Environmental Survey of the Distribution and Metal Contents of Pteris vittata in Arsenic–Lead–Mercury-Contaminated Gold Mining Areas along the Bone River in Gorontalo Province, Indonesia
Abstract
1. Introduction
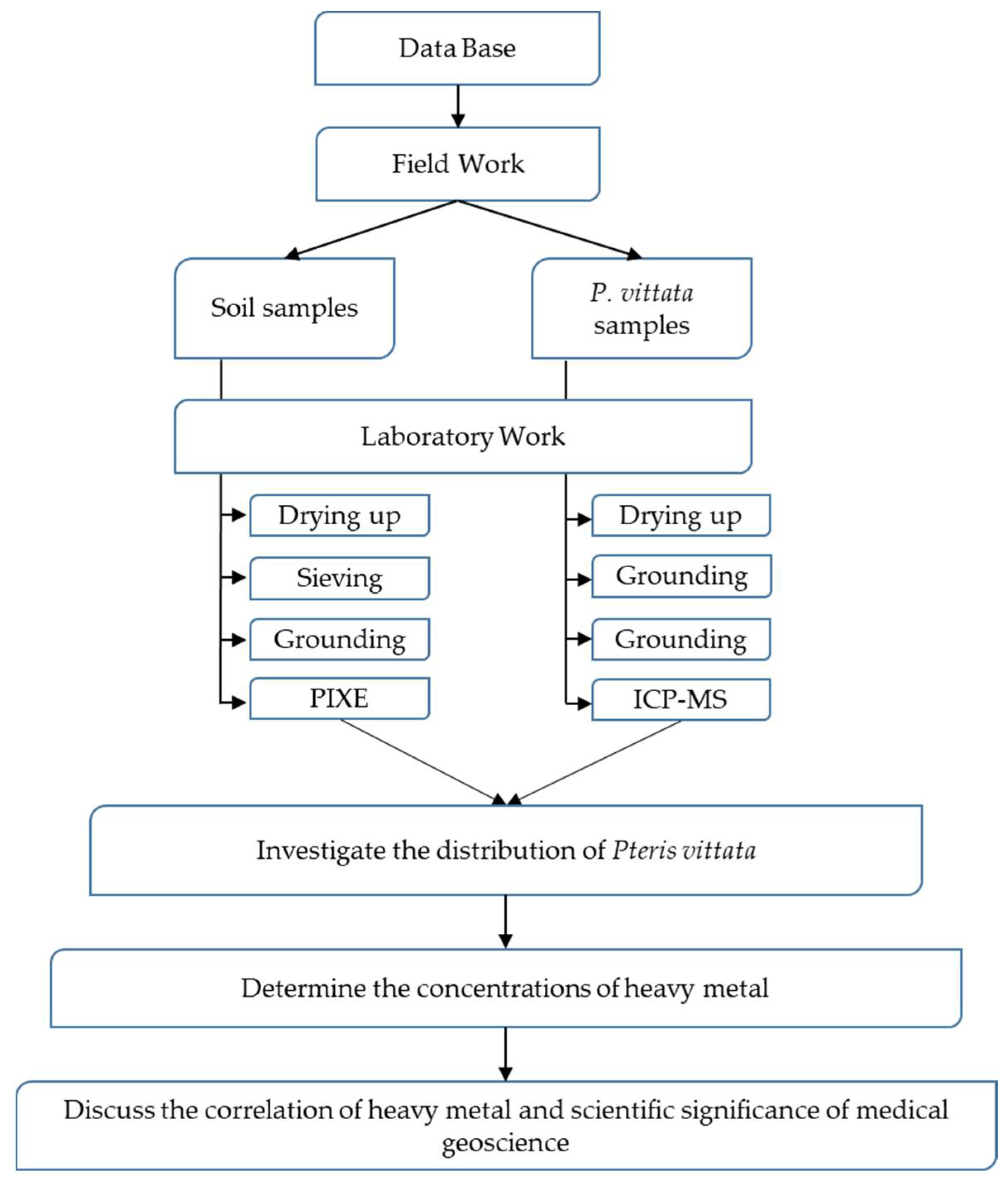
2. Materials and Methods
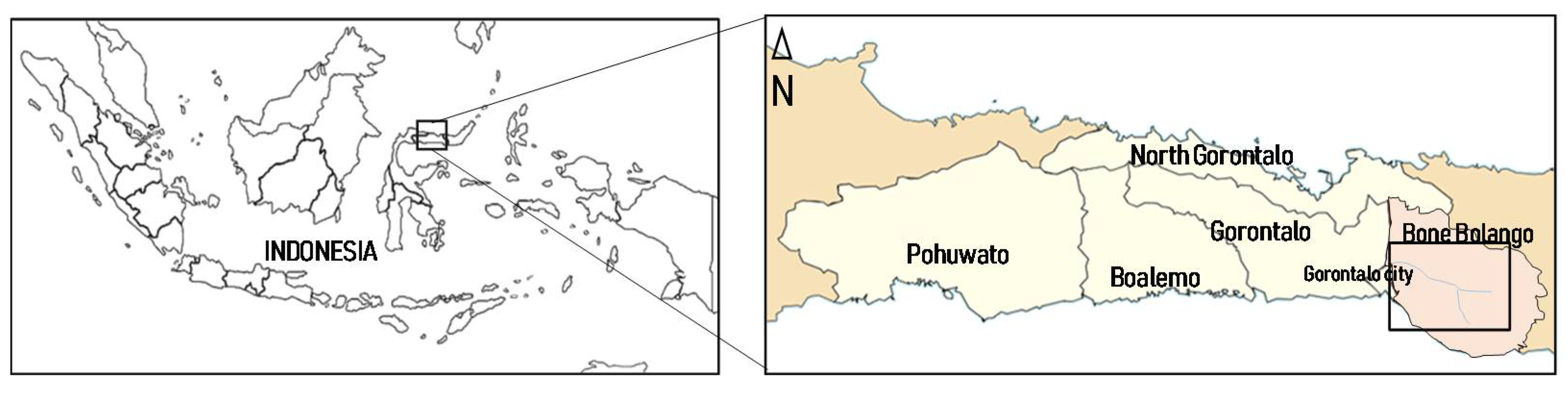
2.1. Distribution of the Population Density of P. vittata and Sampling of the Plants and Soils
2.2. Chemical Analysis
2.3. Quality Control and Statistical Analysis
3. Results and Discussion
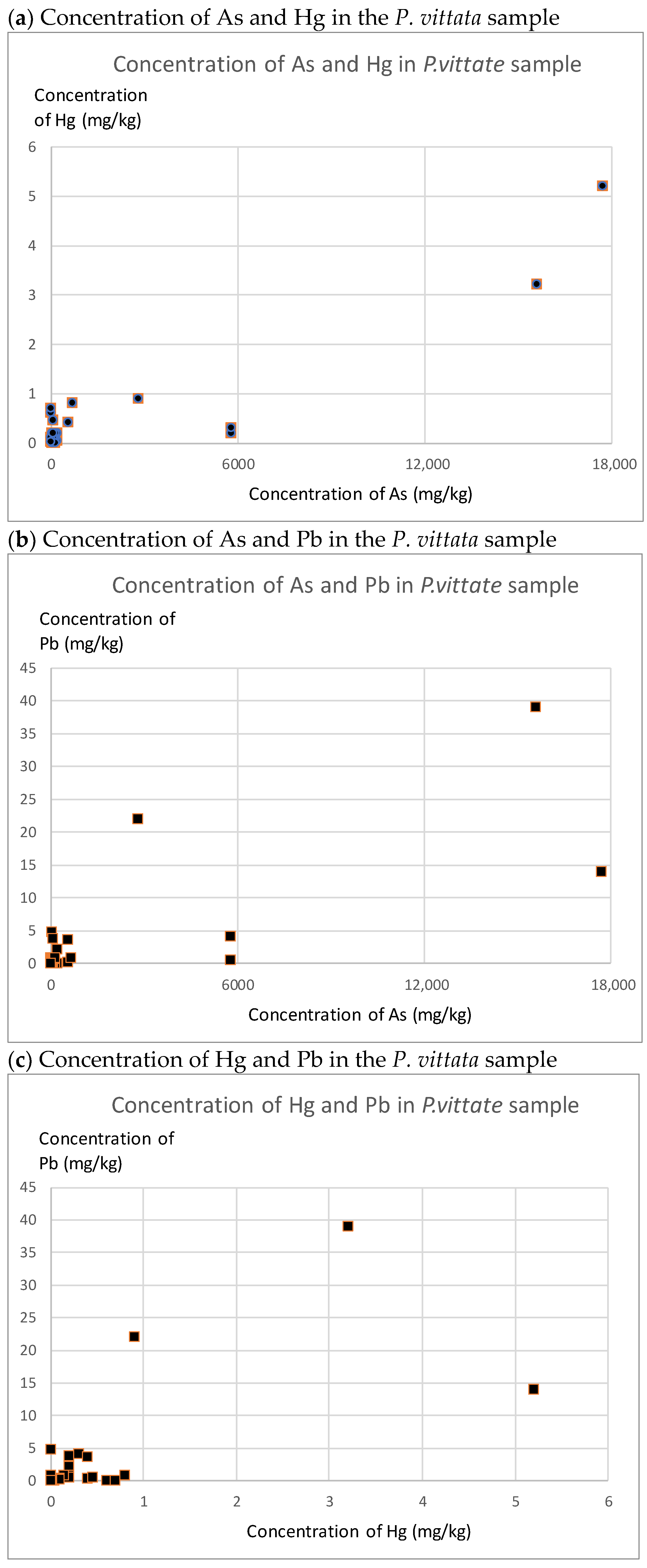
3.1. As, Hg, and Pb Concentrations in Soil and P. vittata
3.2. Relationship between the Distributions of the Population Density of P. vittata and the As Concentration in Soil
3.3. Metal Pollution and Uptake by P. vittata along the Bone River
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- United Nations Environment Programme-UN Environmental Program. The Global Mercury Assessment. 2013. Available online: http://www.unep.org/PDF/PressReleases/GlobalMercuryAssessment2013.pdf (accessed on 14 August 2019).
- McGrew, L. Asia Pacific Foundation of Canada Home Page. Available online: https://www.asiapacific.ca/blog/artisanal-and-small-scale-gold-mining-sector-problems-and (accessed on 29 October 2021).
- GOLD-ISMIA Team. United Nation Development Programme Indonesia Home Page. Available online: https://www.id.undp.org/content/indonesia/en/home/presscenter/articles/2021/090621.html (accessed on 29 October 2021).
