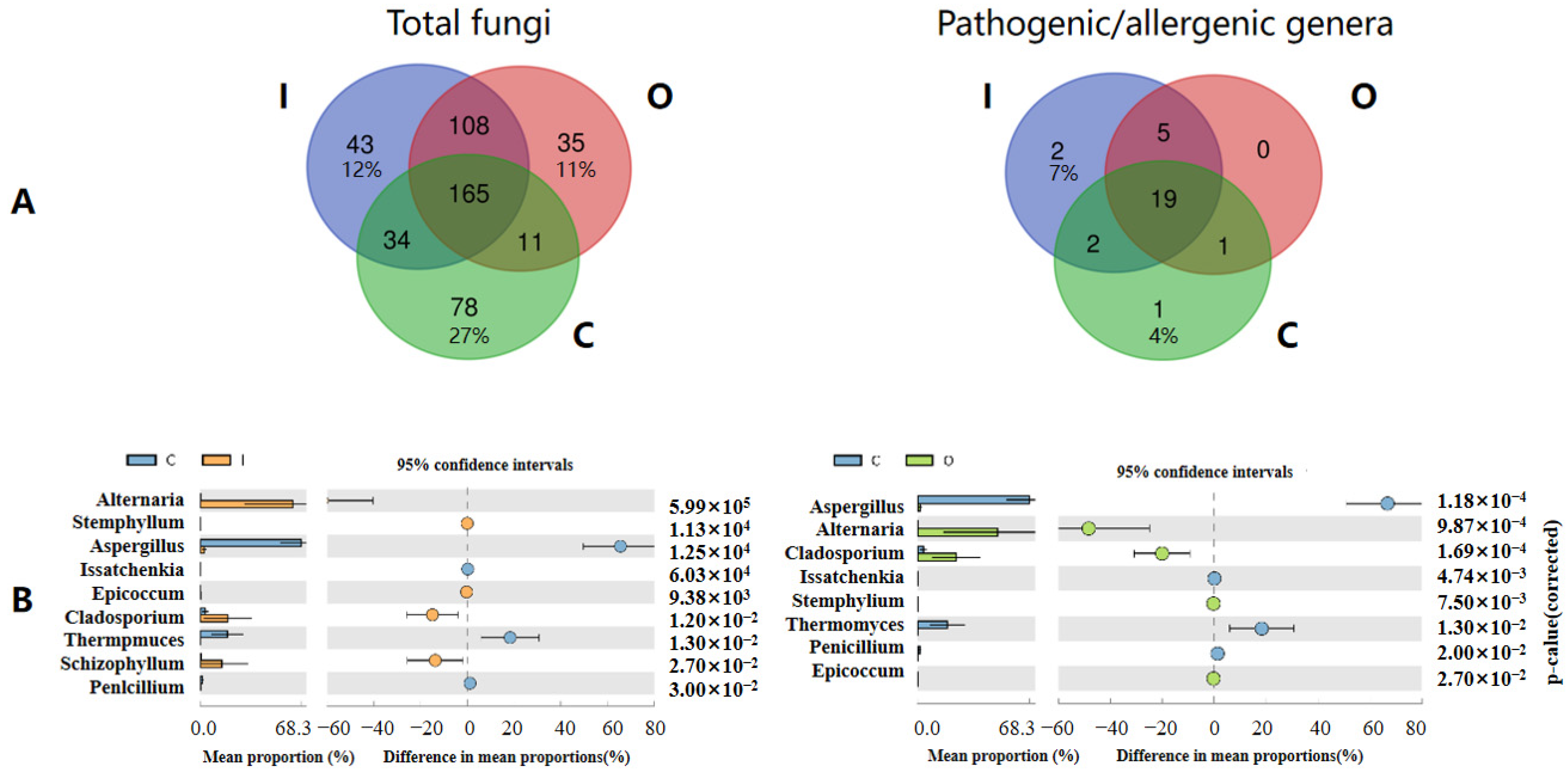
3.2. Connection and Difference of the Fungal Community and Abundance between Composting Piles and Air
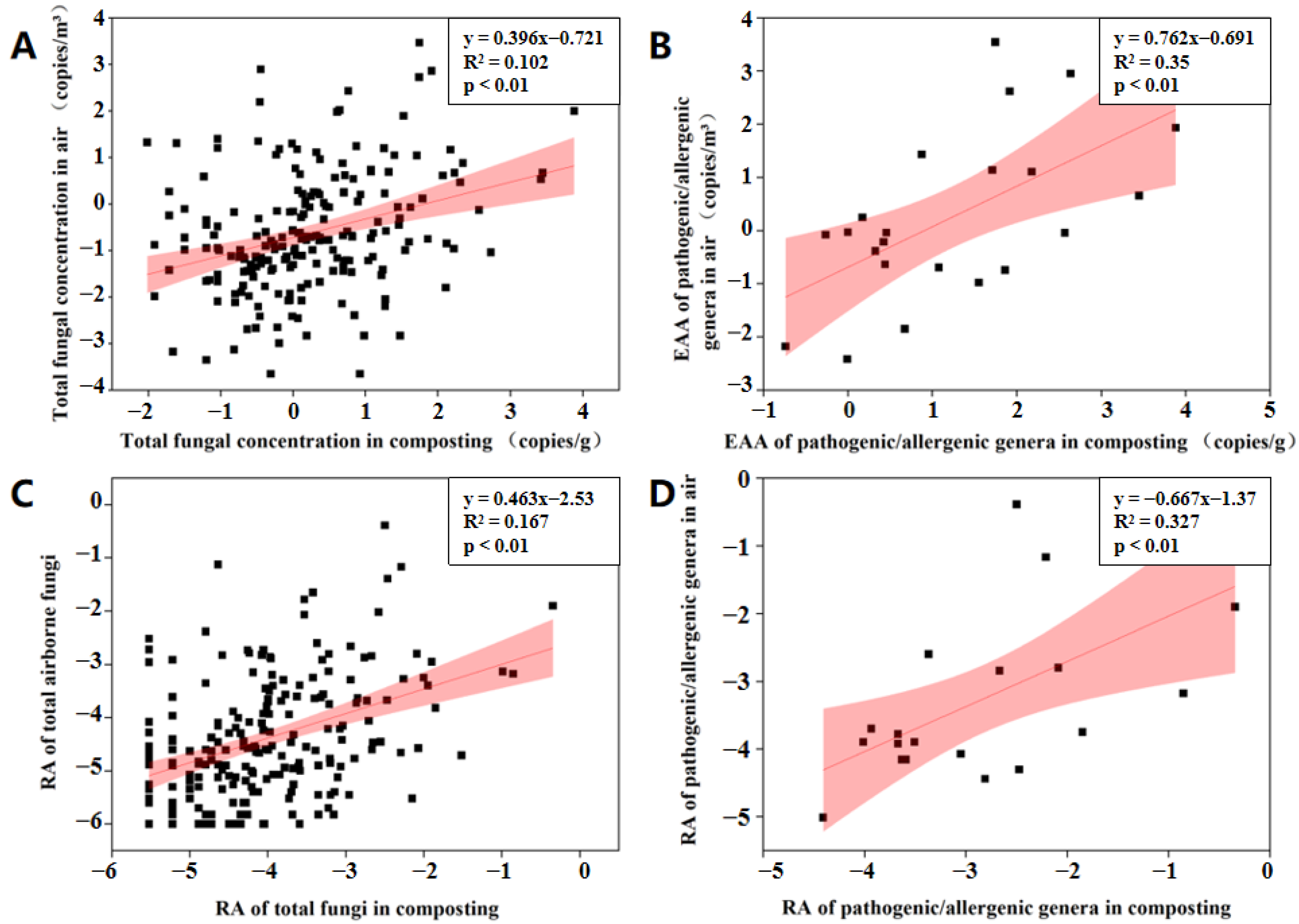
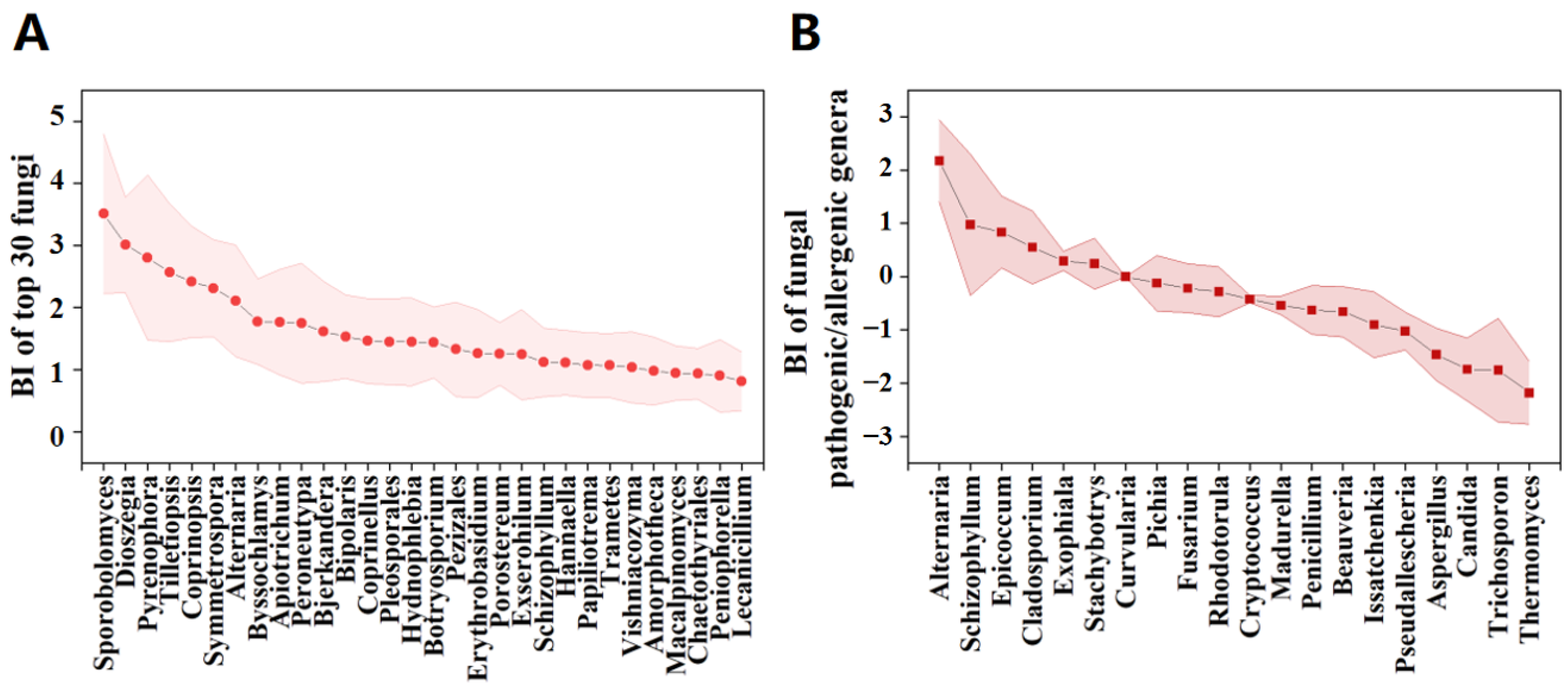
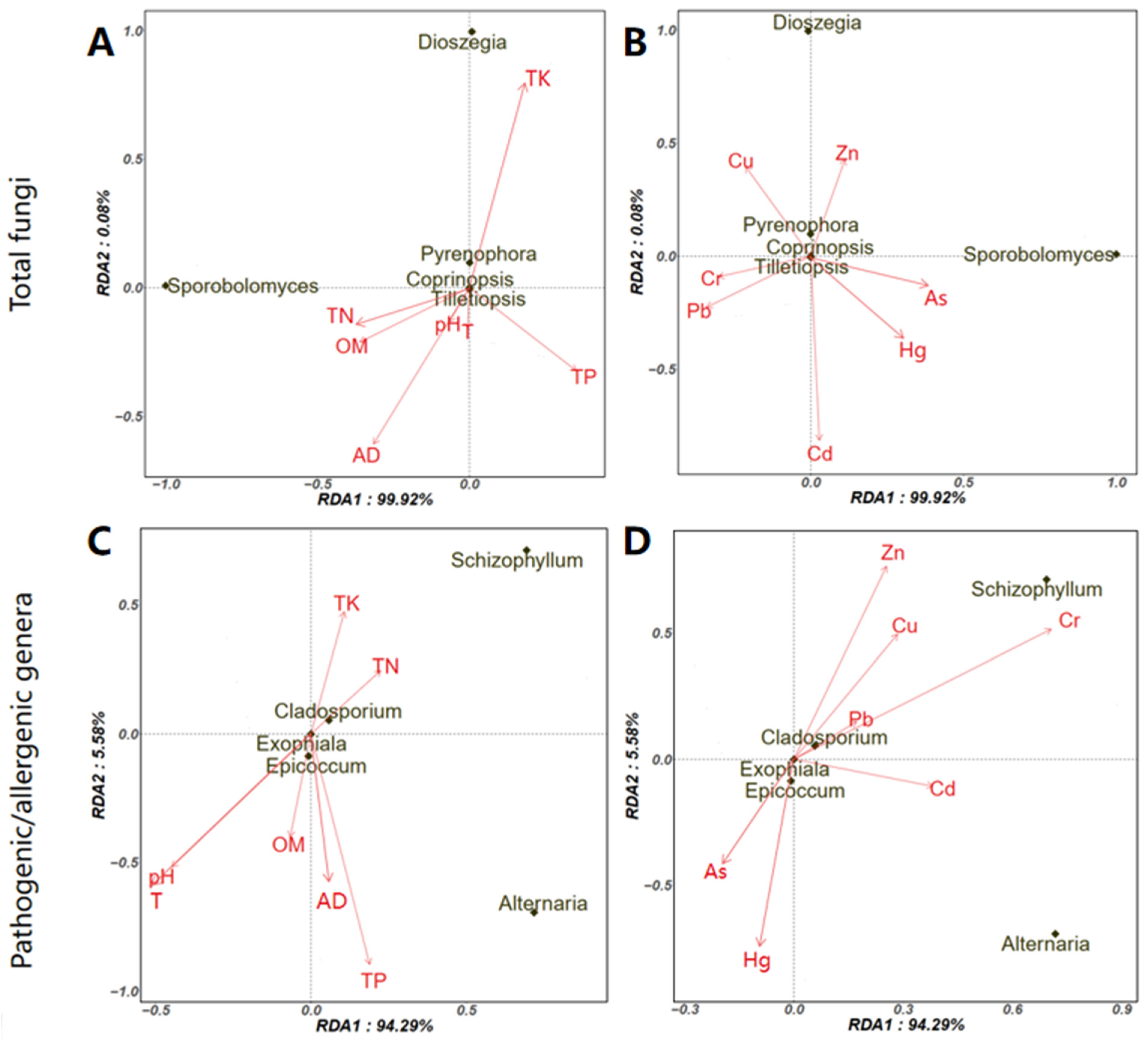
3.3. Potential Factors Affecting the Aerosolization Behavior of Airborne Fungi and Pathogenic/Allergenic Genera
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhang, B.; Tian, H.; Lu, C.; Dangal, S.R.S.; Yang, J.; Pan, S. Global manure nitrogen production and application in cropland during 1860–2014: A 5 arcmin gridded global dataset for Earth system modeling. Earth Syst. Sci. Data 2017, 9, 667–678. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Bao, H.; Xing, W.; Yang, J.; Liu, R.; Wang, X.; Lv, L.; Tong, X.; Wu, F. Succession of fungal dynamics and their influence on physicochemical parameters during pig manure composting employing with pine leaf biochar. Bioresour. Technol. 2020, 297, 122377. [Google Scholar] [CrossRef] [PubMed]
- Robertson, S.; Douglas, P.; Jarvis, D.; Marczylo, E. Bioaerosol exposure from composting facilities and health outcomes in workers and in the community: A systematic review update. Int. J. Hyg. Environ. Health 2019, 222, 364–386. [Google Scholar] [CrossRef] [PubMed]
- Wery, N. Bioaerosols from composting facilities-a review. Front. Cell. Infect. Microbiol. 2014, 4, 42. [Google Scholar] [PubMed]
- Langarica-Fuentes, A.; Zafar, U.; Heyworth, A.; Brown, T.; Fox, G.; Robson, G.D. Fungal succession in an in-vessel composting system characterized using 454 pyrosequencing. FEMS Microbiol. Ecol. 2014, 88, 296–308. [Google Scholar] [CrossRef] [Green Version]
- Mbareche, H.; Veillette, M.; Bonifait, L.; Dubuis, M.-E.; Benard, Y.; Marchand, G.; Bilodeau, G.J.; Duchaine, C. A next generation sequencing approach with a suitable bioinformatics workflow to study fungal diversity in bioaerosols released from two different types of composting plants. Sci. Total Environ. 2017, 601–602, 1306–1314. [Google Scholar] [CrossRef]
