Endocrine Disruptor Bisphenol a Affects the Neurochemical Profile of Nerve Fibers in the Aortic Arch Wall in the Domestic Pig
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rubin, B.S. Bisphenol A: An endocrine disruptor with widespread exposure and multiple effects. J. Steroid. Biochem. Mol. Bio. 2011, 127, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Balistrieri, A.; Hobohm, L.; Srivastava, T.; Meier, A.; Corriden, R. Alterations in human neutrophil function caused by Bisphenol A. Am. J. Physiol. Cell Physiol. 2018, 315, C636–C642. [Google Scholar] [CrossRef] [PubMed]
- Hao, P.P. Determination of Bisphenol A in barreled drinking water by a SPE-LC-MS method. J. Environ. Sci. Health Part A 2020, 55, 697–703. [Google Scholar] [CrossRef] [PubMed]
- Michałowicz, J. Bisphenol A—Sources, toxicity and biotransformation. Environ. Toxicol. Pharmacol. 2014, 37, 738–758. [Google Scholar] [CrossRef] [PubMed]
- Rytel, L.; Gonkowski, S. The influence of Bisphenol A on the nitrergic nervous structures in the domestic porcine uterus. Int. J. Mol. Sci. 2020, 21, 4543. [Google Scholar] [CrossRef] [PubMed]
- Reale, E.; Vernez, D.; Hopf, N.B. Skin absorption of Bisphenol A and its alternatives in thermal paper. Ann. Work Expo. Health 2021, 65, 206–218. [Google Scholar] [CrossRef] [PubMed]
- Szymanska, K.; Gonkowski, S. Neurochemical characterization of the enteric neurons within the porcine jejunum in physiological conditions and under the influence of Bisphenol A (BPA). Neurogastroenterol Motil. 2019, 31, e13580. [Google Scholar] [CrossRef]
- Hafezi, S.A.; Abdel-Rahman, W.M. The endocrine disruptor Bisphenol A (BPA) exerts a wide range of effects in carcinogenesis and response to therapy. Curr. Mol. Pharmacol. 2019, 12, 230–238. [Google Scholar] [CrossRef]
- Monneret, C. What is an endocrine disruptor? Comptes Rendus Biol. 2017, 340, 403–405. [Google Scholar] [CrossRef]
- Rytel, L. The influence of Bisphenol A (BPA) on neuregulin 1-like immunoreactive nerve fibers in the wall of porcine uterus. Int. J. Mol. Sci. 2018, 19, 2962. [Google Scholar] [CrossRef]
- Szymanska, K.; Calka, J.; Gonkowski, S. Nitric oxide as an active substance in the enteric neurons of the porcine digestive tract in physiological conditions and under intoxication with Bisphenol A (BPA). Nitric Oxide 2018, 80, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Santoro, A.; Chianese, R.; Troisi, J.; Richards, S.; Nori, S.L.; Fasano, S.; Guida, M.; Plunk, E.; Viggiano, A.; Pierantoni, R.; et al. Neuro-toxic and reproductive effects of BPA. Curr. Neuropharmacol. 2019, 17, 1109–1132. [Google Scholar] [CrossRef] [PubMed]
- Seachrist, D.D.; Bonk, K.W.; Ho, S.M.; Prins, G.S.; Soto, A.M.; Keri, R.A. A review of the carcinogenic potential of Bisphenol A. Reprod. Toxicol. 2016, 59, 167–182. [Google Scholar] [CrossRef] [PubMed]
