Recurrent Plasmodium vivax Cases of Both Short and Long Latency Increased with Transmission Intensity and Were Distributed Year-Round in the Most Affected Municipalities of the RACCN, Nicaragua, 2013–2018
Abstract
1. Introduction
2. Materials and Methods
2.1. Description of the Study Area
2.2. Data Cleaning and Analysis
3. Results
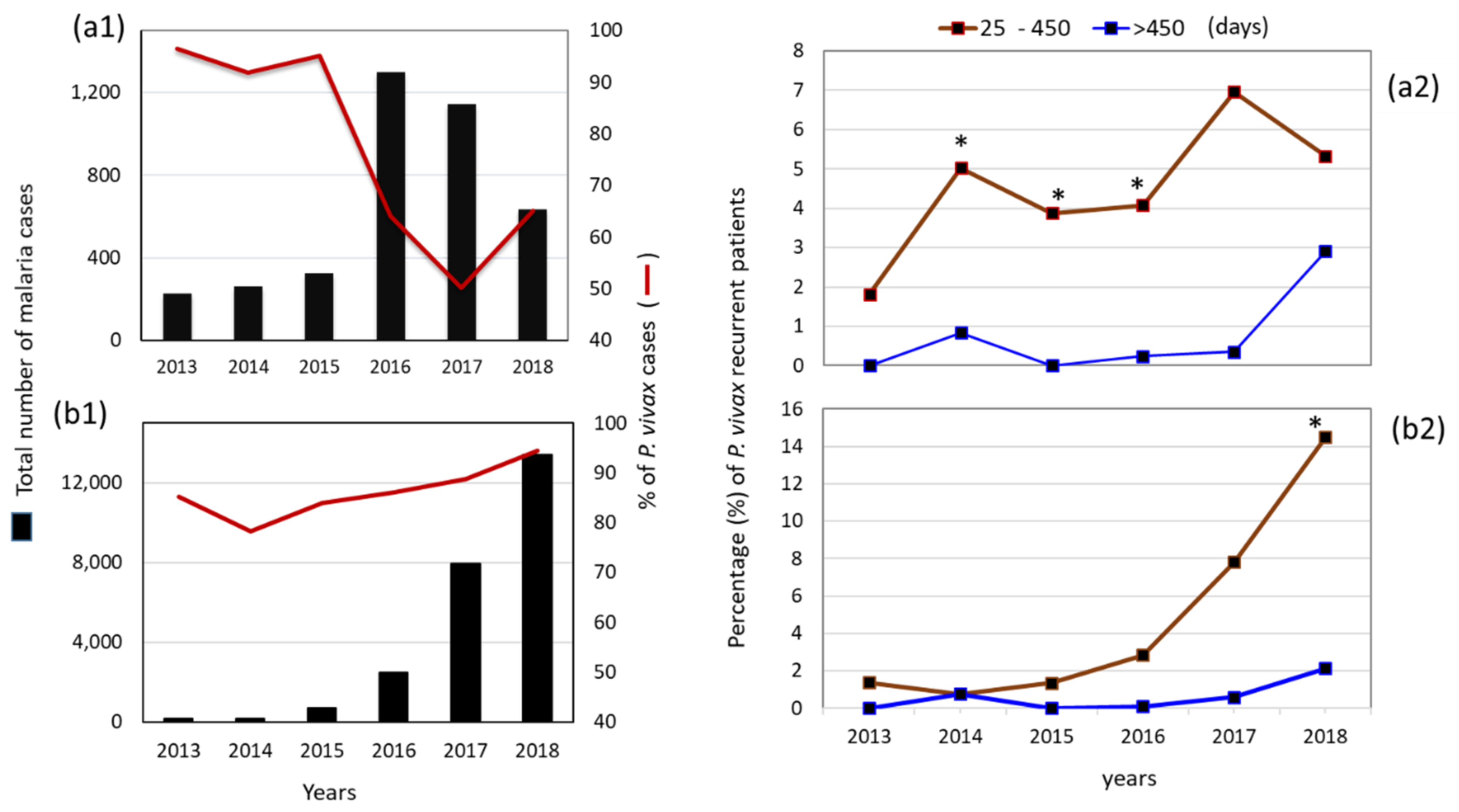
3.1. Malaria Cases per Species per Year at a National Level
3.2. Patients with Recurrent P. vivax Infections Nationwide
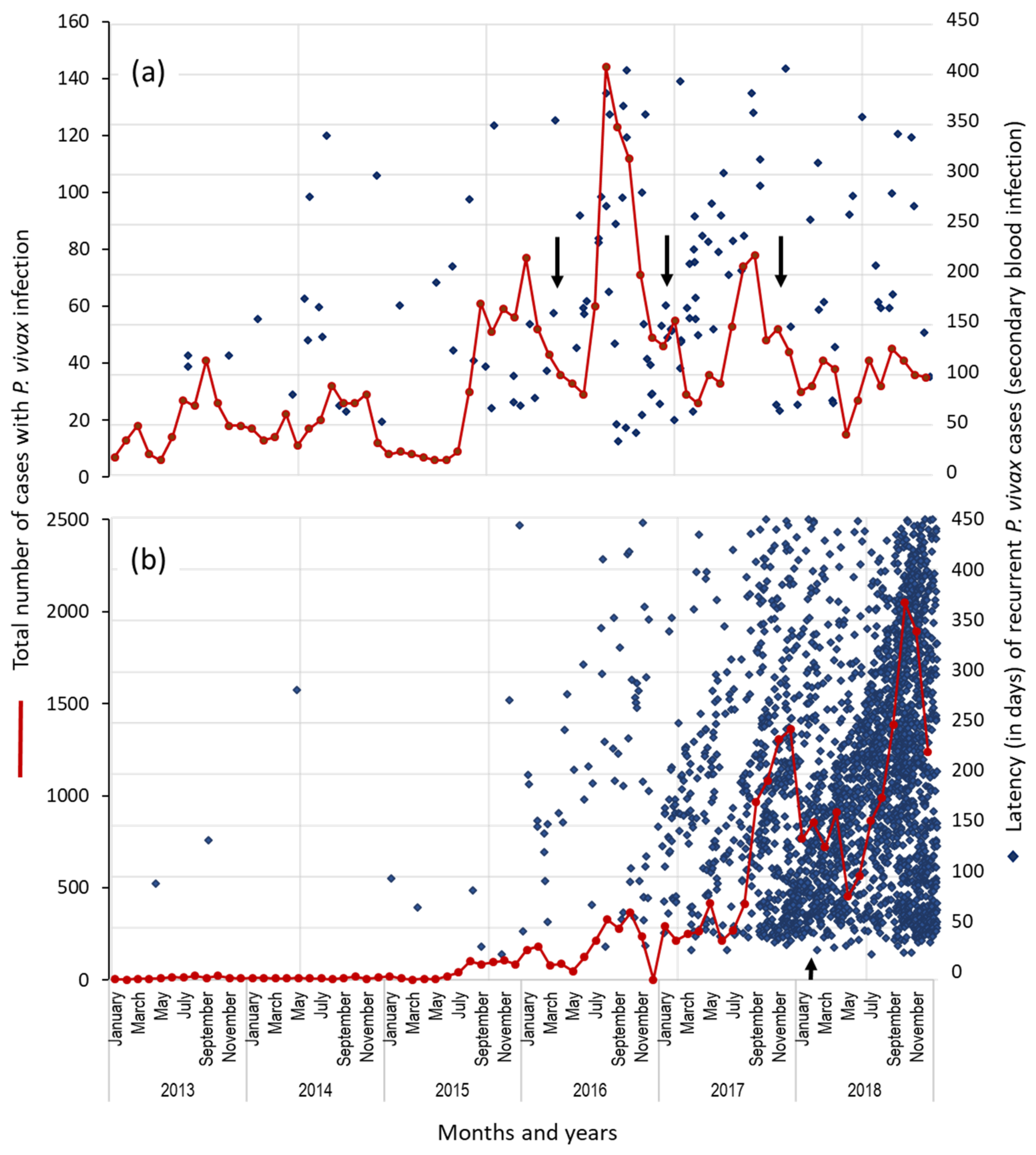
3.3. Proportion and Latency of P. vivax Recurrent Cases in Municipalities: Puerto Cabezas and Rosita, RACCN (2013–2018)
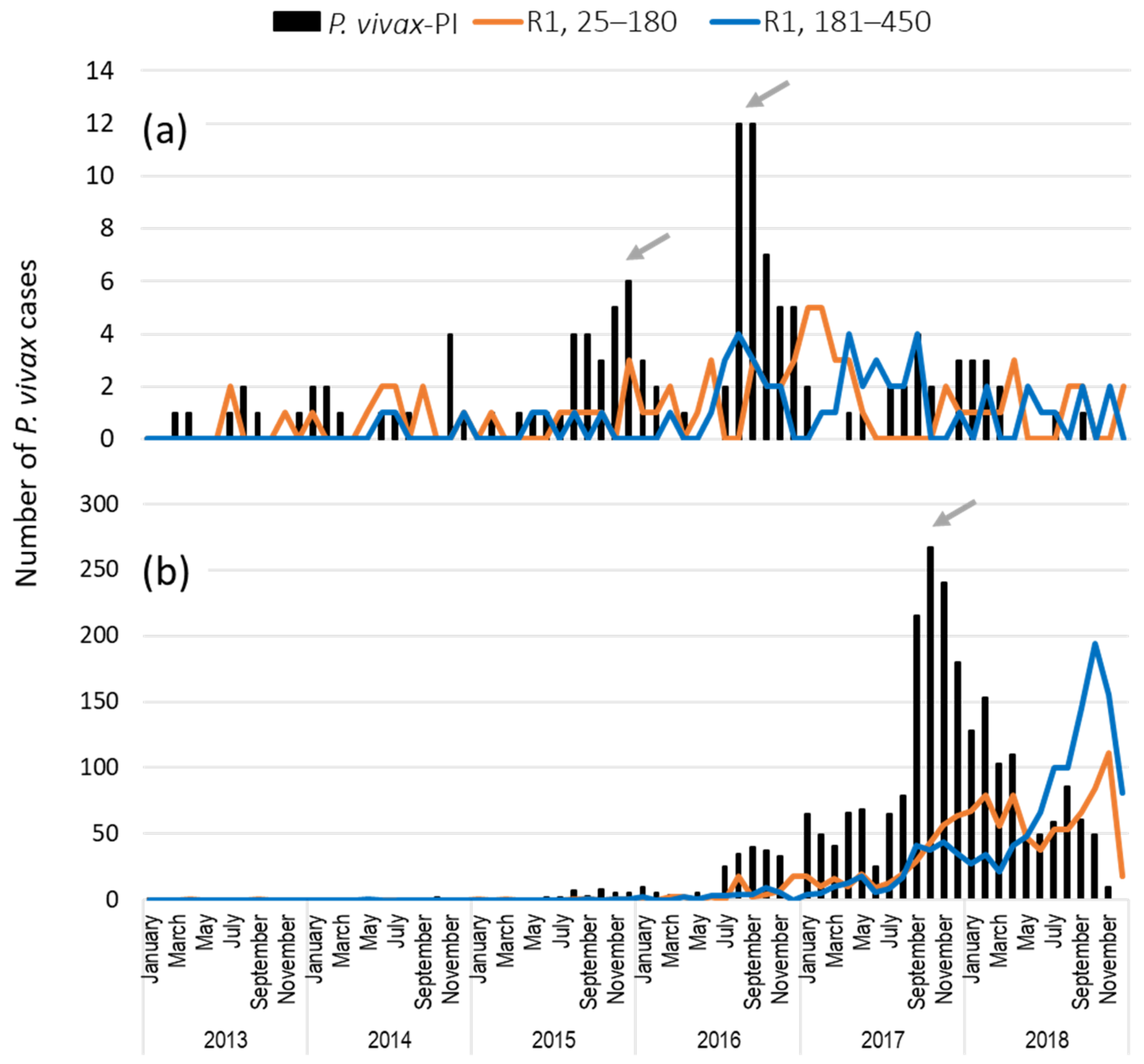
3.4. Temporal Distribution of First Recurrent Episodes (by the Latency) and Total P. vivax Infections in Rosita and Puerto Cabezas, RACCN
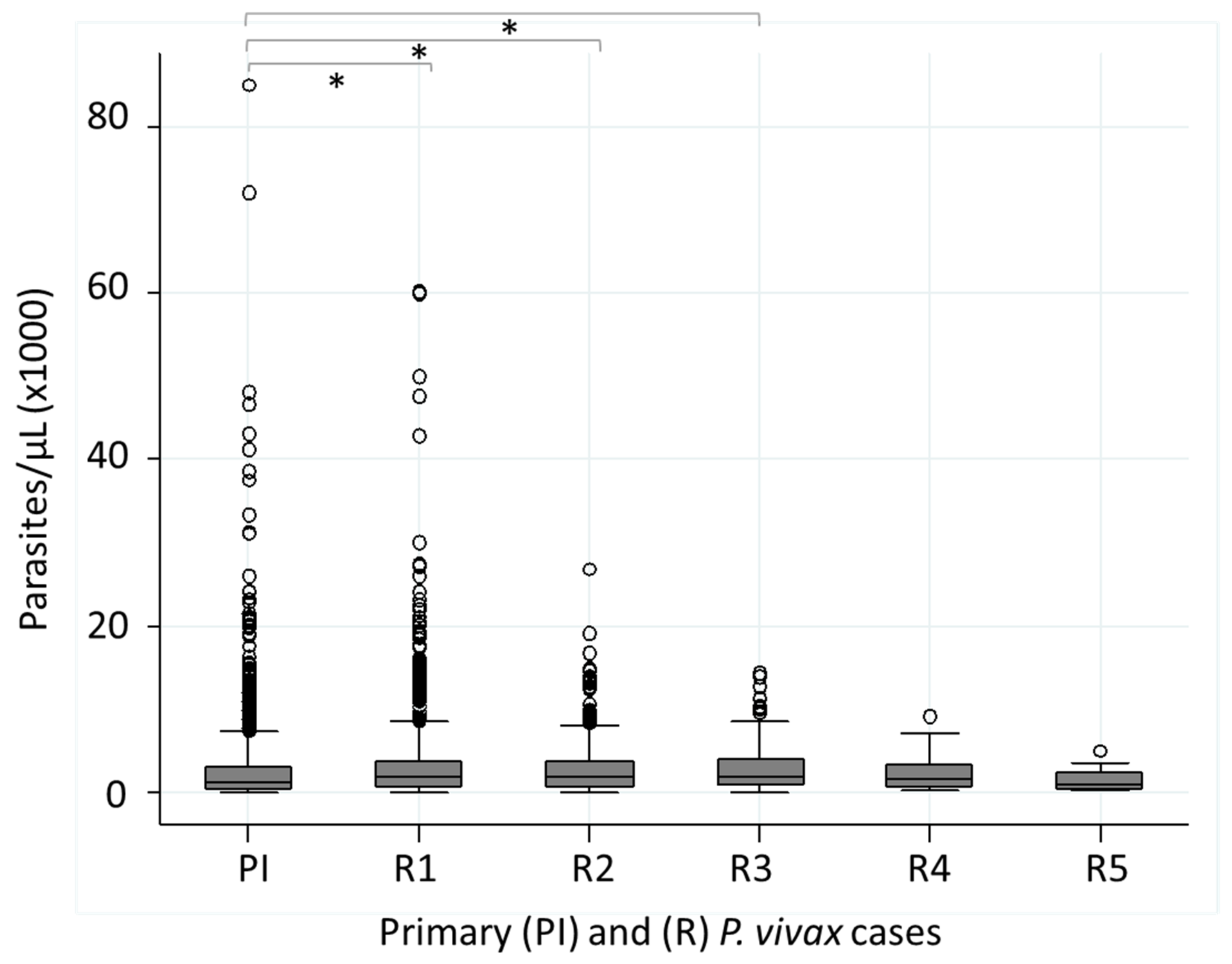