- Gao, M.; Qiu, T.; Sun, Y.; Wang, X. The abundance and diversity of antibiotic resistance genes in the atmospheric environment of composting plants. Environ. Int. 2018, 116, 229–238. [Google Scholar] [CrossRef]
- Ferguson, R.M.W.; Neath, C.E.E.; Nasir, Z.A.; Garcia-Alcega, S.; Tyrrel, S.; Coulon, F.; Dumbrell, A.J.; Colbeck, I.; Whitby, C. Size fractionation of bioaerosol emissions from green-waste composting. Environ. Int. 2021, 147, 106327. [Google Scholar] [CrossRef]
- Domingo, J.L.; Nadal, M. Domestic waste composting facilities: A review of human health risks. Environ. Int. 2009, 35, 382–389. [Google Scholar] [CrossRef]
- Fung, F.; Hughson, W.G. Health effects of indoor fungal bioaerosol exposure. Appl. Occup. Environ. Hyg. 2003, 18, 535–544. [Google Scholar] [CrossRef]
- Eduard, W. A health-based criteria document on fungal spore exposure in the working population. Is it relevant for the general population? Indoor Air 2008, 18, 257–258. [Google Scholar] [CrossRef] [PubMed]
- Pearson, C.; Littlewood, E.; Douglas, P.; Robertson, S.; Gant, T.W.; Hansell, A.L. Exposures and health outcomes in relation to bioaerosol emissions from composting facilities: A systematic review of occupational and community studies. J. Toxicol. Environ. Health Part B 2015, 18, 43–69. [Google Scholar] [CrossRef] [PubMed]
- Taha, M.P.M.; Pollard, S.J.T.; Sarkar, U.; Longhurst, P. Estimating fugitive bioaerosol releases from static compost windrows: Feasibility of a portable wind tunnel approach. Waste Manag. 2005, 25, 445–450. [Google Scholar] [CrossRef] [Green Version]
- Sanchez-Monedero, M.A.; Stentiford, E.I.; Mondini, C. Biofiltration at Composting Facilities: Effectiveness for Bioaerosol Control. Environ. Sci. Technol. 2003, 37, 4299–4303. [Google Scholar] [CrossRef] [PubMed]