- Braun, J.M. Early-life exposure to EDCs: Role in childhood obesity and neurodevelopment. Nat. Rev. Endocrinol. 2017, 13, 161–173. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Wang, H.; Zhou, L.; Fan, D.; Shi, L.; Ji, G.; Gu, A. Oxidative stress in Bisphenol AF-induced cardiotoxicity in zebrafish and the protective role of N-acetyl N-cysteine. Sci. Total Environ. 2020, 731, 139190. [Google Scholar] [CrossRef]
- Gear, R.; Kendziorski, J.A.; Belcher, S.M. Effects of Bisphenol A on incidence and severity of cardiac lesions in the NCTR-Sprague-Dawley rat: A CLARITY-BPA study. Toxicol. Lett. 2017, 275, 123–135. [Google Scholar] [CrossRef]
- Yan, S.; Chen, Y.; Dong, M.; Song, W.; Belcher, S.M.; Wang, H.S. Bisphenol A and 17β-estradiol promote arrhythmia in the female heart via alteration of calcium handling. PLoS ONE 2011, 6, e25455. [Google Scholar] [CrossRef]
- Ma, J.; Hong, K.; Wang, H.S. Progesterone protects against Bisphenol A-induced arrhythmias in female rat cardiac myocytes via rapid signaling. Endocrinology 2017, 158, 778–790. [Google Scholar] [CrossRef]
- Bruno, K.A.; Mathews, J.E.; Yang, A.L.; Frisancho, J.A.; Scott, A.J.; Greyner, H.D.; Molina, F.A.; Greenaway, M.S.; Cooper, G.M.; Bucek, A.; et al. BPA alters estrogen receptor expression in the heart after viral infection activating cardiac mast cells and t cells leading to perimyocarditis and fibrosis. Front. Endocrinol. (Lausanne) 2019, 10, 598. [Google Scholar] [CrossRef]
- Asahi, J.; Kamo, H.; Baba, R.; Doi, Y.; Yamashita, A.; Murakami, D.; Hanada, A.; Hirano, T. Bisphenol A induces endoplasmic reticulum stress-associated apoptosis in mouse non-parenchymal hepatocytes. Life Sci. 2010, 87, 431–438. [Google Scholar] [CrossRef]
- Figueiredo, L.S.; Oliveira, K.M.; Freitas, I.N.; Silva Jr, J.A.; Silva, J.N.; Favero-Santos, B.C.; Bonfleur, M.L.; Carneiro, E.M.; Ribeiro, R.A. Bisphenol-A exposure worsens hepatic steatosis in ovariectomized mice fed on a high-fat diet: Role of endoplasmic reticulum stress and fibrogenic pathways. Life Sci. 2020, 256, 118012. [Google Scholar] [CrossRef] [PubMed]
- Saura, M.; Marquez, S.; Reventun, P.; Olea-Herrero, N.; Arenas, M.I.; Moreno-Gómez-Toledano, R.; Gómez-Parrizas, M.; Muñóz-Moreno, C.; González-Santander, M.; Zaragoza, C.; et al. Oral administration of Bisphenol A induces high blood pressure through angiotensin II/CaMKII-dependent uncoupling of eNOS. FASEB J. 2014, 28, 4719–4728. [Google Scholar] [CrossRef] [PubMed]
- Rasdi, Z.; Kamaludin, R.; Rahim, S.A.; Fuad, S.B.S.A.; Othman, M.H.D.; Siran, R.; Mohd Nor, N.S.; Hasani, N.A.H.; Kadir, S.H.S.A. The impacts of intrauterine Bisphenol A exposure on pregnancy and expression of miRNAs related to heart development and diseases in animal model. Sci. Rep. 2020, 10, 5882. [Google Scholar] [CrossRef] [PubMed]
