1. Introduction
To keep a pandemic like the one caused by COVID-19 [
1] under control, resources such as personal protection equipment (PPE; i.e., face masks, gloves, and disinfectants) are critical both for the population and for health care personnel [
2]. Unfortunately, the demand for PPE increases exponentially with the spread of a new epidemic. As the available resource stock is tailored to the population’s standard use, this poses serious challenges to the associated resource supply chains. Consequently, several countries with high infection rates reached a state of low to no resource availability during the various waves of the COVID-19 pandemic, irrespective of their gross domestic products (GDP) [
3,
4].
Due to an exponential increase in demand during epidemic waves, local production increases are rarely fast enough to cover the high need [
5]. Moreover, local production is not the primary provider in many countries that obtain PPE from abroad. In some places, preventive methods were proposed to mitigate the upcoming shortages [
6], such as setting guidelines for rational and safe prolonged PPE use or reuse [
7] and alternative solutions for protection [
8]. Such mitigation strategies may provide temporary relief but do not address the bigger scalability problem. Moreover, some of these strategies lead to healthcare workers feeling inadequately protected and being fearful of getting infected [
9].
The primary production of medical products comes from global supply chains (GSC). GSCs have been optimized for cost-efficiency and may therefore lack resilience, such as redundant supply lines or large buffers along the chains. For this reason, a major topic of debate is if and how these GSCs should be restructured or replaced [
7,
10,
11]. An example of GSC fragility is the disruption of several technological- and health-product factories in Wuhan during the COVID-19 pandemic and the closing of shipping ports, impacting the international market and availability of these products on a very short time scale [
12,
13].
Consequently, due to the resource scarcity threat in the first wave of the COVID-19 pandemic, multiple countries started to stock, confiscate, and prohibit the exportation of PPE [
14,
15] and diagnostic testing materials. This affected the global market as a whole since no country is fully self-sufficient in all the different types of PPE. Furthermore, it greatly impacted low-income countries that had to deal with increased prices and a scarcity of goods [
14,
15,
16,
17]. Although a plausible solution would be to redesign the GSCs for increased systemic resilience against rare incidents, such as pandemics, the restructuring of infrastructure may very well pose a prohibitive cost. Additionally, it would require a globally concerted effort at an unprecedented scale.
Instead, this paper investigates the question of whether implementing a resource-sharing policy among different regions or countries can enhance essential-resource stock resilience. A PPE-sharing policy differs from increasing the overall resilience of GSCs: instead of ensuring the transport lines from producers to each individual consumer region, a sharing policy entails creating flexible transport lines between the consumer regions. For this reason, it may provide a more feasible approach that is effective at suppressing future pandemics. The current research field into epidemic spreading and COVID-19 lacks studies on sharing strategies for sharing this type of resource. However, the effects of vaccine sharing between two populations have already been studied using a classical multi-compartmental susceptible–infected–recovered (SIR)-based model [
18], concluding that overall health outcomes can greatly benefit from cooperative sharing [
19]. Although both vaccine and PPE availability are important factors in successfully mitigating a pandemic, they are, respectively, aimed at pre-epidemic immunization increase and within-epidemic disease containment. It is therefore important to study the effects of PPE sharing separately. A convincing point to share resources used during epidemics was made using a stochastic model considering ventilator allocation [
20]. However, the explicit disease transmission dynamics within populations could not be taken into account by this approach. Because so far, concrete studies of PPE-resource-sharing effects on these transmission dynamics are thus absent, we want to create an SIR-inspired multi-compartmental model to study this specifically.
In this article we will first present the stylized model we created to investigate cooperative resource sharing, followed by a simulation of sharing scenarios between different GDP-sized community combinations. In brief, the model concerns two communities that can be interpreted as countries, regions, or even cities. We modeled their local epidemics and the spread of infections across the communities, in combination with their (unspecified) PPE resources. PPE availability lowers the infection rate by offering protection and therefore enables suppressing the infection spread to some degree. Of interest are the effects of simulating changes in resource-sharing behavior on the capacity of going through the entire epidemic without fully depleting the PPE stock.
The specific contribution of this study is thus not the novelty of our modeling techniques, but presenting an important message to a broad public by using a stylized model. Motivated by the non-cooperative strategies observed at present, it has the purpose to understand at a high level the impact of sharing essential resources and which factors should be taken into account to reach a better local situation for all communities involved.
2. Methods
Here follows a functional description of the model needed to understand the results. Readers interested in more technical details are referred to the Extended Methods in
Appendix A. Here,
Table A1 can also be found with an overview and justification of the assumed model parameters.
2.1. Coupled SIR and DSP Model
We developed a simplified demand–stock–production (DSP) model to emulate the usage and production of PPE. PPE can be interpreted here as any physical equipment that may help limit disease transmission. To cover the disease dynamics, including the transmission-mitigating effects of PPE, the DSP model is coupled to a classical susceptible–infected–recovered (SIR) model [
21,
22] without births and deaths. As we model the occurrence of one epidemic wave per community at most, we assume a total simulation duration short enough to justify this simplification. N.B.: throughout this paper the letter
S will be used to refer to the PPE ‘stock’ from the DSP model, as susceptibles (from the SIR model) are never referred to outside of the appendix.
For the inter-model coupling, the following two assumptions are used: (1) more protective equipment is needed when the amount of infected increases [
3,
9]; and (2) the unavailability of PPE increases the infection rate of the disease [
4,
5]. This leads to the following two connecting factors: (1) a community’s current PPE demand in the DSP chain depends directly on their number of infected and an assumed constant of necessary PPE per infected case; and (2) the effective reproduction number
of the SIR model depends on the available PPE stock. Concretely, this means that in absence of PPE,
is set to the standard reproduction number
of the virus. In presence of PPE, the
is reduced to a lower value with
. In the appendix, explicit calculations and justifications of the assumed constant values are found. An important assumption made in this model implementation is thus that the
only reduces to
upon full PPE stock depletion, a point we consider further in the discussion.
In all experiments, two communities A and B of n individuals each are simulated. They differ only in maximum stock () and epidemic onset time. The results are described from the point of view of community A. Therefore, we assume epidemic onset in A to always be at , with the introduction of one infected individual into this community. The epidemic onset in B is at a certain time difference from , thus at . This time difference models the time it would take for the epidemic to spread between the two communities directly, or via another (‘confounding’) community. The difference can be days for simultaneous onset and take on both positive and negative values. In this way, both an epidemic starting in A followed by a spread to B and vice versa can be investigated. This enables us to study the effects of changes in the time difference of the epidemic onset between A and B and in their relative order. The differences in maximum stock reflect the different capacities to directly access the necessary PPE, due to, e.g., differences in GDP and storage capacity. By using different maximum stock levels we can investigate how possible asymmetries between communities might impact sharing behavior outcomes.
2.2. Sharing System
We further implemented a PPE-sharing system between the two communities to simulate sharing behavior. Sharing is managed via a switch mechanism that depends on the ratio between the current and maximum stock of each community and a sharing threshold . The sharing threshold is a critical stock ratio agreed upon beforehand by the communities. We assume the communities want to ensure always having at least this proportion of stock available to themselves, to increase their chances of weathering a local epidemic without reaching stock depletion (). If during an epidemic the stock of a community falls below the sharing threshold , they request support from the other community. The switch mechanism activates the sharing system if the other community has the possibility to share, i.e., if their own stock ratio is above the sharing threshold. If the second community starts sharing, they are assumed to continue until reaching the sharing threshold themselves or until the other community’s stock is restored to at least again. In summary, the switch mechanism leads to active PPE sharing if and only if exactly one of the communities’ stock levels is below the sharing threshold and the other community has a stock level above the threshold. A low sharing threshold corresponds to communities being willing to share a large fraction of their stock with one another, while a high indicates that they intend to keep a large fraction of stock for themselves.