- Huffnagle, G.B.; Noverr, M.C. The emerging world of the fungal microbiome. Trends Microbiol. 2013, 21, 334–341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, P.; Wei, S.; Shao, L.; Lü, F. Aerosolization behavior of prokaryotes and fungi during composting of vegetable waste. Waste Manag. 2019, 89, 103–113. [Google Scholar] [CrossRef]
- Le Goff, O.; Bru-Adan, V.; Bacheley, H.; Godon, J.J.; Wery, N. The microbial signature of aerosols produced during the thermophilic phase of composting. J. Appl. Microbiol. 2010, 108, 325–340. [Google Scholar] [CrossRef]
- Gutarowska, B.; Skóra, J.; Stępień, Ł.; Szponar, B.; Otlewska, A.; Pielech-Przybylska, K. Assessment of microbial contamination within working environments of different types of composting plants. J. Air Waste Manag. 2015, 65, 466–478. [Google Scholar] [CrossRef]
- Peccia, J.; Hernandez, M. Incorporating polymerase chain reaction-based identification, population characterization, and quantification of microorganisms into aerosol science: A review. Atmos. Environ. 2006, 40, 3941–3961. [Google Scholar] [CrossRef]
- Tischer, C.G.; Heinrich, J. Exposure assessment of residential mould, fungi and microbial components in relation to children’s health: Achievements and challenges. Int. J. Hyg. Environ. Health 2013, 216, 109–114. [Google Scholar] [CrossRef]
- Zhang, Z.; Qu, Y.; Li, S.; Feng, K.; Wang, S.; Cai, W.; Liang, Y.; Li, H.; Xu, M.; Yin, H. Soil bacterial quantification approaches coupling with relative abundances reflecting the changes of taxa. Sci. Rep. 2017, 7, 4837. [Google Scholar] [CrossRef] [PubMed]
- Finnegan, S.; Droser, M.L. Relative and absolute abundance of trilobites and rhynchonelliform brachiopods across the Lower/Middle Ordovician boundary, eastern Basin and Range. Paleobiology 2005, 31, 480–502. [Google Scholar] [CrossRef]