- Cai, S.; Rao, X.; Ye, J.; Ling, Y.; Mi, S.; Chen, H.; Fan, C.; Li, Y. Relationship between urinary Bisphenol A levels and cardiovascular diseases in the U.S. adult population, 2003–2014. Ecotoxicol. Environ. Saf. 2020, 192, 110300. [Google Scholar] [CrossRef] [PubMed]
- Melzer, D.; Osborne, N.J.; Henley, W.E.; Cipelli, R.; Young, A.; Money, C.; McCormack, P.; Luben, R.; Khaw, K.T.; Wareham, N.J.; et al. Urinary Bisphenol A concentration and risk of future coronary artery disease in apparently healthy men and women. Circulation 2012, 125, 1482–1490. [Google Scholar] [CrossRef]
- Zhang, Y.F.; Shan, C.; Wang, Y.; Qian, L.L.; Jia, D.D.; Zhang, Y.F.; Hao, X.D.; Xu, H.M. Cardiovascular toxicity and mechanism of Bisphenol A and emerging risk of Bisphenol S. Sci. Total Environ. 2020, 723, 137952. [Google Scholar] [CrossRef]
- Murata, M.; Kang, J.H. Bisphenol A (BPA) and cell signaling pathways. Biotechnol. Adv. 2018, 36, 311–327. [Google Scholar] [CrossRef]
- Argunhan, F.; Brain, S.D. The vascular-dependent and -independent actions of calcitonin gene-related peptide in cardiovascular disease. Front. Physiol. 2022, 13, 833645. [Google Scholar] [CrossRef]
- Costa, E.D.; Rezende, B.A.; Cortes, S.F.; Lemos, V.S. Neuronal nitric oxide synthase in vascular physiology and diseases. Front. Physiol. 2016, 7, 206. [Google Scholar] [CrossRef]
- Timotin, A.; Pisarenko, O.; Sidorova, M.; Studneva, I.; Shulzhenko, V.; Palkeeva, M. Myocardial protection from ischemia/reperfusion injury by exogenous galanin fragment. Oncotarget 2017, 8, 21241–21252. [Google Scholar] [CrossRef]
- Serebryakova, L.; Pal’keeva, M.; Studneva, I.; Molokoedov, A.; Veselova, O.; Ovchinnikov, M. Galanin and its N-terminal fragments reduce acute myocardial infarction in rats. Peptides 2019, 111, 127–131. [Google Scholar] [CrossRef] [PubMed]
- Dvoráková, M.C. Cardioprotective role of the VIP signaling system. Drug News Perspect. 2005, 18, 387–391. [Google Scholar] [CrossRef] [PubMed]
- Iliff, J.J.; Alkayed, N.J.; Gloshani, K.J.; Traystman, R.J.; West, G.A. Cocaine- and amphetamine-regulated transcript (CART) peptide: A vasoactive role in the cerebral circulation. J. Cereb. Blood Flow Metab. 2005, 25, 1376–1385. [Google Scholar] [CrossRef]
- Rytel, L.; Całka, J. Aspirin administration affects neurochemical characterization of substance p-like immunoreactive (sp-li) nodose ganglia neurons supplying the porcine stomach. Biomed. Res. Int. 2020, 2020, 1049179. [Google Scholar] [CrossRef]
- Conrad, M.S.; Johnson, R.W. The domestic piglet: An important model for investigating the neurodevelopmental consequences of early life insults. Annu. Rev. Anim. Biosci. 2015, 3, 245–264. [Google Scholar] [CrossRef]
- Lelovas, P.P.; Kostomitsopoulos, N.G.; Xanthos, T.T. A comparative anatomic and physiologic overview of the porcine heart. J. Am. Assoc. Lab. Anim. Sci. 2014, 53, 432–438. [Google Scholar] [PubMed]