- Bose-O’Reilly, S.; Drasch, G.; Beinhoff, C.; Rodrigues-Filho, S.; Roider, G.; Lettmeier, B.; Maydl, A.; Maydl, S.; Siebert, U. Health Assessment of Artisanal Gold Miners in Indonesia. Sci. Total Environ. 2010, 408, 713–725. [Google Scholar] [CrossRef]
- Puluhulawa, F.; Junus, N. A Final Report: Perlindungan Hukum Terhadap Usaha Pertambangan Rakyat di Provinsi Gorontalo, Gorontalo State University: Gorontalo, Indonesia, 2013; unpublished work.
- WHO/IPCS. Methylmercury—Environmental Health Criteria 101. Available online: https://wedocs.unep.org/handle/20.500.11822/29413 (accessed on 3 September 2021).
- Arifin, Y.; Sakakibara, M.; Sera, K. Impacts of Artisanal and Small-Scale Gold Mining (ASGM) on Environment and Human Health of Gorontalo Utara Regency, Gorontalo Province, Indonesia. Geosciences 2015, 5, 160–176. [Google Scholar] [CrossRef]
- Gafur, N.A.; Sakakibara, M.; Sano, S.; Sera, K. A Case Study of Heavy Metal Pollution in Water of Bone River by Artisanal Small-Scale Gold Mine Activities in Eastern Part of Gorontalo, Indonesia. Water 2018, 10, 1507. [Google Scholar] [CrossRef]
- Lihawa, F.; Mahmud, M. The Content of Mercury in Sediments Around Artisanal Smallscale Gold Mining (ASGM) Bumela District, Gorontalo Regency, Gorontalo Province, Indonesia. IOP Conf. Ser. Earth Environ. Sci. 2019, 314, 012016. [Google Scholar] [CrossRef]
- Hedenquist, J.W. Mineralization Associated with Volcanic-Related Hydrothermal Systems in the Circum-Pacific Basin. In Proceedings of the 4th Circum Pacific Energy and Mineral Resources Conference, Singapore, 17–22 August 1986; pp. 513–524. [Google Scholar]