3. Results
The effects of differences in the relative time difference between communities’ epidemic onset, maximum stock height, and sharing threshold were assessed in different scenarios that are outlined below. We will first discuss the case where the collaborating communities have equal maximum stocks and then scenarios where these are unequal.
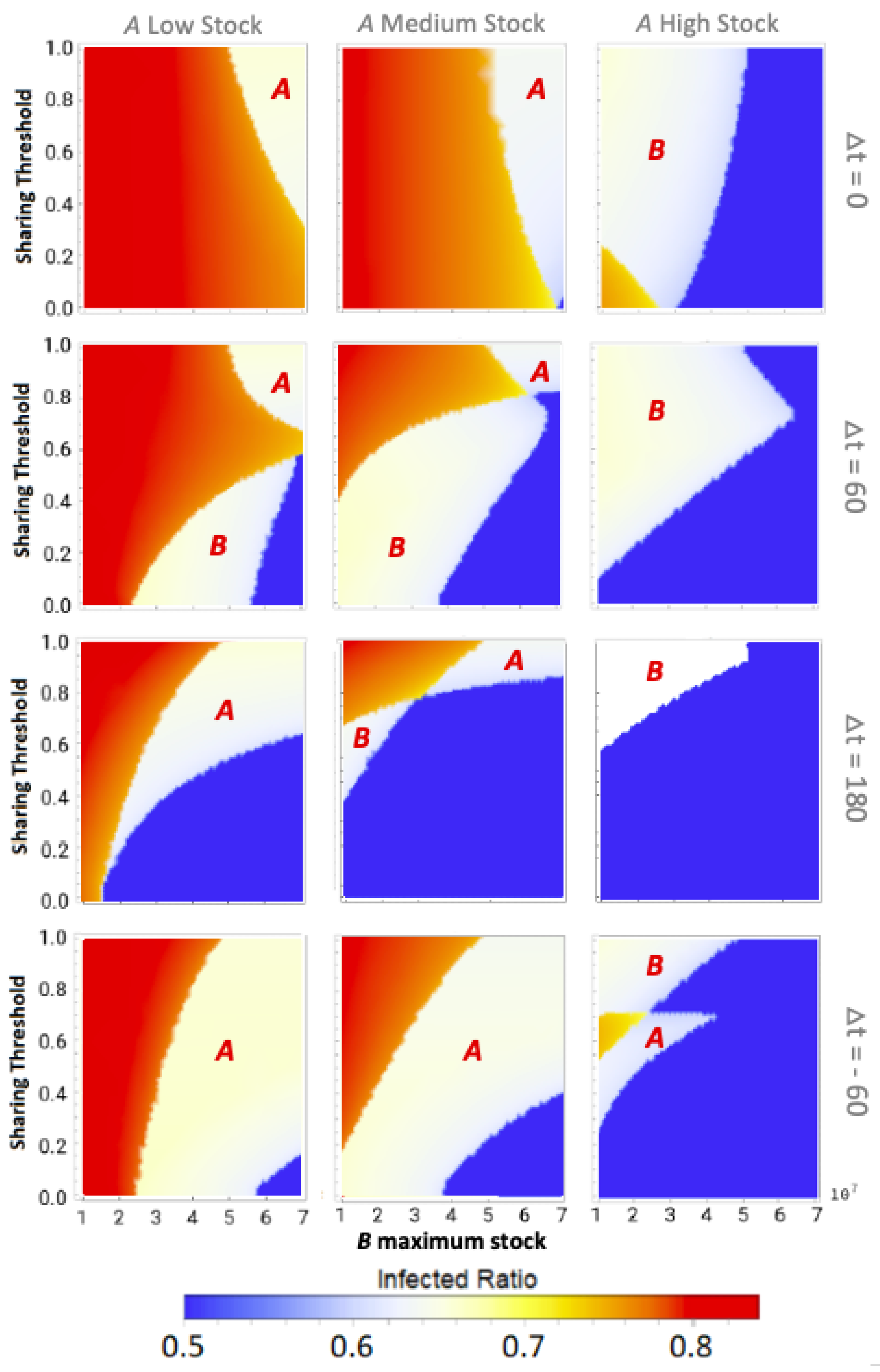
Our main results are shown in the form of phase plots with a distinctive color scheme. We discriminate between three qualitative outcomes in the phase plots, shown in red, white, and blue, in decreasing order of severity. In all phase plots, the total infection incidence is given as the ratio of individuals within the populations that has been infected during the disease spread: the infected ratio. If the stocks of both communities would deplete fully at any point during the epidemic, then their effective infection reproduction numbers would increase enormously. This would lead to the highest possible total infected ratio, corresponding to the red state. If only one community would reach zero stock then this would lead to an intermediate total infected ratio, corresponding to the white intermediate state. Finally, if both communities could weather the epidemic without reaching zero stock, the blue state would be reached with the lowest possible infected ratio. Intermediate transition phases, i.e., those visible in orange, could appear due to small differences in the total infected ratio, for example due to slight differences in the zero-stock-period duration.
3.1. Equal Maximum Stocks
We can observe that sharing between certain equal maximum stock communities can prevent stock depletion and the associated high infected ratio.