- Stämmler, F.; Gläsner, J.; Hiergeist, A.; Holler, E.; Weber, D.; Oefner, P.J.; Gessner, A.; Spang, R. Adjusting microbiome profiles for differences in microbial load by spike-in bacteria. Microbiome 2016, 4, 28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moletta-Denat, M.; Bru-Adan, V.; Delgenes, J.-P.; Hamelin, J.; Wéry, N.; Godon, J.-J. Selective microbial aerosolization in biogas demonstrated by quantitative PCR. Bioresour. Technol. 2010, 101, 7252–7257. [Google Scholar] [CrossRef]
- Veillette, M.; Bonifait, L.; Mbareche, H.; Marchand, G.; Duchaine, C. Preferential aerosolization of Actinobacteria during handling of composting organic matter. J. Aerosol Sci. 2018, 116, 83–91. [Google Scholar] [CrossRef]
- Lu, F.; Hu, T.; Wei, S.; Shao, L.; He, P. Bioaerosolization behavior along sewage sludge biostabilization. Front. Environ. Sci. Eng. 2020, 15, 45. [Google Scholar] [CrossRef]
- Perrott, P.; Turgeon, N.; Gauthier-Levesque, L.; Duchaine, C. Preferential aerosolization of bacteria in bioaerosols generated in vitro. J. Appl. Microbiol. 2017, 123, 688–697. [Google Scholar] [CrossRef]
- Ekinci, K.; Keener, H.; Akbolat, D. Effects of feedstock, airflow rate, and recirculation ratio on performance of composting systems with air recirculation. Bioresour. Technol. 2006, 97, 922–932. [Google Scholar] [CrossRef]
- Zhang, J.; Zeng, G.; Chen, Y.; Yu, M.; Yu, Z.; Hui, L.; Yu, Y.; Huang, H. Effects of physico-chemical parameters on the bacterial and fungal communities during agricultural waste composting. Bioresour. Technol. 2011, 102, 2950–2956. [Google Scholar] [CrossRef]
- Shehata, E.; Cheng, D.-m.; Ma, Q.-q.; Li, Y.-l.; Liu, Y.-w.; Feng, Y.; Ji, Z.-y.; Li, Z.-j. Microbial community dynamics during composting of animal manures contaminated with arsenic, copper, and oxytetracycline. J. Integr. Agric. 2021, 20, 1649–1659. [Google Scholar] [CrossRef]