3.5. Parasitemia (Parasites/µL) in Patients with Recurrent P. vivax Infections
3.6. Comparison of Recurrent P. vivax and P. falciparum Infections (25–450 Days) in RACCN Municipalities: Puerto Cabezas and Rosita
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lover, A.A.; Baird, J.K.; Gosling, R.; Price, R.N. Malaria Elimination: Time to Target All Species. Am. J. Trop. Med. Hyg. 2018, 99, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Battle, K.E.; Karhunen, M.S.; Bhatt, S.; Gething, P.W.; Howes, R.E.; Golding, N.; Van Boeckel, T.P.; Messina, J.P.; Shanks, G.D.; Smith, D.L.; et al. Geographical variation in Plasmodium vivax relapse. Malar. J. 2014, 13, 144. [Google Scholar] [CrossRef] [PubMed]
- WHO. Guidelines for the Treatment of Malaria. 2015. Available online: https://www.ncbi.nlm.nih.gov/books/NBK294440/ (accessed on 16 May 2022).
- Howes, R.E.; Battle, K.E.; Mendis, K.N.; Smith, D.L.; Cibulskis, R.E.; Baird, J.K.; Hay, S.I. Global Epidemiology of Plasmodium vivax. Am. J. Trop. Med. Hyg. 2016, 95, 15–34. [Google Scholar] [CrossRef] [PubMed]
- WHO. Guidelines for Malaria. Available online: https://apps.who.int/iris/handle/10665/343751 (accessed on 16 May 2022).
- Cedillos, R.A.; Warren, M.; Jeffery, G.M. Field evaluation of primaquine in the control of Plasmodium vivax. Am. J. Trop. Med. Hyg. 1978, 27, 466–472. [Google Scholar] [CrossRef] [PubMed]
- Mason, J. Patterns of Plasmodium vivax recurrence in a high-incidence coastal area of El Salvador, C.A. Am. J. Trop. Med. Hyg. 1975, 24, 581–585. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Ceron, L.; Mu, J.; Santillan, F.; Joy, D.; Sandoval, M.A.; Camas, G.; Su, X.; Choy, E.V.; Torreblanca, R. Molecular and epidemiological characterization of Plasmodium vivax recurrent infections in southern Mexico. Parasites Vectors 2013, 6, 109. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Ceron, L.; Rodriguez, M.H.; Sandoval, M.A.; Santillan, F.; Galindo-Virgen, S.; Betanzos, A.F.; Rosales, A.F.; Palomeque, O.L. Effectiveness of combined chloroquine and primaquine treatment in 14 days versus intermittent single dose regimen, in an open, non-randomized, clinical trial, to eliminate Plasmodium vivax in southern Mexico. Malar. J. 2015, 14, 426. [Google Scholar] [CrossRef][Green Version]
- Contacos, P.G.; Collins, W.E.; Jeffery, G.M.; Krotoski, W.A.; Howard, W.A. Studies on the characterization of Plasmodium vivax strains from Central America. Am. J. Trop. Med. Hyg. 1972, 21, 707–712. [Google Scholar] [CrossRef]
- WHO. World Malaria Report. 2021. Available online: https://www.who.int/teams/global-malaria-programme/reports/world-malaria-report-2021 (accessed on 16 May 2022).