- Cullen, J.M.; Lu, G.; Shannon, A.H.; Su, G.; Sharma, A.; Salmon, M.; Fashandi, A.Z.; Spinosa, M.D.; Montgomery, W.G.; Johnston, W.F.; et al. A novel swine model of abdominal aortic aneurysm. J. Vasc. Surg. 2019, 70, 252–260. [Google Scholar] [CrossRef] [PubMed]
- Fuxe, K.; Sedva, G. The distribution of adrenergic nerve fibres to the blood vessels in skeletal muscle. Acta Physiol. Scand. 1965, 64, 75–86. [Google Scholar] [CrossRef]
- Krzastek, S.C.; Farhi, J.; Gray, M.; Smith, R.P. Impact of environmental toxin exposure on male fertility potential. Transl. Androl. Urol. 2020, 9, 2797–2813. [Google Scholar] [CrossRef]
- Jiang, Y.; Li, J.; Xu, S.; Zhou, Y.; Zhao, H.; Li, Y.; Xiong, C.; Sun, X.; Liu, H.; Liu, W.; et al. Prenatal exposure to Bisphenol A and its alternatives and child neurodevelopment at 2 years. J. Hazard. Mater. 2020, 388, 121774. [Google Scholar] [CrossRef]
- Di Paola, D.; Capparucci, F.; Lanteri, G.; Cordaro, M.; Crupi, R.; Siracusa, R.; D'Amico, R.; Fusco, R.; Impellizzeri, D.; Cuzzocrea, S.; et al. Combined toxicity of xenobiotics Bisphenol A and heavy metals on zebrafish embryos (Danio rerio). Toxics 2021, 9, 9–344. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, K.; Gagné, M.; Nong, A.; Aylward, L.L.; Hays, S.M. Biomonitoring equivalents for Bisphenol A (BPA). Regul. Toxicol. Pharmacol. 2010, 58, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Hengstler, J.G.; Foth, H.; Gebel, T.; Kramer, P.J.; Lilienblum, W.; Schweinfurth, H.; Völkel, W.; Wollin, K.M.; Gundert-Remy, U. Critical evaluation of key evidence on the human health hazards of exposure to Bisphenol A. Crit. Rev. Toxicol. 2011, 41, 263–291. [Google Scholar] [CrossRef] [PubMed]
- Santovito, A.; Cannarsa, E.; Schleicherova, D.; Cervella, P. Clastogenic effects of Bisphenol A on human cultured lymphocytes. Hum. Exp. Toxicol. 2018, 37, 69–77. [Google Scholar] [CrossRef]
- Rytel, L.; Gonkowski, S.; Janowski, T.; Wojtkiewicz, J.; Pomianowski, A. The neurochemical characterization of parasympathetic nerve fibers in the porcine uterine wall under physiological conditions and after exposure to Bisphenol A (BPA). Neurotox. Res. 2019, 35, 867–882. [Google Scholar] [CrossRef]
- González, N.; Marquès, M.; Cunha, S.C.; Fernandes, J.O.; Domingo, J.L.; Nadal, M. Biomonitoring of co-exposure to bisphenols by consumers of canned foodstuffs. Environ. Int. 2020, 140, 105760. [Google Scholar] [CrossRef]
- Fleisch, A.F.; Sheffield, P.E.; Chinn, C.; Edelstein, B.L.; Landrigan, P.J. Bisphenol A and related compounds in dental materials. Pediatrics 2010, 126, 760–768. [Google Scholar] [CrossRef]
- Smedh, U.; Scott, K.A.; Moran, T.H. Fourth ventricular CART peptide induces c-fos in the area postrema and nucleus of the solitary tract via a CRF-receptor dependent mechanism. Neurosci. Lett. 2015, 609, 124–128. [Google Scholar] [CrossRef]