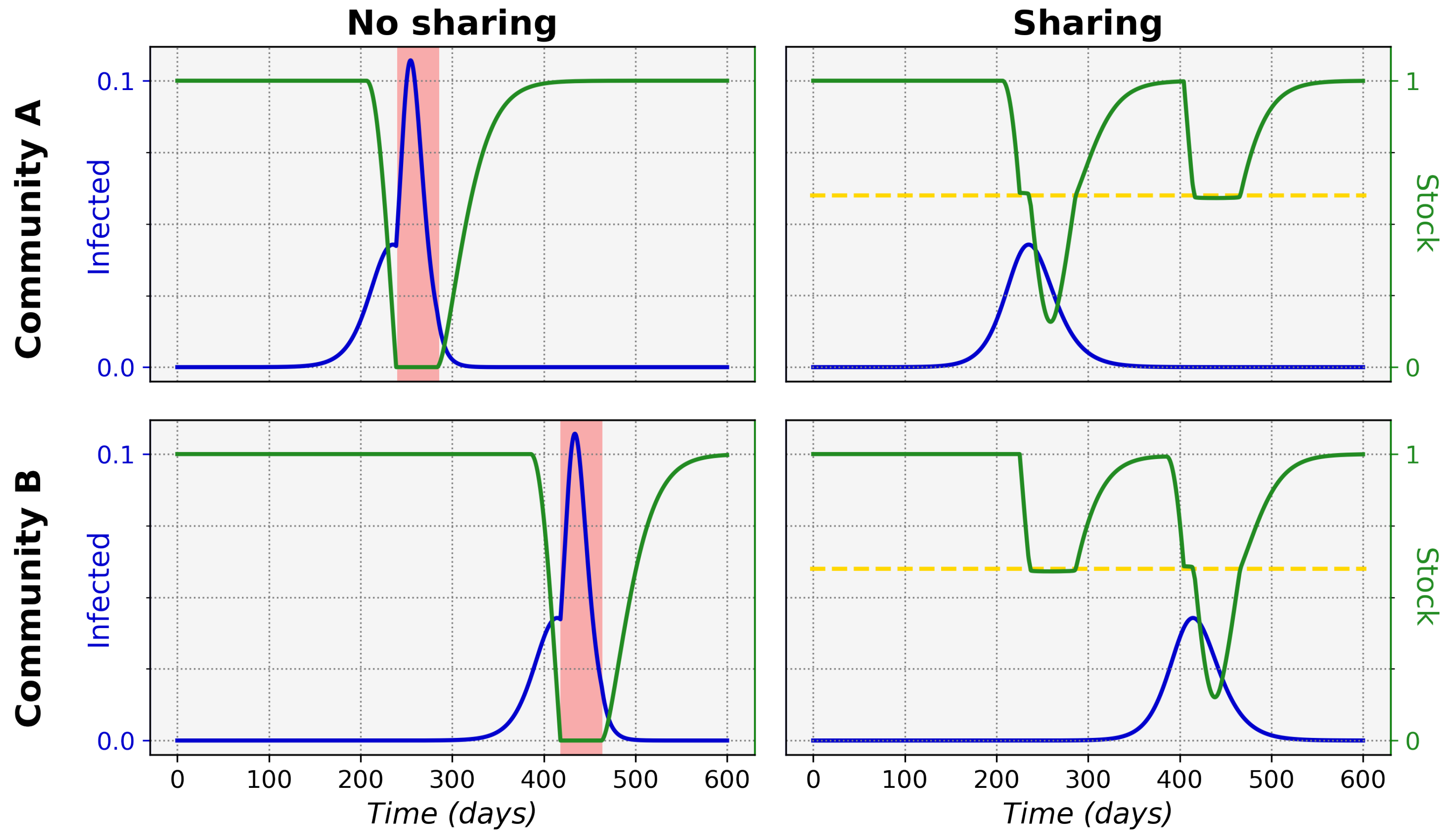
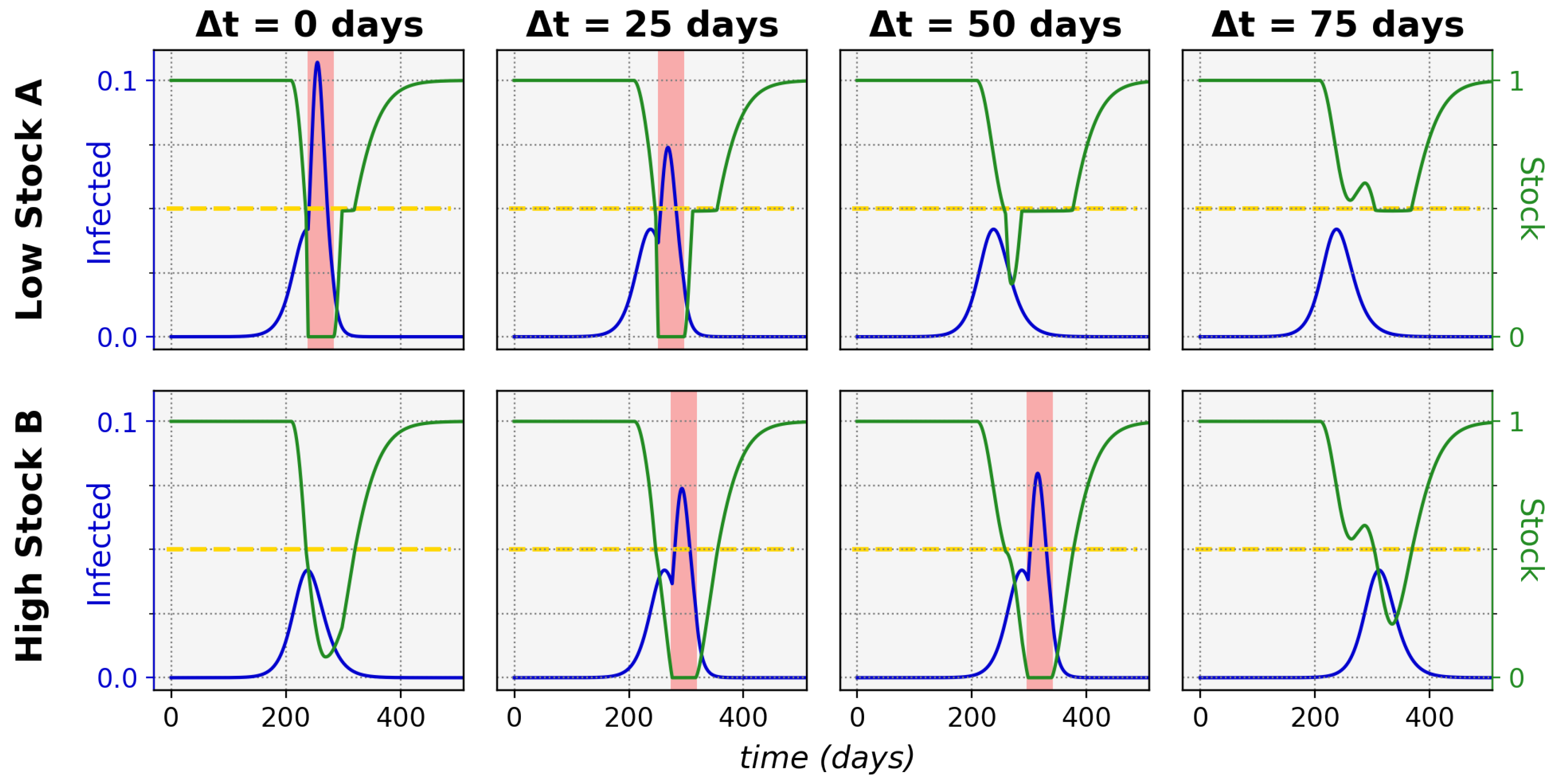
Figure 1 shows typical epidemic time evolutions for two equal maximum stock communities and the difference between non-sharing and sharing. This clearly depicts how non-sharing (left column) for these communities leads to stock depletion. During a stock depletion period (indicated by the red shading)
becomes larger and therefore leads to stronger exponential growth and a high peak of infections. In the sharing condition (right column) we can see that stock sharing with the community in need can prevent full stock depletion and thus prevent the
increase, keeping the associated incidence peak to a manageable level. This ultimately leads to a significantly lower total of infected cases in both communities (area under the infected curves).