- Mbareche, H.; Veillette, M.; Bilodeau, G.; Duchaine, C. Comparison of the performance of ITS1 and ITS2 as barcodes in amplicon-based sequencing of bioaerosols. PeerJ 2020, 8, e8523. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Edgar, R.C. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 2013, 10, 996–998. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C.; Haas, B.J.; Clemente, J.C.; Quince, C.; Knight, R. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics 2011, 27, 2194–2200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Pena, A.G.; Goodrich, J.K.; Gordon, J.I. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bremner, J.M. Determination of nitrogen in soil by the Kjeldahl method. J. Agric. Sci. 1960, 55, 11–33. [Google Scholar] [CrossRef]
- Guo, H.; Nasir, M.; Lv, J.; Dai, Y.; Gao, J. Understanding the variation of microbial community in heavy metals contaminated soil using high throughput sequencing. Ecotoxicol. Environ. Saf. 2017, 144, 300–306. [Google Scholar] [CrossRef]
- Parks, D.; Tyson, G.; Hugenholtz, P.; Beiko, R. STAMP: Statistical analysis of taxonomic and functional profiles. Bioinformatics 2014, 30, 3123–3124. [Google Scholar] [CrossRef] [Green Version]
- Bonifait, L.; Marchand, G.; Veillette, M.; M’Bareche, H.; Dubuis, M.-E.; Pépin, C.; Cloutier, Y.; Bernard, Y.; Duchaine, C. Workers’ exposure to bioaerosols from three different types of composting facilities. J. Occup. Environ. Hyg. 2017, 14, 815–822. [Google Scholar] [CrossRef]
- Glass, N.L.; Schmoll, M.; Cate, J.H.D.; Coradetti, S. Plant Cell Wall Deconstruction by Ascomycete Fungi. Annu. Rev. Microbiol. 2013, 67, 477–498. [Google Scholar] [CrossRef]
- Pal, C.; Bengtsson-Palme, J.; Kristiansson, E.; Larsson, D.G.J. The structure and diversity of human, animal and environmental resistomes. Microbiome 2016, 4, 54. [Google Scholar] [CrossRef] [Green Version]
- Sebők, F.; Dobolyi, C.; Bobvos, J.; Szoboszlay, S.; Kriszt, B.; Magyar, D. Thermophilic fungi in air samples in surroundings of compost piles of municipal, agricultural and horticultural origin. Aerobiologia 2015, 32, 255–263. [Google Scholar] [CrossRef]