- Ma, L.Q.; Komar, K.M.; Tu, C.; Zhang, W.; Cai, Y.; Kennelley, E.D. A Fern That Hyperaccumulates Arsenic. Nature 2001, 409, 579. [Google Scholar] [CrossRef] [PubMed]
- Natarajan, S.; Stamps, R.H.; Saha, U.K.; Ma, L.Q. Phytofltration of Arsenic-Contaminated Groundwater Using Pteris vittata L.: Effect of Plant Density and Nitrogen and Phosphorus Levels. Int. J. Phytoremediat. 2008, 10, 220–233. [Google Scholar] [CrossRef]
- Sakakibara, M.; Watanabe, A.; Inoue, M.; Sano, S.; Kaise, T. Phytoextraction and Phytovolatilization of Arsenic from as-Contaminated Soils by Pteris vittata. In Proceedings of the Annual International Conference on Soils, Sediments, Water and Energy, Amherst, MA, USA, 18–21 October 2010; Volume 12, pp. 264–272. [Google Scholar]
- Fayiga, A.O.; Ma, L.Q.; Cao, X. Rathinasabapathi, B. Effects of heavy metals on growth and arsenic accumulation in the arsenic hyperaccumulator Pteris vittata L. Environ Pollut. 2004, 132, 289–296. [Google Scholar] [CrossRef]
- Lei, M.; Wan, X.; Guo, G.; Yang, J.; Chen, T. Phytoextraction of arsenic-contaminated soil with Pteris vittata in Henan Province, China: Comprehensive evaluation of remediation efficiency correcting for atmospheric depositions. Environ. Sci. Pollut. Res. Int. 2018, 25, 124–131. [Google Scholar] [CrossRef]
- Zeng, P.; Guo, Z.; Xiao, X.; Peng, C.; Feng, W.; Xin, L.; Xu, Z. Phytoextraction potential of Pteris vittata L. co-planted with woody species for As, Cd, Pb and Zn in contaminated soil. Sci. Total Environ. 2019, 650 Pt 1, 594–603. [Google Scholar] [CrossRef]
- Ma, J.; Lei, E.; Lei, M.; Liu, Y.; Chen, T. Remediation of Arsenic contaminated soil using malposed intercropping of Pteris vittata L. and maize. Chemosphere 2018, 194, 737–744. [Google Scholar] [CrossRef] [PubMed]