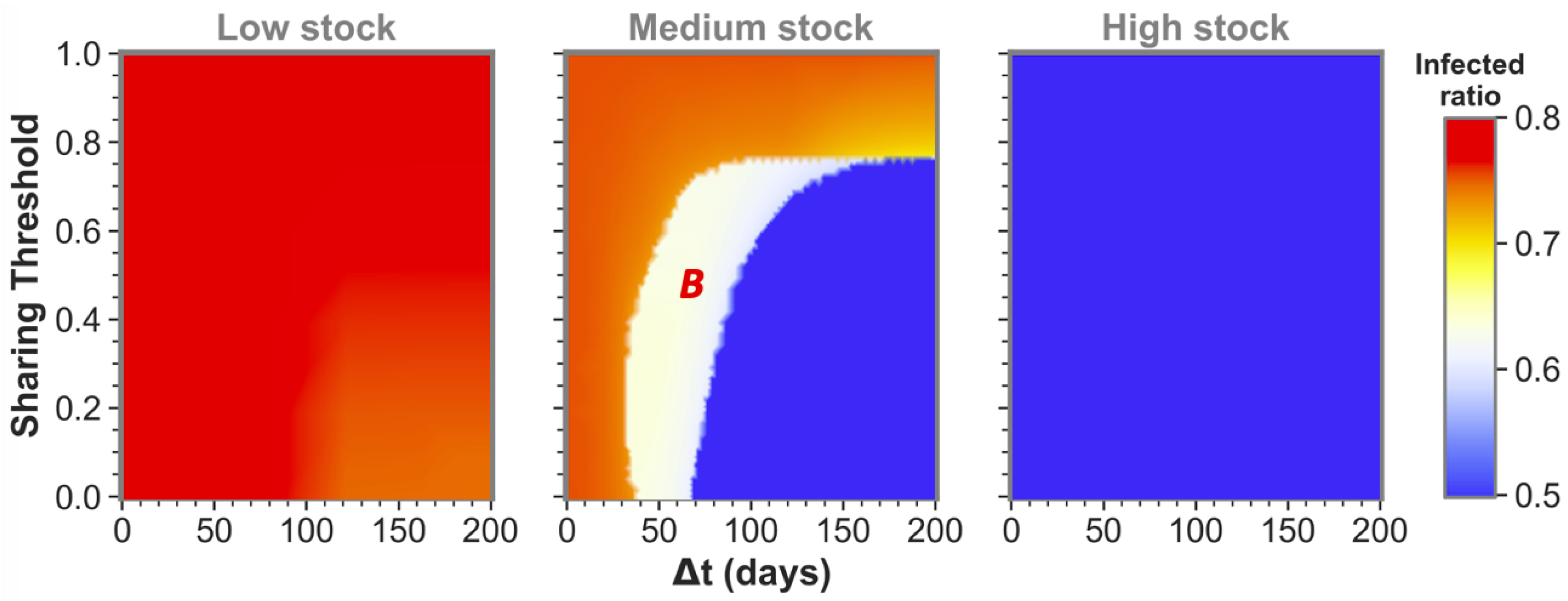
The size of the time difference (
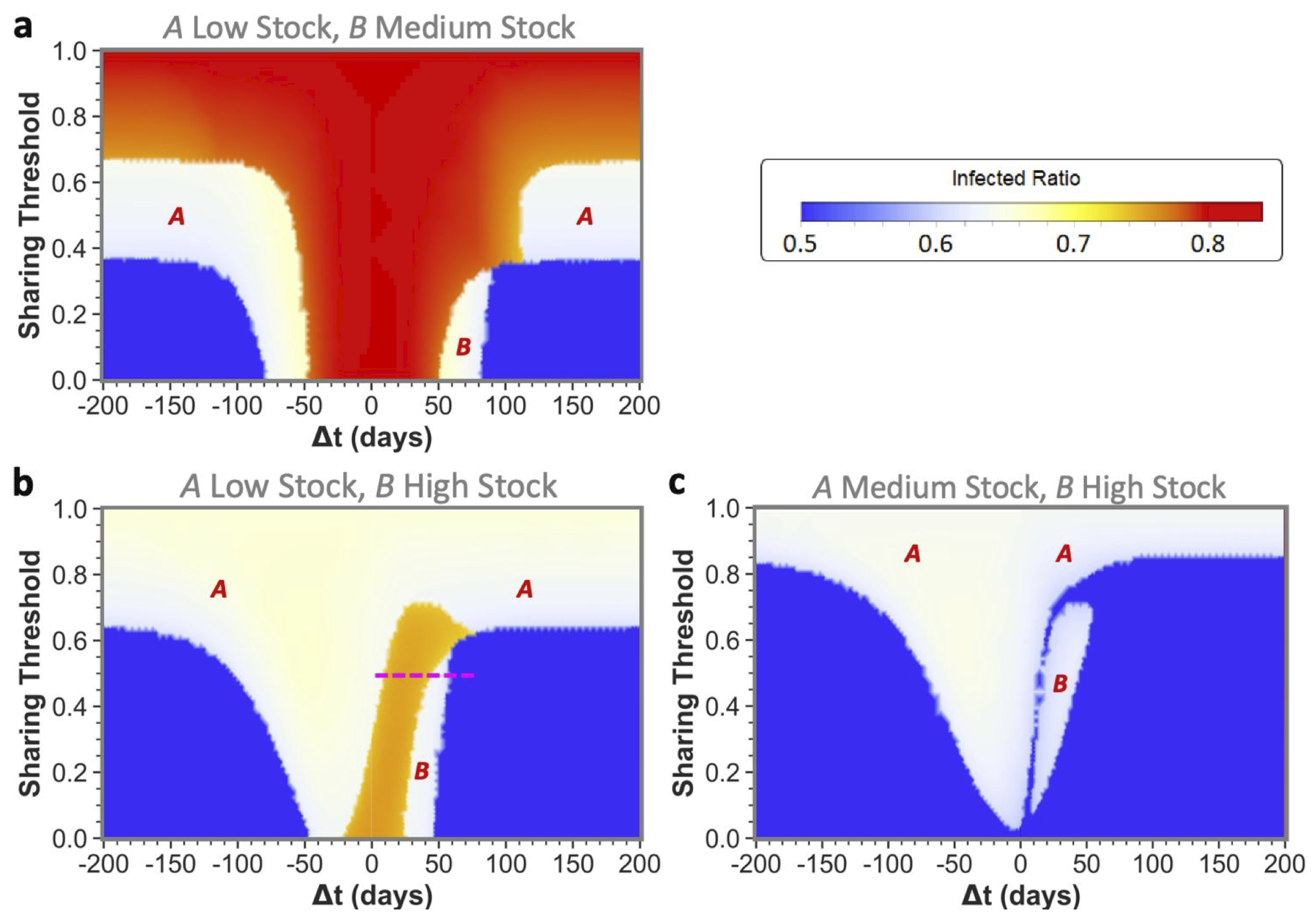
) between epidemic onsets heavily influences if communities as the ones shown here can reach low infected ratios for one or even both communities by sharing their stock. This is illustrated by the middle pane of
Figure 2 for medium-stock communities (defined to have a maximum stock of
). When not sharing (
these communities end up in the red state, both having the highest infected ratio. However, if the sharing threshold is low enough and there is some time difference between the epidemic onsets, the communities can reach the white state in which only one of the two communities (
B in this case) reaches stock depletion. Although this is already a successful sharing example for
A, the time difference is still too short for the combined stocks to be restored sufficiently for the second epidemic in
B. That being said, we can see that when the time difference between
A and
B epidemic onsets is increased further, both communities can achieve a low infected ratio (blue state). In the latter case, there was enough time to replenish the stock levels between the consecutive epidemics to save both communities.
We defined the medium stock category with this maximum stock of
to explicitly show the above described behavior when cooperating with a community that is equal in maximum stock. The other distinctive categories we use throughout this paper are low-stock (
) and high-stock (
) communities. Low stock is set at this specific level as these communities show high infected ratios (red state) individually and for all possible conditions when sharing with another low-stock group, as can be seen in the left pane of
Figure 2. Conversely, high-stock communities always reach low infected ratios (blue state in the right pane) irrespective of their sharing behavior with an equal-stock community. As mentioned before, virtually no actual countries exist where (all possible) PPE stock levels can be high enough to endure a whole epidemic of this sort. The high stock category is therefore included merely for illustrational purposes.