- WHO. World Malaria Report. 2019. Available online: https://www.who.int/publications/i/item/9789241565721 (accessed on 16 May 2022).
- PAHO. Report on the Situation of Malaria in the Americas. 2017. Available online: https://www3.paho.org/hq/index.php?option=com_docman&view=download&category_slug=datos-estadisticos-mapas-8110&alias=48336-situation-of-malaria-in-the-region-of-the-americas-2017-1&Itemid=270&lang=es (accessed on 16 May 2022).
- BANCO MUNDIAL. Población Total Nicaragua. Available online: https://datos.bancomundial.org/indicador/SP.POP.TOTL?locations=NI (accessed on 16 May 2022).
- Gobierno de Nicaragua. Perfil Demográfico Nicaragua. 2020. Available online: https://pronicaragua.gob.ni/media/publications/Perfil_Demografico_2020_PWsyOuB.pdf (accessed on 16 May 2022).
- Plan Nacional de Nicaragua en el Marco del Plan Mesoamericano Para Mejorar el Control de la Malaria Hacia su Eliminación. 2015. Available online: http://www.proyectomesoamerica.org:8088/smsp/phocadownload/Institucional/PlanesNacionales/PNMalaria/NIC%20PN%20Malaria.pdf (accessed on 16 May 2022).
- Biología y Ecología de Anopheles albimanus Wiedemann en Centroamérica. Bol. Oficina Sanit. Panam. 1996, 121, 32.
- Sinka, M.E.; Rubio-Palis, Y.; Manguin, S.; Patil, A.P.; Temperley, W.H.; Gething, P.W.; Van Boeckel, T.; Kabaria, C.W.; Harbach, R.E.; Hay, S.I. The dominant Anopheles vectors of human malaria in the Americas: Occurrence data, distribution maps and bionomic precis. Parasites Vectors 2010, 3, 72. [Google Scholar] [CrossRef]
- Sequeira, M.E.H.; Amador, J.J.; Domingo, G.; Quintanilla, M.; de los Santos, T. Malaria in Nicaragua: A Review of Control Status, Trends, and Needs. Available online: https://path.azureedge.net/media/documents/TS_nicaragua_malaria_rpt.pdf (accessed on 16 May 2022).
- Covell, G. Relationship between malarial parasitaemia and symptoms of the disease: A review of the literature. Bull. World Health Organ. 1960, 22, 605–619. [Google Scholar] [PubMed]
- Stepniewska, K.; Taylor, W.R.; Mayxay, M.; Price, R.; Smithuis, F.; Guthmann, J.P.; Barnes, K.; Myint, H.Y.; Adjuik, M.; Olliaro, P.; et al. In vivo assessment of drug efficacy against Plasmodium falciparum malaria: Duration of follow-up. Antimicrob. Agents. Chemother. 2004, 48, 4271–4280. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Galappaththy, G.N.; Tharyan, P.; Kirubakaran, R. Primaquine for preventing relapse in people with Plasmodium vivax malaria treated with chloroquine. Cochrane Database Syst. Rev. 2013, 10, CD004389. [Google Scholar] [CrossRef]
- Kitchener, S.; Nasveld, P.; Bennett, S.; Torresi, J. Adequate primaquine for vivax malaria. J. Travel. Med. 2005, 12, 133–135. [Google Scholar] [CrossRef][Green Version]
- Carmona Fonseca, J. Primaquine and relapses of Plasmodium vivax. Meta analysis of controlled clinical trials. Rev. Bras. Epidemiol. 2015, 18, 174–193. [Google Scholar] [CrossRef]
- Ministerio de Salud. Esquema Nacional de Tratamiento de Malaria. 2014. Available online: https://www.paho.org/es/documentos/esquema-nacional-tratamiento-malaria-miniosterio-salud-nicaragua-2014 (accessed on 16 May 2022).