- Feeney, P.; Rodríguez, S.F.; Molina, R.; McGillicuddy, E.; Hellebust, S.; Quirke, M.; Daly, S.; O’Connor, D.; Sodeau, J. A comparison of on-line and off-line bioaerosol measurements at a biowaste site. Waste Manag. 2018, 76, 323–338. [Google Scholar] [CrossRef] [PubMed]
- Alvarado, C.S.; Gandara, A.; Flores, C.; Perez, H.R.; Green, C.F.; Hurd, W.W.; Gibbs, S.G. Seasonal changes in airborne fungi and bacteria at a dairy cattle concentrated animal feeding operation in the southwest United States. J. Environ. Health 2009, 71, 40–44. [Google Scholar] [PubMed]
- O’Connor, D.; Daly, S.M.; Sodeau, J.R. On-line monitoring of airborne bioaerosols released from a composting/green waste site. Waste Manag. 2015, 42, 23–30. [Google Scholar] [CrossRef]
- Agency, E. M9: Environmental Monitoring of Bioaerosols at Regulated Facilities; Environment Agency: Bristol, UK, 2018. [Google Scholar]
- Janbon, G.; Quintin, J.; Lanternier, F.; d’Enfert, C. Studying fungal pathogens of humans and fungal infections: Fungal diversity and diversity of approaches. Microbes Infect. 2019, 21, 237–245. [Google Scholar] [CrossRef]
- Lü, F.; Shao, L.-M.; Zhang, H.; Fu, W.-D.; Feng, S.-J.; Zhan, L.-T.; Chen, Y.-M.; He, P.-J. Application of advanced techniques for the assessment of bio-stability of biowaste-derived residues: A minireview. Bioresour. Technol. 2018, 248, 122–133. [Google Scholar] [CrossRef]
- Niculita-Hirzel, H.; Hantier, G.; Storti, F.; Plateel, G.; Roger, T. Frequent Occupational Exposure to Fusarium Mycotoxins of Workers in the Swiss Grain Industry. Toxins 2016, 8, 370. [Google Scholar] [CrossRef] [Green Version]
- Hashemi, H.; Abbasi, F.; Samaei, M.R.; Khodadadi, H. Determination of Fungi Species Variety in Thermal Phases of Compost Production and Related Operational Parameters. J. Environ. Eng. 2018, 144, 04018065. [Google Scholar] [CrossRef]