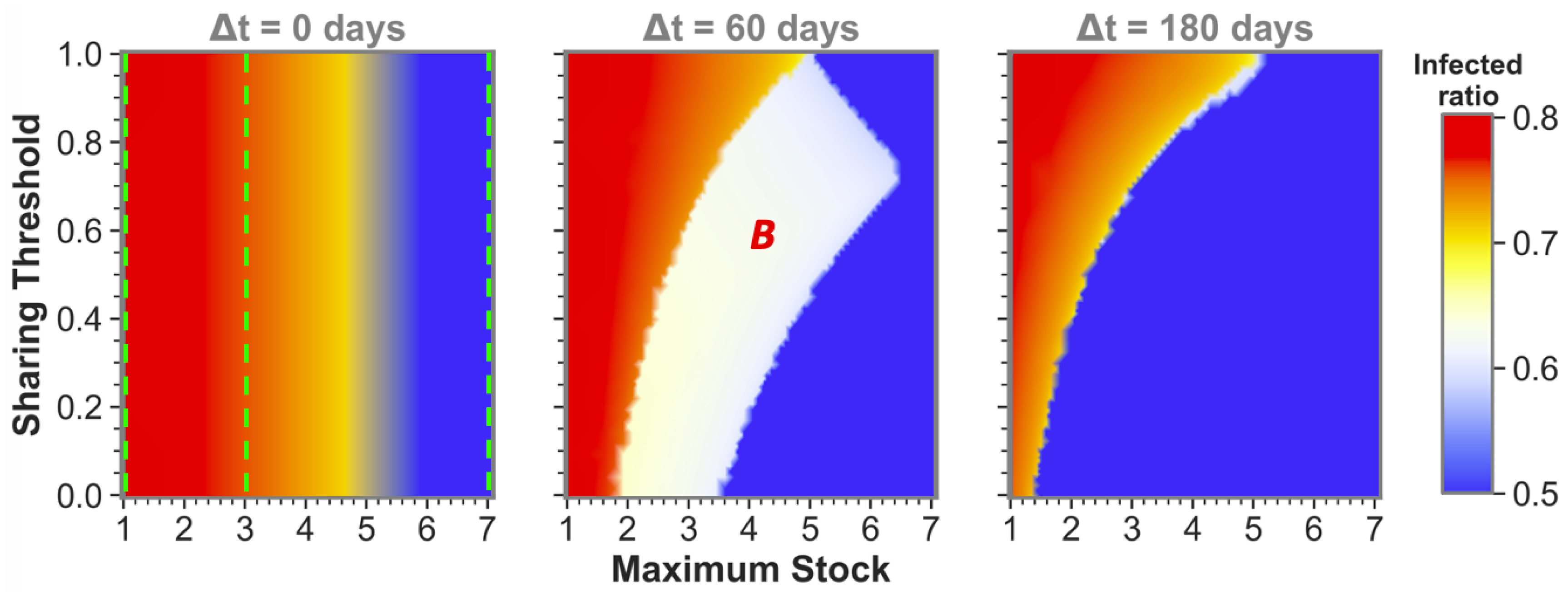
The observation that sufficient time difference increases the chance of having one or both sharing communities reach a low infected ratio also holds for more intermediate maximum stock communities. This becomes apparent from
Figure 3, which shows sharing between more intermediate maximum stock sizes for three time-difference categories that can be seen as cross-sections (at
,
and
) of the phase plots in
Figure 2 and vice versa (at the maximum stock categories, see green dashed lines). We can observe that equal-stock communities can already benefit from sharing from a maximum stock as low as
for big enough time differences. We also see that the lower the maximum stock, the more important implementing a low sharing threshold is for sharing success. The white area in which community
B (the community that gets infected secondly) still reaches high infected numbers even diminishes for the lower maximum stock communities when
.
In summary, the most important result for equal maximum stock community sharing is that communities with insufficient stock to weather the epidemic by themselves can, when agreeing on sufficiently low sharing thresholds, pass the epidemic without reaching the maximal infected ratio. Crucially, for these communities, a large-scale epidemic can be averted only for either or both of them when the time difference between epidemic onsets is sufficiently long. This requires a tailored strategy depending on the dynamics of the disease at hand, but always with a strong focus on mitigating disease spread between different regions by, for example, imposing travel restrictions early in epidemic outbreaks.
3.2. Unequal Maximum Stocks
Upon reading the equal maximum stock results, one could start fearing that sharing poses a risk to the second community. However, as we will further show by presenting the unequal maximum stock sharing results, this fear is unjustified for two reasons: (1) for a low- or medium-stock community, sharing never leads to worse results than non-sharing for both equal and unequal maximum stock combinations; and (2) for higher-stock communities there is hardly any risk in unequal sharing-even when the total shared stock turns out to be insufficiently high for both communities, a higher-stock second community can still weather the epidemic with a low infected ratio (
Figure 4).
Only for very specific low time differences between epidemic onsets, a secondly affected higher-stock community could possibly be worse off, i.e., experience so-called ‘second community disadvantage’ (in the white areas indicated with
B in the figure). This should again stress the importance of implementing measures to sufficiently slow down infection spread between communities, rather than discouraging higher-stock communities from sharing. Furthermore, in
Figure 4c, we can even observe that a low enough sharing threshold fully abolished the chance for high-stock communities of having the second community disadvantage. However, as this was only the case for these very specific disease dynamics and stock levels, communities should not solely rely on a low sharing threshold and still see increasing the onset time difference as a top priority. The substantial reduction of the second community disadvantage for higher-stock communities is a major difference from what we have seen for equal community sharing, in which the white state always meant full depletion for the community affected secondly by the epidemic.
An additional interesting observation is that sharing between unequal communities could help both communities weather the epidemic for all maximum stock height combinations. Even the low- and medium-stock communities can together end up in blue states by sharing! To reach blue states, the ordering of epidemic onsets is not of major importance, as we can see from the approximate similar sizes of the blue states for both positive and negative time differences. What is of major importance to obtain from these results, is (again) the time differences between these onsets and agreeing on a low sharing threshold.
In other aspects however, the epidemic onset ordering does impact sharing outcomes, which is indicated by some asymmetries that can be observed in the phase plots around : an epidemic that starts in the highest-stock community (negative ’s) will in general less often lead to the highest total infected ratios (red and orange states) than vice versa. It should be noted, however, that the lowest infected ratio regions (blue states) are often bigger when the lower-stock community is infected first (positive ’s). If starting in the lower-stock community, we see that increasing the time difference between onsets is of utmost importance to avoid high total infected ratios. The longer this difference between the epidemics, the more time for stock regeneration.
An interesting illustration of the effects of increasing the time difference between onset in a lower-stock and higher-stock community is shown in
Figure 5, for which the relevant sharing threshold and time-difference settings were indicated in
Figure 4 with the pink dashed line.
By increasing the threshold, the system passes from a state of depleted stock for the first community, via the maximal total infected ratio and a state of second community disadvantage, to both communities reaching low infected ratios. This effect is indeed asymmetric around if time evolutions would be taken for the same threshold, but with negative ’s (the mirror-image of the pink dashed line), we would only observe the state in which the lower-stock community depletes fully. We can further observe that the asymmetry in the phase plots vanishes in the larger time-difference regions. This is because, after sufficient time, the first epidemic dynamics die out and the stocks replenish enough, resulting in virtually no interaction effects between the consecutive epidemics.