- Rodriguez, M.H.; Betanzos-Reyes, A.F.; Hernandez-Avila, J.E.; Mendez-Galvan, J.F.; Danis-Lozano, R.; Altamirano-Jimenez, A. The participation of secondary clinical episodes in the epidemiology of vivax malaria during pre- and post-implementation of focal control in the state of Oaxaca, Mexico. Am. J. Trop. Med. Hyg. 2009, 80, 889–895. [Google Scholar] [CrossRef]
- Hurtado, L.A.; Rigg, C.A.; Calzada, J.E.; Dutary, S.; Bernal, D.; Koo, S.I.; Chaves, L.F. Population Dynamics of Anopheles albimanus (Diptera: Culicidae) at Ipeti-Guna, a Village in a Region Targeted for Malaria Elimination in Panama. Insects 2018, 9, 164. [Google Scholar] [CrossRef]
- Haghdoost, A.A.; Mazhari, S.; Bahaadini, K. Estimating the relapse risk of Plasmodium vivax in Iran under national chemotherapy scheme using a novel method. J. Vector. Borne Dis. 2006, 43, 168–172. [Google Scholar]
- Taylor, W.R.; Hoglund, R.M.; Peerawaranun, P.; Nguyen, T.N.; Hien, T.T.; Tarantola, A.; von Seidlein, L.; Tripura, R.; Peto, T.J.; Dondorp, A.M.; et al. Development of weight and age-based dosing of daily primaquine for radical cure of vivax malaria. Malar. J. 2021, 20, 366. [Google Scholar] [CrossRef]
- Mac Donald-Ottevanger, M.S.; Adhin, M.R.; Jitan, J.K.; Bretas, G.; Vreden, S.G. Primaquine double dose for 7 days is inferior to single-dose treatment for 14 days in preventing Plasmodium vivax recurrent episodes in Suriname. Infect. Drug Resist. 2018, 11, 3–8. [Google Scholar] [CrossRef]
- Orjuela-Sanchez, P.; da Silva, N.S.; da Silva-Nunes, M.; Ferreira, M.U. Recurrent parasitemias and population dynamics of Plasmodium vivax polymorphisms in rural Amazonia. Am. J. Trop. Med. Hyg. 2009, 81, 961–968. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, L.; Rodrigues, E.; Ribatski-Silva, D.; Teixeira, L.; Ferreira, A.; Fernandes, C. Factors associated with recurrent Plasmodium vivax malaria in Porto Velho, Rondônia State, Brazil, 2009. Cad. Saude Publica 2014, 30, 1403–1417. [Google Scholar]
- Daher, A.; Silva, J.; Stevens, A.; Marchesini, P.; Fontes, C.J.; Ter Kuile, F.O.; Lalloo, D.G. Evaluation of Plasmodium vivax malaria recurrence in Brazil. Malar. J. 2019, 18, 18. [Google Scholar] [CrossRef] [PubMed]
- PAHO Malaria Technical Advisory Group. Strategies to Decrease Relapses in P. vivax Cases. Available online: https://www3.paho.org/hq/index.php?option=com_docman&view=download&slug=malaria-technical-group-session-6-strategy-for-reducing-the-relapses-of-p-vivax-for-the-control-and-elimination-of-malaria-in-the-americas&Itemid=270&lang=es (accessed on 16 May 2022).
- Mueller, I.; Galinski, M.R.; Tsuboi, T.; Arevalo-Herrera, M.; Collins, W.E.; King, C.L. Natural acquisition of immunity to Plasmodium vivax: Epidemiological observations and potential targets. Adv. Parasitol. 2013, 81, 77–131. [Google Scholar] [CrossRef]
- Michon, P.; Cole-Tobian, J.L.; Dabod, E.; Schoepflin, S.; Igu, J.; Susapu, M.; Tarongka, N.; Zimmerman, P.A.; Reeder, J.C.; Beeson, J.G.; et al. The risk of malarial infections and disease in Papua New Guinean children. Am. J. Trop. Med. Hyg. 2007, 76, 997–1008. [Google Scholar] [CrossRef]
- Collins, W.E.; Jeffery, G.M.; Roberts, J.M. A retrospective examination of reinfection of humans with Plasmodium vivax. Am. J. Trop. Med. Hyg. 2004, 70, 642–644. [Google Scholar] [CrossRef]
- Cucunuba, Z.M.; Guerra, A.P.; Rahirant, S.J.; Rivera, J.A.; Cortes, L.J.; Nicholls, R.S. Asymptomatic Plasmodium spp. infection in Tierralta, Colombia. Mem. Inst. Oswaldo Cruz. 2008, 103, 668–673. [Google Scholar] [CrossRef]
- Camargo, E.P.; Alves, F.; Pereira da Silva, L.H. Symptomless Plasmodium vivax infections in native Amazonians. Lancet 1999, 353, 1415–1416. [Google Scholar] [CrossRef]