- Matsumura, K.; Tsuchihashi, T.; Abe, I. Central human cocaine- and amphetamine-regulated transcript peptide 55-102 increases arterial pressure in conscious rabbits. Hypertension 2001, 38, 1096–1100. [Google Scholar] [CrossRef]
- Han, C.; Hong, Y.C. Bisphenol A, hypertension, and cardiovascular diseases: Epidemiological, laboratory, and clinical trial evidence. Curr. Hypertens. Rep. 2016, 18, 11. [Google Scholar] [CrossRef]
- Bharne, A.P.; Upadhya, M.A.; Shelkar, G.P.; Singru, P.S.; Subhedar, N.K.; Kokare, D.M. Neuroprotective effect of cocaine- and amphetamine-regulated transcript peptide in spinal cord injury in mice. Neuropharmacology 2013, 67, 126–135. [Google Scholar] [CrossRef] [PubMed]
- Mao, P.; Ardeshiri, A.; Jacks, R.; Yang, S.; Hurn, P.D.; Alkayed, N.J. Mitochondrial mechanism of neuroprotection by CART. Eur. J. Neurosci. 2007, 26, 624–632. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.; Chen, Y.; Li, J.; Liu, Z.; Wang, Z.; Chen, J.; Cao, W.; Xu, Y. Cocaine-and amphetamine-regulated transcript modulates peripheral immunity and protects against brain injury in experimental stroke. Brain Behav. Immun. 2011, 25, 260–269. [Google Scholar] [CrossRef] [PubMed]
- Gonkowski, S. Bisphenol A (BPA)-induced changes in the number of serotonin-positive cells in the mucosal layer of porcine small intestine-the preliminary studies. Int. J. Mol. Sci. 2020, 21, 1079. [Google Scholar] [CrossRef]
- Meli, R.; Monnolo, A.; Annunziata, C.; Pirozzi, C.; Ferrante, M.C. Oxidative stress and BPA toxicity: An antioxidant approach for male and female reproductive dysfunction. Antioxidants 2020, 9, 405. [Google Scholar] [CrossRef]
- Guo, Y.; Zhang, Q.; Chen, H.; Jiang, Y.; Gong, P. The protective role of calcitonin gene-related peptide (CGRP) in high-glucose-induced oxidative injury in rat aorta endothelial cells. Peptides 2019, 121, 170121. [Google Scholar] [CrossRef]
- Abushik, P.A.; Bart, G.; Korhonen, P.; Leinonen, H.; Giniatullina, R.; Sibarov, D.A.; Levonen, A.L.; Malm, T.; Antonov, S.M.; Giniatullin, R. Pro-nociceptive migraine mediator CGRP provides neuroprotection of sensory, cortical and cerebellar neurons via multi-kinase signaling. Cephalalgia 2017, 37, 1373–1383. [Google Scholar] [CrossRef]
- Bowen, E.J.; Schmidt, T.W.; Firm, C.S.; Russo, A.F.; Durham, P.L. Tumor necrosis factor-alpha stimulation of calcitonin gene-related peptide expression and secretion from rat trigeminal ganglion neurons. J. Neurochem. 2006, 96, 65–77. [Google Scholar] [CrossRef]
- Wisskirchen, F.M.; Gray, D.W.; Marshall, I. Receptors mediating CGRP-induced relaxation in the rat isolated thoracic aorta and porcine isolated coronary artery differentiated by h(alpha) CGRP(8-37). Br. J. Pharmacol. 1999, 128, 283–292. [Google Scholar] [CrossRef]
- Iwatani, Y.; Kosugi, K.; Isobe-Oku, S.; Atagi, S.; Kitamura, Y.; Kawasaki, H. Endothelium removal augments endothelium-independent vasodilatation in rat mesenteric vascular bed. Br. J. Pharmacol. 2008, 154, 32–40. [Google Scholar] [CrossRef]