The results discussed here solely focus on the three stock categories, as we defined before, to ease result interpretation. For more intermediate stock values, we refer to
Figure A1 in
Appendix B, where the effect of an increasing
on infected ratio is shown for different fixed
and different onset-time-difference categories.
Summarizing, the unequal maximum stock sharing results presented here again stress the positive combined effect of a low sharing threshold and a sufficient time difference between epidemic onsets. Additionally they show (1) that there is no downside from sharing for lower-stock communities and (2) how high-stock communities may substantially improve the overall epidemic resilience for both communities combined without much increased risk for their own population, as long as sufficient measures are implemented to reduce epidemic spread between the communities.
4. Discussion
We have introduced a stylized PPE production model with a sharing mechanism for communities facing an epidemic. The model convincingly shows that involved PPE stock sharing can greatly improve total and local epidemic resilience for all combinations of maximum stock communities. Crucially, these sharing strategies should be accompanied by disease spread mitigation measures to increase the time between epidemic peaks. This ensures that both communities have access to large quantities of stock when they need it most. First, we saw that completely blocking the circulation of essential supplies can lead to local stock shortages and subsequently epidemic proliferation. Then, we showed that for all realistically possible scenarios (where communities never have enough stock to fully endure exceptional situations, such as the COVID-19 pandemic, by themselves), sharing leads to better overall and local epidemic outcomes than non-sharing. Even if a high-stock community would exist, sharing can never lead to a more negative outcome for any cooperating community as long as the epidemic onset time differences are long enough. It is good to additionally realize that investing in the global reduction of infection rates helps to restrict the total viral circulation and the chance of reinfection in each region. Therefore, these investments are beneficial to all and crucial for final pandemic mitigation. The results thus strongly advocate for a willingness to share big stock proportions, with a special focus on accompanying measures to slow down epidemic spread between communities. In this way, pandemics can be fought way more efficiently and successfully for all parties involved with their respective PPE production limitations.
We emphasize that the numbers used in the examples are not intended to be realistic and are merely for illustrational purposes. In particular, what time difference would be ‘sufficient’ will depend on the properties of the virus, the modes in which people have contacts with other people, the infrastructure and production capabilities of the PPE, the preventive measures in place, and so on.
As mentioned before: increasing the delay between epidemic onset should evidently be a major focus when implementing this sharing mechanism. To achieve this, participating countries must directly focus on, e.g., limiting international travel when a community gets infected. Interestingly, the process of resource sharing itself could then start posing a threat, by increasing the chance of importing infection cases [
23]. This could limit the beneficial effects of implementing the proposed sharing method if not attended to well enough. We believe, however, that setting clear safety guidelines for this process can lower the risk sufficiently to still make sharing the most viable option. For interested policymakers, the model could be extended with the possibility to import infection cases during sharing. In this way, this possible risk and the necessary precautions could be investigated in more detail.
There are some simplifications in our model, which could possibly be accounted for in further research. For example, the effective reproduction number only changes when the community’s stock fully depletes (). In a more realistic scenario, there would be a gradual increase in as the stock becomes more scarce and less people can be protected. This would result in smoother phase plots, abolishing the three clear outcome states observed in this paper. Nevertheless, the general result that sharing reduces the infected ratio will then still hold. This more realistic approach would therefore make the model interpretations more complex without adding new insights. Further, parameters such as the basic reproduction number , the population size n, and PPE production rate P could differ substantially between communities. At the moment, the only characteristic we varied between communities was their maximum stock , but it could be interesting to study the effects of greater heterogeneity between communities on the results. One last simplification worth mentioning is the instantaneous transfer of huge PPE stock quantities during sharing. In real life, infrastructure limitations could form a barrier to implement the proposed sharing strategies by slowing down the redistribution process and limiting sharing effectiveness. Although it was beyond the scope of this paper, it could thus be interesting to extend the model with some geographical transportation limits and delays for different distances to ascertain the boundary conditions for sharing to be effective.
Further interesting extensions could be to include more communities and to tailor the DSP model to specific and possibly multiple resource types. When multiple communities are involved, the necessary individual sharing amount to sufficiently help a community in need reduces as the load can be divided over contributing groups. In our model we have seen that two low-stock communities had too little combined stock to save either of them, even when sharing major proportions of stock. But a bigger group of solely low-stock communities could potentially have enough combined stock to successfully avoid high infected ratios. However, one should note that in this more complex scenario, a more complex sharing strategy decision structure also arises. To model the politics of this, game-theoretic approaches could be used [
24,
25]. For the extensions suggested here, the modeler should further be aware of the increasing computational costs of solving
ODEs for
n communities and
r resource types. Lastly, in all its simplicity, our model could serve as a basis for models of higher complexity that more closely resemble real-world scenarios. After fitting the data of manufacturers, resource usage, and epidemic dynamics, optimal sharing strategies could potentially be estimated with some precision for combinations of collaborating communities, depending on the uncertainties involved. A similar work of fitting is done to predict SARS-CoV-2 evolution [
26].
Overall, we can see that increasing global resilience to future pandemics is a complex problem that requires an interdisciplinary effort. Although myriad extensions could be made to this model, these additions would have unnecessarily distracted the reader from our main aim of communicating to policymakers how cooperation can improve epidemic resilience. The strengths of this model are its simplicity and comprehensibility, conveying a clear message and recommendations.
5. Conclusions
In summary, this work demonstrates how trade barriers for essential healthcare resources can worsen pandemic outcomes, while cooperation between different communities can successfully avoid resource scarcity and prevent high-infected incidences for all parties involved. We used a stylized model encompassing disease spread and a resource supply chain in two communities. If enough measures were taken to slow down infection spread between them, both communities were able to avoid resource exhaustion at any point during their epidemics by cooperative sharing. This shows that collaboration is a viable solution to avoid resource scarcity. This message is similarly true for vaccines, as we have learned during the current COVID-19 pandemic [
19].
The model serves as a proof-of-concept that illustrates how sharing protective equipment resources between communities is a crucial step in epidemic mitigation. Although the model is stylized, we believe that its general insights are easy to understand and essential to disseminate to all stakeholders involved. It advocates for involved sharing strategies between all types of communities, showing that with a high willingness to share and a strong focus on slowing international infection spread, total epidemic mitigation outcomes can be improved greatly. It also successfully demonstrates that high GDP countries should not be afraid to offer help to countries that need it, as potential negative effects for their own population can be abolished if the right disease spread mitigation measures are in place. Furthermore, reducing viral circulation globally reduces each region’s chance of reinfection and is thus in the interest of all. By taking these insights into account, we believe that global initiatives can help prevent essential-resource exhaustion during pandemics. Our study thus shows that mutual resource sharing can potentially support all participating communities during their local epidemics and ultimately lead to the collaborative mitigation of a pandemic.
Author Contributions
Conceptualization, J.E., B.D.L.C., J.F.U., M.O.R., H.W. and R.Q.; Methodology, J.E.; Formal analysis, K.d.N.; Investigation, K.d.N. and J.E.; Supervision, R.Q.; Writing—original draft, K.d.N. and J.E.; Writing—review & editing, K.d.N., B.D.L.C., J.F.U., M.O.R., H.W. and R.Q.; Visualization, K.d.N. and J.E.; Funding acquisition, M.O.R. and R.Q. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by ZonMw project ‘Dynamic symptoms networks’ project 350 grant number 09120012010063.