- Qiu, X.; Zhou, G.; Zhang, J.; Wang, W. Microbial community responses to biochar addition when a green waste and manure mix are composted: A molecular ecological network analysis. Bioresour. Technol. 2019, 273, 666–671. [Google Scholar] [CrossRef]
- Kang, W.; Kim, I.-H.; Lee, T.-J.; Kim, K.-Y.; Kim, D. Effect of temperature on bacterial emissions in composting of swine manure. Waste Manag. 2014, 34, 1006–1011. [Google Scholar] [CrossRef]
- Galès, A.; Bru-Adan, V.; Godon, J.-J.; Delabre, K.; Catala, P.; Ponthieux, A.; Chevallier, M.; Birot, E.; Steyer, J.-P.; Wéry, N. Predominance of single bacterial cells in composting bioaerosols. Atmos. Environ. 2015, 107, 225–232. [Google Scholar] [CrossRef]


| Time | TK (%) | TN (%) | TP (%) | OM (%) | pH | AD (%) | T (°C) |
|---|---|---|---|---|---|---|---|
| 2020.06.14 | 6.49 ± 0.01 | 1.58 ± 0.00 | 0.73 ± 0.00 | 19.65 ± 0.12 | 8.93 ± 0.03 | 1.90 ± 0.00 | 72.50 ± 0.00 |
| 2020.06.16 | 6.31 ± 0.00 | 1.80 ± 0.01 | 0.70 ± 0.00 | 33.70 ± 0.16 | 8.67 ± 0.03 | 2.00 ± 0.00 | 72.00 ± 0.00 |
| 2020.06.18 | 6.16 ± 0.02 | 1.81 ± 0.01 | 0.76 ± 0.00 | 28.85 ± 0.04 | 8.69 ± 0.00 | 1.90 ± 0.00 | 70.00 ± 0.00 |
| 2020.06.20 | 5.98 ± 0.00 | 1.90 ± 0.00 | 0.80 ± 0.00 | 27.30 ± 0.08 | 8.50 ± 0.02 | 1.70 ± 0.00 | 68.50 ± 0.00 |
| 2020.06.22 | 6.48 ± 0.03 | 2.01 ± 0.01 | 0.81 ± 0.00 | 28.10 ± 0.08 | 8.78 ± 0.04 | 1.90 ± 0.00 | 70.00 ± 0.00 |
| 2020.06.24 | 6.01 ± 0.02 | 2.02 ± 0.01 | 0.67 ± 0.00 | 30.05 ± 0.04 | 8.74 ± 0.03 | 2.00 ± 0.00 | 70.00 ± 0.00 |
| Mean value | 6.24 ± 0.21 | 1.85 ± 0.15 | 0.75 ± 0.05 | 27.94 ± 4.24 | 8.72 ± 0.13 | 1.90 ± 0.00 | 70.50 ± 0.00 |
| Time | Pb | Cd | Cr | Cu | Zn | Hg | As |
|---|---|---|---|---|---|---|---|
| 2020.06.14 | 18.05 ± 0.58 | 1.37 ± 0.02 | 20.42 ± 0.03 | 41.80 ± 0.08 | 231.26 ± 4.68 | 0.06 ± 0.00 | 2.03 ± 0.07 |
| 2020.06.16 | 14.09 ± 0.87 | 1.12 ± 0.04 | 16.66 ± 0.25 | 44.32 ± 0.24 | 223.14 ± 0.91 | 0.06 ± 0.00 | 1.80 ± 0.01 |