- Feiteiro, J.; Mariana, M.; Glória, S.; Cairrao, E. Inhibition of L-type calcium channels by Bisphenol A in rat aorta smooth muscle. J. Toxicol. Sci. 2018, 43, 579–586. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Li, M.; Wu, C.; Zhou, C.; Zhang, J.; Zhu, Q.; Shen, T. Bisphenol A promotes macrophage proinflammatory subtype polarization via upregulation of IRF5 expression in vitro. Toxicol. Vitr. 2019, 60, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Gracia-Sancho, J.; Maeso-Díaz, R.; Fernández-Iglesias, A.; Navarro-Zornoza, M.; Bosch, J. New cellular and molecular targets for the treatment of portal hypertension. Hepatol. Int. 2015, 9, 183–191. [Google Scholar] [CrossRef]
- Jimenez-Andrade, J.M.; Mantyh, W.G.; Bloom, A.P.; Freeman, K.T.; Ghilardi, J.R.; Kuskowski, M.A.; Mantyh, P.W. The effect of aging on the density of the sensory nerve fiber innervation of bone and acute skeletal pain. Neurobiol. Aging. 2012, 33, 921–932. [Google Scholar] [CrossRef][Green Version]
- Ferrari, A.U.; Radaelli, A.; Mori, T.; Mircoli, L.; Perlini, S.; Meregalli, P.; Fedele, L.; Mancia, G. Nitric oxide-dependent vasodilation and the regulation of arterial blood pressure. J. Cardiovasc. Pharmacol. 2001, 38, S19–S22. [Google Scholar] [CrossRef]
- Lee, S.H.; Ok, S.H.; Kang, D.; Kim, H.J.; Ahn, S.H.; Bae, S.I.; Kim, J.Y.; Kim, E.J.; Kim, S.; Hwag, Y.; et al. Nitric oxide-dependent vasodilation induced by minoxidil in isolated rat aorta. Gen. Physiol. Biophys. 2021, 40, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, V.; Mancuso, C.; Calvani, M.; Rizzarelli, E.; Butterfield, D.A.; Stella, A.M. Nitric oxide in the central nervous system: Neuroprotection versus neurotoxicity. Nat. Rev. Neurosci. 2007, 8, 766–775. [Google Scholar] [CrossRef] [PubMed]
- Kajimoto, M.; Nuri, M.; Sleasman, J.R.; Charette, K.A.; Nelson, B.R.; Portman, M.A. Inhaled nitric oxide reduces injury and microglia activation in porcine hippocampus after deep hypothermic circulatory arrest. J. Thorac. Cardiovasc. Surg. 2021, 161, e485–e498. [Google Scholar] [CrossRef]
- Filpa, V.; Carpanese, E.; Marchet, S.; Pirrone, C.; Conti, A.; Rainero, A.; Moro, E.; Chiaravalli, A.M.; Zucchi, I.; Moriondo, A.; et al. Nitric oxide regulates homeoprotein OTX1 and OTX2 expression in the rat myenteric plexus after intestinal ischemia-reperfusion injury. Am. J. Physiol. Gastrointest. Liver Physiol. 2017, 312, G374–G389. [Google Scholar] [CrossRef]
- Hocher, B.; Schwarz, A.; Slowinski, T.; Bachmann, S.; Pfeilschifter, J.; Neumayer, H.H.; Bauer, C. In-vitro interation of nitric oxide and endothelin. J. Hypertens. 2004, 22, 111–119. [Google Scholar] [CrossRef]
- Sharma, N.; Al-Omran, A.; Parvathy, S.S. Role of nitric oxide in inflammatory diseases. Inflammopharmacology 2017, 15, 252–259. [Google Scholar] [CrossRef] [PubMed]