- Lin, J.T.; Saunders, D.L.; Meshnick, S.R. The role of submicroscopic parasitemia in malaria transmission: What is the evidence? Trends. Parasitol. 2014, 30, 183–190. [Google Scholar] [CrossRef]
- Silva, R.G.; Nunes, J.E.; Canduri, F.; Borges, J.C.; Gava, L.M.; Moreno, F.B.; Basso, L.A.; Santos, D.S. Purine nucleoside phosphorylase: A potential target for the development of drugs to treat T-cell- and apicomplexan parasite-mediated diseases. Curr. Drug Targets 2007, 8, 413–422. [Google Scholar] [CrossRef]
- Gutierrez, S.; Gonzalez-Ceron, L.; Montoya, A.; Sandoval, M.A.; Torres, M.E.; Cerritos, R. Genetic structure of Plasmodium vivax in Nicaragua, a country in the control phase, based on the carboxyl terminal region of the merozoite surface protein-1. Infect. Genet. Evol. 2016, 40, 324–330. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Ceron, L.; Montoya, A.; Corzo-Gomez, J.C.; Cerritos, R.; Santillan, F.; Sandoval, M.A. Genetic diversity and natural selection of Plasmodium vivax multi-drug resistant gene (pvmdr1) in Mesoamerica. Malar. J. 2017, 16, 261. [Google Scholar] [CrossRef] [PubMed]
- Douglas, N.M.; Nosten, F.; Ashley, E.A.; Phaiphun, L.; van Vugt, M.; Singhasivanon, P.; White, N.J.; Price, R.N. Plasmodium vivax recurrence following falciparum and mixed species malaria: Risk factors and effect of antimalarial kinetics. Clin. Infect. Dis. 2011, 52, 612–620. [Google Scholar] [CrossRef] [PubMed]
- Ashley, E.A.; Phyo, A.P.; Carrara, V.I.; Tun, K.M.; Nosten, F.; Smithuis, F.; White, N.J. Plasmodium vivax Relapse Rates Following Plasmodium falciparum Malaria Reflect Previous Transmission Intensity. J. Infect. Dis. 2019, 220, 100–104. [Google Scholar] [CrossRef]
- Commons, R.J.; Simpson, J.A.; Watson, J.; White, N.J.; Price, R.N. Estimating the Proportion of Plasmodium vivax Recurrences Caused by Relapse: A Systematic Review and Meta-Analysis. Am. J. Trop. Med. Hyg. 2020, 103, 1094–1099. [Google Scholar] [CrossRef]
- Sagara, I.; Sangare, D.; Dolo, G.; Guindo, A.; Sissoko, M.; Sogoba, M.; Niambele, M.B.; Yalcoue, D.; Kaslow, D.C.; Dicko, A.; et al. A high malaria reinfection rate in children and young adults living under a low entomological inoculation rate in a periurban area of Bamako, Mali. Am. J. Trop. Med. Hyg. 2002, 66, 310–313. [Google Scholar] [CrossRef]
- Larranaga, N.; Mejia, R.E.; Hormaza, J.I.; Montoya, A.; Soto, A.; Fontecha, G.A. Genetic structure of Plasmodium falciparum populations across the Honduras-Nicaragua border. Malar. J. 2013, 12, 354. [Google Scholar] [CrossRef]
- Rodriguez, M.H.; Betanzos-Reyes, A.F. Plan to improve malaria control towards its elimination in Mesoamerica. Salud. Publica Mex. 2011, 53 (Suppl. S3), S333–S348. [Google Scholar]
- Balieiro, A.A.S.; Siqueira, A.M.; Melo, G.C.; Monteiro, W.M.; Sampaio, V.S.; Mueller, I.; Lacerda, M.V.G.; Villela, D.A.M. Short-Time Recurrences of Plasmodium vivax Malaria as a Public Health Proxy for Chloroquine-Resistance Surveillance: A Spatio-Temporal Study in the Brazilian Amazon. Int. J. Environ. Res. Public Health 2021, 18, 5061. [Google Scholar] [CrossRef]
- Llanos-Cuentas, A.; Lacerda, M.V.G.; Hien, T.T.; Velez, I.D.; Namaik-Larp, C.; Chu, C.S.; Villegas, M.F.; Val, F.; Monteiro, W.M.; Brito, M.A.M.; et al. Tafenoquine versus Primaquine to Prevent Relapse of Plasmodium vivax Malaria. N. Engl. J. Med. 2019, 380, 229–241. [Google Scholar] [CrossRef]
- WHO. New Opportunities to Prevent Plasmodium vivax Malaria Relapse. Available online: https://www.who.int/news/item/25-02-2019-new-opportunities-to-prevent-p-vivax-malaria-relapse#:~:text=For%20the%20treatment%20of%20P,a%20full%202%2Dweek%20period (accessed on 16 May 2022).