| 2020.06.18 | 14.81 ± 0.81 | 1.13 ± 0.02 | 20.60 ± 0.15 | 49.62 ± 0.04 | 277.25 ± 0.35 | 0.03 ± 0.00 | 1.72 ± 0.06 |
| 2020.06.20 | 15.93 ± 0.12 | 1.33 ± 0.01 | 31.56 ± 0.09 | 49.55 ± 0.18 | 278.00 ± 2.48 | 0.03 ± 0.00 | 1.33 ± 0.01 |
| 2020.06.22 | 14.55 ± 0.58 | 1.26 ± 0.01 | 22.83 ± 0.13 | 48.27 ± 0.01 | 253.77 ± 0.92 | 0.03 ± 0.00 | 1.30 ± 0.02 |
| 2020.06.24 | 13.81 ± 0.99 | 1.26 ± 0.05 | 19.36 ± 0.19 | 46.39 ± 0.19 | 270.23 ± 0.01 | 0.01 ± 0.00 | 1.20 ± 0.01 |
| Mean value | 15.20 ± 0.66 | 1.25 ± 0.02 | 21.90 ± 0.14 | 46.66 ± 0.12 | 255.61 ± 1.56 | 0.04 ± 0.00 | 1.56 ± 0.03 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, R.; Yu, A.; Qiu, T.; Guo, Y.; Gao, H.; Sun, X.; Gao, M.; Wang, X. Aerosolization Behaviour of Fungi and Its Potential Health Effects during the Composting of Animal Manure. Int. J. Environ. Res. Public Health 2022, 19, 5644. https://doi.org/10.3390/ijerph19095644
Wang R, Yu A, Qiu T, Guo Y, Gao H, Sun X, Gao M, Wang X. Aerosolization Behaviour of Fungi and Its Potential Health Effects during the Composting of Animal Manure. International Journal of Environmental Research and Public Health. 2022; 19(9):5644. https://doi.org/10.3390/ijerph19095644
Chicago/Turabian StyleWang, Ruonan, Aoyuan Yu, Tianlei Qiu, Yajie Guo, Haoze Gao, Xingbin Sun, Min Gao, and Xuming Wang. 2022. "Aerosolization Behaviour of Fungi and Its Potential Health Effects during the Composting of Animal Manure" International Journal of Environmental Research and Public Health 19, no. 9: 5644. https://doi.org/10.3390/ijerph19095644
APA StyleWang, R., Yu, A., Qiu, T., Guo, Y., Gao, H., Sun, X., Gao, M., & Wang, X. (2022). Aerosolization Behaviour of Fungi and Its Potential Health Effects during the Composting of Animal Manure. International Journal of Environmental Research and Public Health, 19(9), 5644. https://doi.org/10.3390/ijerph19095644