- Fahrenkrug, J. VIP and PACAP. Results Probl. Cell Differ. 2010, 50, 221–234. [Google Scholar] [CrossRef] [PubMed]
- Chan, S.L.; Fiscus, R.R. Vasorelaxations induced by calcitonin gene-related peptide, vasoactive intestinal peptide, and acetylcholine in aortic rings of endothelial and inducible nitric oxide synthase-knockout mice. J. Cardiovasc. Pharmacol. 2003, 41, 434–443. [Google Scholar] [CrossRef] [PubMed]
- Syed, A.U.; Koide, M.; Braas, K.M.; May, V.; Wellman, G.C. Pituitary adenylate cyclase-activating polypeptide (PACAP) potently dilates middle meningeal arteries: Implications for migraine. J. Mol. Neurosci. 2012, 48, 574–583. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Szabadfi, K.; Danyadi, B.; Kiss, P.; Tamas, A.; Fabian, E.; Gabriel, R.; Reglodi, D. Protective effects of vasoactive intestinal peptide (VIP) in ischemic retinal degeneration. J. Mol. Neurosci. 2012, 48, 501–507. [Google Scholar] [CrossRef]
- Gomariz, R.P.; Juarranz, Y.; Abad, C.; Arranz, A.; Leceta, J.; Martinez, C. VIP-PACAP system in immunity: New insights for multitarget therapy. Ann. N. Y. Acad. Sci. 2006, 1070, 51–74. [Google Scholar] [CrossRef]
- Long, J.B.; Rigamonti, D.D.; Dosaka, K.; Kraimer, J.M.; Martinez-Arizala, A. Somatostatin causes vasoconstriction, reduces blood flow and increases vascular permeability in the rat central nervous system. J. Pharmacol. Exp. Ther. 1992, 260, 1425–1432. [Google Scholar]
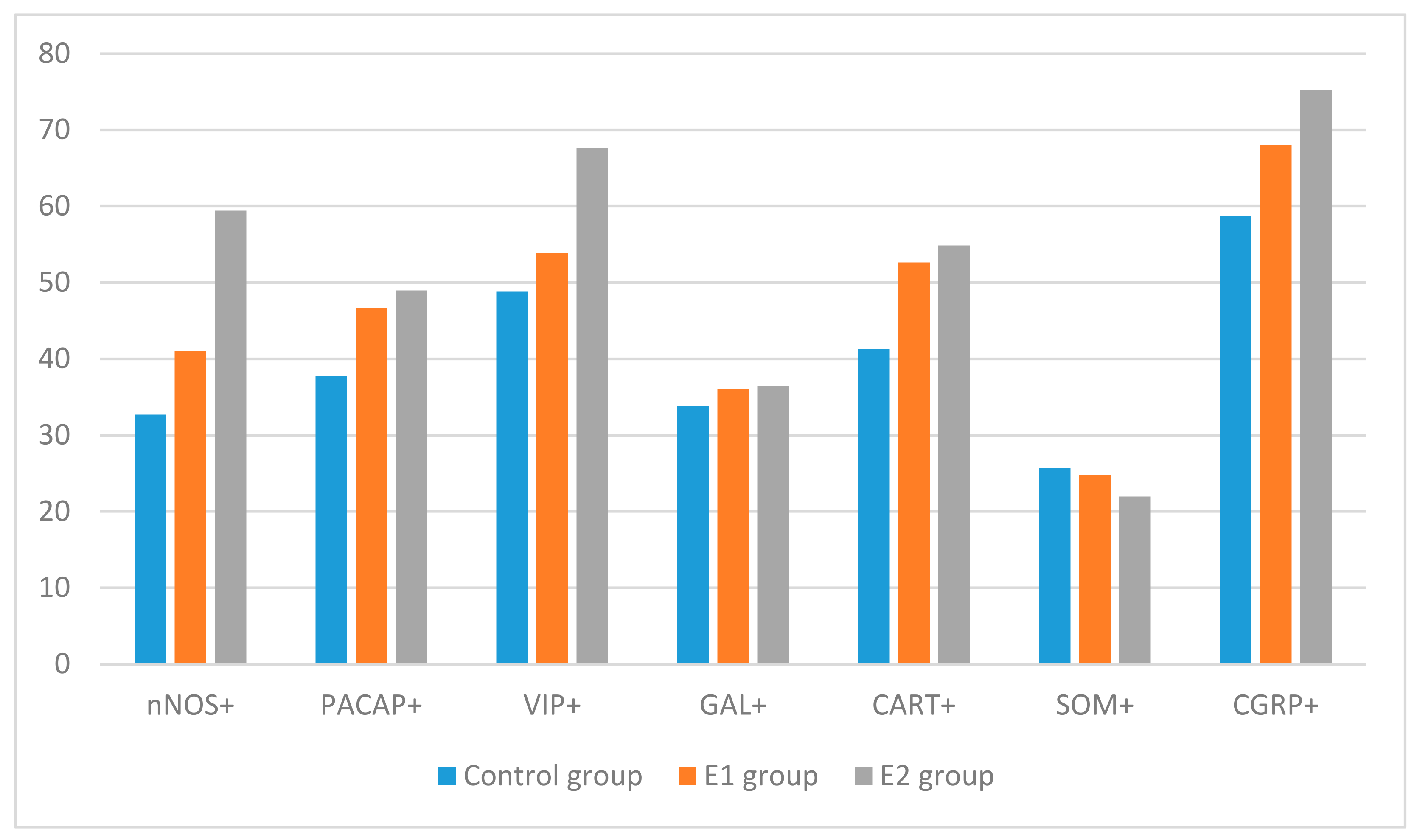


| Antigen | Species of Origin | Code | Dilution | Supplier |
|---|---|---|---|---|
| PRIMARY ANTIBODIES | ||||
| PGP 9.5 | Mouse | ab72911 | 1:1000 | Abcam |
| GAL | Rabbit | AB2233 | 1:2000 | Milipore |
| nNOS | Rabbit | AB5380 | 1:4000 | Chemicon |
| VIP | Rabbit | VA 1285 | 1:4000 | Biogene |
| PACAP | Rabbit | ab216627 | 1:4000 | Abcam |
| CGRP | Rabbit | AB5920 | 1:4000 | AbDserotec |
| CART | Rabbit | HPA046278 | 1:2000 | Merck |
| SOM | Rabbit | ab111912 | 1:2000 | Abcam |
| SECONDARY ANTIBODIES | ||||
| Alexa Fluor 546 | Donkey Anti-Rabbit | A10040 | 1:1000 | Invitrogen |
| Alexa Fluor 488 | Donkey Anti-Mouse | A21202 | 1:1000 | Invitrogen |
| Control Group | |||||||
|---|---|---|---|---|---|---|---|
| Number of Animals | PGP 9.5+/ GAL+ | PGP 9.5+/ SOM+ | PGP 9.5+/ VIP+ | PGP 9.5+/ nNOS+ | PGP 9.5+/ PACAP+ | PGP 9.5+/ CGRP+ | PGP 9.5+/ CART+ |
| 1 | 35.03 | 25.18 | 45.08 | 39.05 | 47.96 | 64.99 | 46.17 |
| 2 | 37.38 | 27.56 | 52.82 | 36.73 | 30.08 | 54.58 | 40.07 |
| 3 | 38.56 | 23.08 | 52.17 | 32.17 | 32.95 | 54.44 | 42.96 |
| 4 | 27.82 | 28.32 | 52.48 | 27.19 | 39.44 | 60.76 | 38.75 |
| 5 | 29.99 | 24.68 | 41.38 | 28.24 | 38.05 | 58.51 | 38.54 |
| Minimum | 27.82 | 23.08 | 41.38 | 27.19 | 30.08 | 54.44 | 38.54 |
| Maximum | 38.56 | 28.32 | 52.82 | 39.05 | 47.96 | 64.99 | 46.17 |
| Mean | 33.76 | 25.76 | 48.79 | 32.68 | 37.70 | 58.66 | 41.30 |