Acknowledgments
R.Q., M.O.R. and H.W. acknowledge the ZonMw project ‘Dynamic Symptoms Networks’ (project number 09120012010063), as well as the contributions made by A. G. Hoekstra and A. Titton.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A. Extended Methods
Appendix A.1. General Remark
At multiple instances, a gradual input needs to be translated into a clear on/off output around some boundary value, while ensuring a solvable ODE system. To elegantly solve for this, a sigmoid function is used, defined by , where k indicates the function’s steepness. The sigmoid is represented simply as wherever the standard steepness of was used.
Appendix A.2. Adapted SIR
We use the classical SIR ordinary differential equation formulation without births or deaths [
21] to calculate the number of susceptible
U, infected
I, and recovered
R individuals (the regular expression for susceptibles
S is already used to refer to ‘stock’ in this paper). Additionally, we introduce the possibility of a delay in epidemic onset, by multiplying the differential equations by the sigmoid function
.
indicates the number of days between
and the start of an epidemic with
.
Here
indicates the infection rate and
the recovery rate.
is calculated from the effective reproduction number
. This is defined as the ratio
and is a measure of the infectiousness of the virus: the average amount of people getting infected from one infected individual. It is assumed that the availability of PPE lowers the virus’ infectiousness. Therefore the effective reproduction number is calculated from the basic reproduction number
(infection spread without any protective measures).
with
the stock at time
t and
r being the reduction in infectiousness if the stock is non-zero. It is thus assumed that
is reduced to some fixed lower value depending only on
r and
as long as
, but reverts back to the original
value as soon as the stock has depleted (
).
Appendix A.3. DSP Model
The DSP chain was modeled via the following differential equations for demand
D, available stock
S, and production
P:
Here, w is the PPE needed per infected individual. The demand thus grows linearly with the amount of infected. Interestingly, this demand equation can be replaced by any positive and continuous demand equation of interest and could thus be adapted to be reused in other supply chain contexts. It is assumed that there is a baseline PPE demand for regular non-epidemic-related use. The baseline production is matched to this standard demand (). Before epidemic onset, the stock is thus kept at a dynamic equilibrium, which we assume to be at its full capacity . The stock difference is calculated from the difference between production and demand, but demand can only be subtracted if there is still stock available to prevent a negative stock. When the demand changes due to differences in the amount of infected, the production difference follows the difference in demand with a cap of the maximum production per day (). Again, sigmoid functions are used to control for different scenario’s in three ways: (1) there can only be an increase in production when the current production is below maximum production; (2) when the stock is below , the production is increased further than the demand to enable stock replenishment; and (3) when production is higher than demand, an additional force is added to prevent a stock overshoot, which gradually pushes the production back to the demand level.
We can see how the coupling between the SIR and DSP model parts is realized via two factors: (1) the DSP demand Equation (
A3) that is dependent on
; and (2) the
calculation (
A2) with the current
, giving the
in the SIR equations (
A1).
Appendix A.4. Sharing Mechanism
The epidemic and production mechanism of each community
i can be separately described by the coupled SIR and DSP equations above. For product sharing between communities, a switch mechanism is constructed. As inputs, the current stock - maximum stock ratios (
) for both communities and the sharing threshold
are taken. Sharing must be enabled when exactly one of the two communities’ stocks drops below this critical threshold. The switch system is implemented as follows:
Due to the need for increased sensitivity, the steepness of the sigmoid was set higher than before, at
. The sharing mechanism was added to the separate community ODEs by adding shared stock (
) to Equation (
A4) as follows:
Here, the size of the shared PPE stock is calculated as
. The amount of shared stock is thus related to the difference between the stocks of both communities. With the system described here, we can simulate a whole spectrum of willingness to share, from a scenario with no sharing (
) to sharing the total stock (
). Mathematica [
27] was used to solve the system and plot the results. In
Table A1, a brief outline is shown of the parameter settings used in the model.
Table A1.
Parameter settings used for the simulations in this paper.
Table A1.
Parameter settings used for the simulations in this paper.
|
Parameter | Symbol | Value | Comment |
|---|
| Population size | n | | This parameter has no effect in the model but makes results more easily interpretable. It is roughly the population size of the Netherlands. |
| Basic reproduction number | | | is the reproduction number of the virus in the absence of countermeasures and without immunity, i.e., at the start of the pandemic. In their June 2020 report [28] the WHO estimated this number in the range 2–4. In the Netherlands, the peak estimate [29] near the start of the pandemic is around but this varies significantly across countries and time. The most important thing about this parameter is that it should be larger than 1 in order to lead to exponential growth and thus a population-wide epidemic. |
| Recovery rate | | | This rate defines the average period ( days) during which an individual is infectious. In the same WHO report, the curve of virus-shedding appears to be high for about six days. More recent sources report a median of 8 days [30]. Together with the infection rate, this parameter induces the reproduction number, which in our simulation is the more important number. |
| Initial production | | | This parameter has an arbitrary unit which has no effect on the model but makes results more easily interpretable. According to a news article from April 2020, the Netherlands were using about face masks per day, which is the most well-known resource. Our value here is thus chosen to be on the order of magnitude of the number of face masks. |
| Maximum production | | | A community can increase the local production to this quantity. This includes all sources, such as increasing local production as well as importing more resources from global suppliers. |
| Necessary PPE per infected case | w | 4 | Keeping to face masks for interpretability, numbers vary wildly: from over a hundred masks per day per corona patient in the intensive care unit to one or zero for infected persons who are either unaware of their infection or remain in quarantine at home. For interpretability, we turn to what would in principle be needed to prevent one infected person from infecting others as much as possible. According to various sources, a disposable mask should only be used for about three hours. This would mean that in principle, in order to cover one’s face for 12 h per day, about 4 masks would be needed, which is the (arbitrary) quantity we chose here. |
| Reduction in for | r | | This factor multiplies with the infection rate and thus reduces the reproduction number. Its value is arbitrary with the most important property being that . In other words, this means that having sufficient PPE would stop the exponential growth of the epidemic. |
Appendix B. Additional Figure
Figure A1.
Influence of B maximum stock and sharing threshold on total infected ratio for different epidemic-onset time differences and fixed A maximum stock. The epidemic is introduced in A at , and in B at . As a special case, the negative thus indicates epidemic onset in B prior to its onset in A. The maximum stock of A is fixed at either Low stock (), Medium stock (), or High stock (). , , , , , , , .
Figure A1.
Influence of B maximum stock and sharing threshold on total infected ratio for different epidemic-onset time differences and fixed A maximum stock. The epidemic is introduced in A at , and in B at . As a special case, the negative thus indicates epidemic onset in B prior to its onset in A. The maximum stock of A is fixed at either Low stock (), Medium stock (), or High stock (). , , , , , , , .
References
- Heymann, D.L.; Shindo, N. COVID-19: What is next for public health? Lancet 2020, 395, 542–545. [Google Scholar] [CrossRef] [Green Version]
- Klompas, M.; Rhee, C.; Baker, M.A. Universal Use of N95 Respirators in Healthcare Settings When Community Coronavirus Disease 2019 Rates Are High. Clin. Infect. Dis. 2021, 74, 529–531. [Google Scholar] [CrossRef] [PubMed]
- Ranney, M.L.; Griffeth, V.; Jha, A.K. Critical supply shortages—the need for ventilators and personal protective equipment during the COVID-19 pandemic. N. Engl. J. Med. 2020, 382, e41. [Google Scholar] [CrossRef] [PubMed]
- McMahon, D.E.; Peters, G.A.; Ivers, L.C.; Freeman, E.E. Global resource shortages during COVID-19: Bad news for low-income countries. PLoS Neglected Trop. Dis. 2020, 14, e0008412. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.l.; Huang, J.; Zhang, C.J.; He, Z.; Ming, W.K. Facemask shortage and the novel coronavirus disease (COVID-19) outbreak: Reflections on public health measures. EClinicalMedicine 2020, 21, 100329. [Google Scholar] [CrossRef]
- Livingston, E.; Desai, A.; Berkwits, M. Sourcing personal protective equipment during the COVID-19 pandemic. JAMA 2020, 323, 1912–1914. [Google Scholar] [CrossRef] [Green Version]
- Baldwin, R.; Evenett, S.J. COVID-19 and Trade Policy: Why Turning Inward Will Not Work. 2020. Available online: https://voxeu.org/content/covid-19-and-trade-policy-why-turning-inward-won-t-work (accessed on 10 January 2022).