- Raymond, R.C.; Madan, K.O.; John, B.W.; Joan, E.B.; William, D.M. Analysis of Gopher Tortoise Population Estimation Techniques; Engineer Research and Development Center: Gainesville, FL, USA, 2005; Available online: https://citeseerx.ist.psu.edu/viewdoc/download?doi=10.1.1.665.3013&rep=rep1&type=pdf (accessed on 21 November 2016).
- Sera, K.; Yanagisawa, T.; Tsunoda, H.; Futatsugawa, S.; Hatakeyama, S.; Saitoh, Y.; Suzuki, S.; Orihara, H. Bio-PIXE at the Takizawa Facility (Bio-PIXE with a Baby Cyclotron). Int. J. Pixe 1992, 2, 325–330. [Google Scholar] [CrossRef]
- Carlos, A.G.; Robert, J. National institute of Standards & Technology Certificate of Analysis. Standard Reference Materials® 1643f. 2015. Available online: https://www-s.nist.gov/srmors/certificates/1643F.pdf (accessed on 5 October 2016).
- SPEX Certificate. Certificate of Reference Material XSTC-13. Available online: http://www.seishin-syoji.co.jp/files/libs/567/201604271251467620.pdf (accessed on 16 October 2018).
- Carlos, A.G.; Robert, J.; National institute of Standards & Technology Certificate of Analysis. Standard Reference Materials® 2782. 2015. Available online: https://www-s.nist.gov/m-srmors/certificates/2782.pdf (accessed on 5 October 2016).
- NIES. NIES No. 9 Sargasso. Certificate of Analysis; National Institute of Environmental Studies: Tsukuba, Japan, 1988.
- USEPA (U.S. Environmental Protection Agency). Clean Water Act; Section 503; US EPA: Washington, DC, USA, 1993; Volume 58.