| SEM | 2.09 | 0.96 | 2.34 | 2.31 | 3.07 | 1.99 | 1.45 |
| E1 Group | |||||||
|---|---|---|---|---|---|---|---|
| Number of Animals | PGP 9.5+/ GAL+ | PGP 9.5+/ SOM+ | PGP 9.5+/ VIP+ | PGP 9.5+/ nNOS+ | PGP 9.5+/ PACAP+ | PGP 9.5+/ CGRP+ | PGP 9.5+/ CART+ |
| 1 | 36.99 | 24.25 | 52.97 | 44.73 | 43.48 | 75.27 | 47.81 |
| 2 | 29.92 | 19.87 | 52.98 | 42.93 | 38.09 | 69.94 | 59.52 |
| 3 | 41.63 | 21.67 | 60.63 | 29.24 | 51.25 | 67.94 | 51.00 |
| 4 | 38.38 | 28.50 | 47.41 | 39.22 | 43.17 | 60.02 | 48.59 |
| 5 | 33.59 | 29.66 | 55.31 | 48.85 | 56.99 | 67.14 | 56.34 |
| Minimum | 29.92 | 19.87 | 47.41 | 29.24 | 38.09 | 60.02 | 47.81 |
| Maximum | 41.63 | 29.66 | 60.63 | 48.85 | 56.99 | 75.27 | 59.52 |
| Mean | 36.10 | 24.79 | 53.86 | 40.99 | 46.60 | 68.07 | 52.65 |
| SEM | 2.01 | 1.89 | 2.13 | 3.32 | 3.34 | 2.46 | 2.27 |
| E2 Group | |||||||
|---|---|---|---|---|---|---|---|
| Number of Animals | PGP 9.5+/ GAL+ | PGP 9.5+/ SOM+ | PGP 9.5+/ VIP+ | PGP 9.5+/ nNOS+ | PGP 9.5+/ PACAP+ | PGP 9.5+/ CGRP+ | PGP 9.5+/ CART+ |
| 1 | 39.78 | 26.50 | 64.95 | 65.03 | 43.30 | 77.31 | 54.13 |
| 2 | 29.91 | 22.32 | 77.93 | 58.97 | 56.51 | 78.47 | 62.39 |
| 3 | 37.98 | 16.17 | 59.53 | 67.24 | 50.76 | 78.99 | 57.48 |
| 4 | 36.22 | 23.84 | 67.94 | 51.87 | 46.17 | 70.62 | 46.71 |
| 5 | 38.02 | 21.04 | 67.46 | 54.01 | 48.10 | 70.80 | 53.58 |
| Minimum | 29.91 | 16.17 | 59.53 | 51.87 | 43.30 | 70.62 | 46.71 |
| Maximum | 39.78 | 26.50 | 77.93 | 67.24 | 56.51 | 78.99 | 62.39 |
| Mean | 36.38 | 21.97 | 67.66 | 59.42 | 48.97 | 75.24 | 54.86 |
| SEM | 1.71 | 1.71 | 2.99 | 2.99 | 2.25 | 1.87 | 2.57 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rytel, L.; Könyves, L.; Gonkowski, S. Endocrine Disruptor Bisphenol a Affects the Neurochemical Profile of Nerve Fibers in the Aortic Arch Wall in the Domestic Pig. Int. J. Environ. Res. Public Health 2022, 19, 5964. https://doi.org/10.3390/ijerph19105964
Rytel L, Könyves L, Gonkowski S. Endocrine Disruptor Bisphenol a Affects the Neurochemical Profile of Nerve Fibers in the Aortic Arch Wall in the Domestic Pig. International Journal of Environmental Research and Public Health. 2022; 19(10):5964. https://doi.org/10.3390/ijerph19105964
Chicago/Turabian StyleRytel, Liliana, László Könyves, and Slawomir Gonkowski. 2022. "Endocrine Disruptor Bisphenol a Affects the Neurochemical Profile of Nerve Fibers in the Aortic Arch Wall in the Domestic Pig" International Journal of Environmental Research and Public Health 19, no. 10: 5964. https://doi.org/10.3390/ijerph19105964
APA StyleRytel, L., Könyves, L., & Gonkowski, S. (2022). Endocrine Disruptor Bisphenol a Affects the Neurochemical Profile of Nerve Fibers in the Aortic Arch Wall in the Domestic Pig. International Journal of Environmental Research and Public Health, 19(10), 5964. https://doi.org/10.3390/ijerph19105964