- Kondrashin, A.; Baranova, A.M.; Ashley, E.A.; Recht, J.; White, N.J.; Sergiev, V.P. Mass primaquine treatment to eliminate vivax malaria: Lessons from the past. Malar. J. 2014, 13, 51. [Google Scholar] [CrossRef] [PubMed]
- Mendis, K. Mass drug administration should be implemented as a tool to accelerate elimination: Against. Malar. J. 2019, 18, 279. [Google Scholar] [CrossRef] [PubMed]

| Plasmodium Species | Years (Number of Cases) | Total | |||||
|---|---|---|---|---|---|---|---|
| 2013 | 2014 | 2015 | 2016 | 2017 | 2018 | ||
| P. vivax | 950 | 924 | 1880 | 4343 | 8959 | 14,501 | 31,557 |
| P. falciparum | 211 | 139 | 320 | 1104 | 2033 | 1319 | 5126 |
| Mixed Pv–Pf | 0 | 0 | 3 | 17 | 35 | 49 | 104 |
| Total | 1161 | 1063 | 2203 | 5464 | 11,027 | 15,869 | 36,787 |
| % P. vivax | 81.8 | 86.9 | 85.3 | 79.5 | 81.2 | 91.4 | 85.8 |
| % P. falciparum | 18.2 | 13.1 | 14.5 | 20.3 | 18.4 | 8.3 | 13.9 |
| % Pv–Pf | 0 | 0 | 0.1 | 0.3 | 0.3 | 0.3 | 0.3 |
| NI | 4 | 2 | 0 | 70 | 0 | 1 | 77 |
| Number of P. vivax Infections | Nationwide (%) n = 3163 Patients | RACCN, Puerto Cabezas (%) n = 2789 Patients | RACCN, Rosita (%) n = 139 Patients |
|---|---|---|---|
| 2 | 77.2 | 75.3 | 90.7 |
| 3 | 16.7 | 17.9 | 7.2 |
| 4 | 4 | 4.4 | 2.1 |
| 5 | 1.2 | 1.3 | 0.7 |
| 6 | 0.6 | 0.7 | - |
| 7 | 0.09 | 0.1 | - |
| (a) | ||||||
| Parameters | Season/Period | Statistics (Comparing All Groups) | ||||
| Rain | Dry | Rain | Dry | Rain | ||
| June–November 2015 | December 2015–May 2016 | June–November 2016 | December 2016–May 2017 | June–November 2017 | ||
| Number of P. vivax cases | 216 | 302 | 533 | 241 | 338 | χ2 = 9.6, p = 0.046 |
| Linked to recurrent patients (%) | 8.3 | 3.9 | 7 | 3 | 3.7 | |
| Number and latency of R1: | χ2 = 11.04, p = 0.026 | |||||
| n | 6 | 9 | 21 | 28 | 13 | |
| Short: 25–180 days (%) | 66.6 | 88.8 | 33.3 | 71.4 | 15.3 | |
| Long: 181–450 days (%) | 33.3 | 11.2 | 66.7 | 28.6 | 84.7 | |
| Statistics: | χ2 = 7.7, p = 0.005 | χ2 = 16.6, p < 0.0001 | ||||
| χ2 = 1.1, p = 0.69 | χ2 = 9.03, p = 0.007 | |||||
| (b) | ||||||
| Parameters | Season/Period | Statistics (Comparing All Groups) | ||||
| Dry | Rain | Dry | Rain | |||
| December 2016–May 2017 | June–November 2017 | December 2017–May 2018 | June–November 2018 | |||
| Number of P. vivax cases | 1439 | 4251 | 5062 | n.d. | χ2 = 76.7, p < 0.0001 | |
| Linked to recurrent patients (%) | 20 | 20 | 14 * | n.d. | ||
| Number and latency of R1: | χ2 = 211.8, p < 0.0001 | |||||
| n | 142 | 325 | 598 | 1111 | ||
| Short: 25–180 days (%) | 64.7 | 52.6 | 65 | 35 | ||
| Long: 181–450 days (%) | 35.3 | 47.4 | 35 | 65 | ||
| Statistics: | χ2 = 5.9, p = 0.014 | χ2 = 132.2, p < 0.0001 | ||||
| χ2 = 10.1, p = 0.001 | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soto, A.M.; González-Cerón, L.; Santillán-Valenzuela, F.; Parrales, M.E.; Montoya, A. Recurrent Plasmodium vivax Cases of Both Short and Long Latency Increased with Transmission Intensity and Were Distributed Year-Round in the Most Affected Municipalities of the RACCN, Nicaragua, 2013–2018. Int. J. Environ. Res. Public Health 2022, 19, 6195. https://doi.org/10.3390/ijerph19106195
Soto AM, González-Cerón L, Santillán-Valenzuela F, Parrales ME, Montoya A. Recurrent Plasmodium vivax Cases of Both Short and Long Latency Increased with Transmission Intensity and Were Distributed Year-Round in the Most Affected Municipalities of the RACCN, Nicaragua, 2013–2018. International Journal of Environmental Research and Public Health. 2022; 19(10):6195. https://doi.org/10.3390/ijerph19106195
Chicago/Turabian StyleSoto, Aida M., Lilia González-Cerón, Frida Santillán-Valenzuela, María E. Parrales, and Alberto Montoya. 2022. "Recurrent Plasmodium vivax Cases of Both Short and Long Latency Increased with Transmission Intensity and Were Distributed Year-Round in the Most Affected Municipalities of the RACCN, Nicaragua, 2013–2018" International Journal of Environmental Research and Public Health 19, no. 10: 6195. https://doi.org/10.3390/ijerph19106195
APA StyleSoto, A. M., González-Cerón, L., Santillán-Valenzuela, F., Parrales, M. E., & Montoya, A. (2022). Recurrent Plasmodium vivax Cases of Both Short and Long Latency Increased with Transmission Intensity and Were Distributed Year-Round in the Most Affected Municipalities of the RACCN, Nicaragua, 2013–2018. International Journal of Environmental Research and Public Health, 19(10), 6195. https://doi.org/10.3390/ijerph19106195