- Freire-Paspuel, B.; Vega, P.; Velez, A.; Castillo, P.; Gomez-Santos, I.; Cruz, M.; Garcia-Bereguiain, M.A. Cotton tipped plastic swabs for SARS-CoV-2 RT-qPCR diagnosis to prevent supplies shortage. medRxiv 2020. [Google Scholar] [CrossRef]
- Hoernke, K.; Djellouli, N.; Andrews, L.; Lewis-Jackson, S.; Manby, L.; Martin, S.; Vanderslott, S.; Vindrola-Padros, C. Frontline healthcare workers’ experiences with personal protective equipment during the COVID-19 pandemic in the UK: A rapid qualitative appraisal. BMJ Open 2021, 11, e046199. [Google Scholar] [CrossRef]
- Gereffi, G. What does the COVID-19 pandemic teach us about global value chains? The case of medical supplies. J. Int. Bus. Policy 2020, 3, 287–301. [Google Scholar] [CrossRef]
- Ivanov, D.; Dolgui, A. Viability of intertwined supply networks: Extending the supply chain resilience angles towards survivability. A position paper motivated by COVID-19 outbreak. Int. J. Prod. Res. 2020, 58, 2904–2915. [Google Scholar] [CrossRef] [Green Version]
- Ayittey, F.K.; Ayittey, M.K.; Chiwero, N.B.; Kamasah, J.S.; Dzuvor, C. Economic impacts of Wuhan 2019-nCoV on China and the world. J. Med Virol. 2020, 92, 473–475. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, S.; Kumar, R.; Panchal, R.; Tiwari, M.K. Impact of COVID-19 on logistics systems and disruptions in food supply chain. Int. J. Prod. Res. 2020, 59, 1993–2008. [Google Scholar] [CrossRef]
- Bown, C. COVID-19: Trump’s curbs on exports of medical gear put Americans and others at risk. Trade Invest. Policy Watch. Peterson Inst. Int. Econ. 2020, 9, 31. [Google Scholar]
- Evenett, S. Tackling COVID-19 together: The trade policy dimension. Glob. Trade Alert 2020. Available online: https://www.globaltradealert.org/reports/51 (accessed on 10 January 2022).
- Carter, S.E.; Gobat, N.; Zambruni, J.P.; Bedford, J.; van Kleef, E.; Jombart, T.; Mossoko, M.; Nkakirande, D.B.; Colorado, C.N.; Ahuka-Mundeke, S. What questions we should be asking about COVID-19 in humanitarian settings: Perspectives from the Social Sciences Analysis Cell in the Democratic Republic of the Congo. BMJ Glob. Health 2020, 5, e003607. [Google Scholar] [CrossRef] [PubMed]
- Espitia, A.; Rocha, N.; Ruta, M. Trade and the COVID-19 Crisis in Developing Countries. VOX CEPR Policy Portal 2020, 9. Available online: https://voxeu.org/article/trade-and-covid-19-crisis-developing-countries (accessed on 10 January 2022).
- Huntingford, C.; Rawson, T.; Bonsall, M.B. Optimal COVID-19 Vaccine Sharing Between Two Nations That Furthermore, Have Extensive Travel Exchanges. Front. Public Health 2021, 9, 1112. [Google Scholar] [CrossRef]
- Wagner, C.E.; Saad-Roy, C.M.; Morris, S.E.; Baker, R.E.; Mina, M.J.; Farrar, J.; Holmes, E.C.; Pybus, O.G.; Graham, A.L.; Emanuel, E.J.; et al. Vaccine nationalism and the dynamics and control of SARS-CoV-2. medRxiv 2021, 373, eabj7364. [Google Scholar] [CrossRef]
- Mehrotra, S.; Rahimian, H.; Barah, M.; Luo, F.; Schantz, K. A Model of Supply-Chain Decisions for Resource Sharing with an Application to Ventilator Allocation to Combat COVID-19. Nav. Res. Logist. (NRL) 2020, 67, 303–320. [Google Scholar] [CrossRef]
- Shulgin, B.; Stone, L.; Agur, Z. Pulse vaccination strategy in the SIR epidemic model. Bull. Math. Biol. 1998, 60, 1123–1148. [Google Scholar] [CrossRef]
- Allen, L.J. Some discrete-time SI, SIR, and SIS epidemic models. Math. Biosci. 1994, 124, 83–105. [Google Scholar] [CrossRef]
- Killeen, G.F.; Kiware, S.S. Why lockdown? Why national unity? Why global solidarity? Simplified arithmetic tools for decision-makers, health professionals, journalists and the general public to explore containment options for the 2019 novel coronavirus. Infect. Dis. Model. 2020, 5, 442–458. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, F.; Flache, A.; Squazzoni, F. Solidarity in collaboration networks when everyone competes for the strongest partner: A stochastic actor-based simulation model. J. Math. Sociol. 2020, 44, 249–266. [Google Scholar] [CrossRef] [Green Version]
- Caparrós, A.; Finus, M. The Corona-Pandemic: A Game-Theoretic Perspective on Regional and Global Governance. Environ. Resour. Econ. 2020, 76, 913–927. [Google Scholar] [CrossRef] [PubMed]
- Neves, A.G.; Guerrero, G. Predicting the evolution of the COVID-19 epidemic with the A-SIR model: Lombardy, Italy and Sao Paulo state, Brazil. Phys. D Nonlinear Phenom. 2020, 413, 132693. [Google Scholar] [CrossRef]
- Wolfram, S. The MATHEMATICA® Book, Version 4; Cambridge University Press: Cambridge, UK, 1999. [Google Scholar]
- World Health Organization. COVID-19—A Global pandemic. What Do We Know about SARS-CoV-2 and COVID-19? 2020. Available online: https://www.who.int/docs/default-source/coronaviruse/risk-comms-updates/update-28-COVID-19-what-we-know-may-2020.pdf (accessed on 3 August 2021).
- Rijksoverheid Nederland. Reproductiegetal. Coronadashboard Rijksoverheid Nederland. 2021. Available online: https://coronadashboard.rijksoverheid.nl/landelijk/reproductiegetal (accessed on 5 August 2021).
- van Kampen, J.J.A.; van de Vijver, D.A.M.C.; Fraaij, P.L.A.; Haagmans, B.L.; Lamers, M.M.; Okba, N.; van den Akker, J.P.C.; Endeman, H.; Gommers, D.A.M.P.J.; Cornelissen, J.J.; et al. Duration and key determinants of infectious virus shedding in hospitalized patients with coronavirus disease-2019 (COVID-19). Nat. Commun. 2021, 12, 267. [Google Scholar] [CrossRef]
| Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).