- Anh, B.T.K.; Minh, N.N.; Ha, N.T.H.; Kim, D.D.; Kien, N.T.; Trung, N.Q.; Cuong, T.T.; Danh, L.T. Field Survey and Comparative Study of Pteris vittata and Pityrogramma Calomelanos Grown on Arsenic Contaminated Lands with Different Soil pH. Bull. Environ. Contam. Toxicol. 2018, 100, 720–726. [Google Scholar] [CrossRef]
- Hindersah, R.; Risamasu, R.; Kalay, A.M.; Dewi, T.; Makatita, I. Mercury Contamination in Soil, Tailing and Plants on Agricultural Fields near Closed Gold Mine in Buru Island, Maluku. J. Degrad. Min. Lands Manag. 2018, 5, 1027–1034. [Google Scholar] [CrossRef][Green Version]
- Basu, N.; Clarke, E.; Green, A.; Calys-Tagoe, B.; Chan, L.; Dzodzomenyo, M.; Fobil, J.; Long, R.N.; Neitzel, R.L.; Obiri, S.; et al. Integrated Assessment of Artisanal and Small-Scale Gold Mining in Ghana—Part 1: Human Health Review. Int. J. Environ. Res. Public Health 2015, 12, 5143–5176. [Google Scholar] [CrossRef]
- Fashola, M.O.; Ngole-Jeme, V.M.; Babalola, O.O. Heavy Metal Pollution from Gold Mines: Environmental Effects and Bacterial Strategies for Resistance. Int. J. Environ. Res. Public Health 2016, 13, 1047. [Google Scholar] [CrossRef]
- Rajaee, M.; Obiri, S.; Green, A.; Long, R.; Cobbina, S.J.; Nartey, V.; Buck, D.; Antwi, E.; Basu, N. Integrated Assessment of Artisanal and Small-Scale Gold Mining in Ghana—Part 2: Natural Sciences Review. Int. J. Environ. Res. Public Health 2015, 12, 8971–9011. [Google Scholar] [CrossRef]
- Wilson, M.L.; Renne, E.; Roncoli, C.; Agyei-Baffour, P.; Tenkorang, E.Y. Integrated Assessment of Artisanal and Small-Scale Gold Mining in Ghana—Part 3: Social Sciences and Economics. Int. J. Environ. Res. Public Health 2015, 12, 8133–8156. [Google Scholar] [CrossRef] [PubMed]
- Naveed, N.H.; Batool, A.I.; Rehman, F.; Hameed, U. Leaves of Roadside Plants as Bioindicator of Traffic Related Lead Pollution During Different Seasons In. Afr. J. Environ. Sci. Technol. 2010, 4, 770–774. [Google Scholar] [CrossRef]
- Peer, W.A.; Baxter, I.R.; Richards, E.L.; Freeman, J.L.; Murphy, A.S. Phytoremediation and Hyperaccumulator Plants. Top. Curr. Genet. 2005, 14, 299–340. [Google Scholar] [CrossRef]
- Statescu, F.; Cotiusca-Zauca, D. Heavy Metal Soil Contamination. Environ. Eng. Manag. J. 2018, 5, 1205–1213. [Google Scholar] [CrossRef]
- Rathinasabapathi, B. Ferns represent an untapped biodiversity for improving crops for environmental stress tolerance. New Phytol. 2006, 172, 385–390. [Google Scholar] [CrossRef]
- Harianja, A.H.; Saragih, G.S.; Fauzi, R.; Hidayat, M.Y.; Syofyan, Y.; Tapriziah, E.R.; Kartiningsih, S.E. Mercury Exposure in Artisanal and Small-Scale Gold Mining Communities in Sukabumi, Indonesia. J. Health Pollut. 2020, 10, 201209. [Google Scholar] [CrossRef]
- Junaidi, M.; Krisnayanti, B.D.; Juharfa; Anderson, C. Risk of Mercury Exposure from Fish Consumption at Artisanal Small-Scale Gold Mining Areas in West Nusa Tenggara, Indonesia. J. Health Pollut. 2019, 9, 190302. [Google Scholar] [CrossRef]
- Abbas, H.H.; Sakakibara, M.; Sera, K.; Arma, L.H. Mercury Exposure and Health Problems in Urban Artisanal Gold Mining (UAGM) in Makassar, South Sulawesi, Indonesia. Geosciences 2017, 7, 44. [Google Scholar] [CrossRef]
- Male, Y.T.; Reichelt-Brushett, A.J.; Pocock, M.; Nanlohy, A. Recent mercury contamination from artisanal gold mining on Buru Island, Indonesia—Potential future risks to environmental health and food safety. Mar. Pollut. Bull. 2013, 77, 428–433. [Google Scholar] [CrossRef]




| No. | Elements | |||||
|---|---|---|---|---|---|---|
| Concentration (μg/g) | Concentration (μg/g) | Concentration (μg/g) | ||||
| As | Error | Hg | Error | Pb | Error | |
| 1 | 15.0 | 24.0 | 11.0 | 17.0 | 46.0 | 18.0 |
| 2 | 1.80 | 4.20 | ND | ND | 70.0 | 16.0 |
| 3 | ND | ND | ND | ND | 90.0 | 31.0 |
| 4 | 4060 | 149 | ND | ND | 1700 | 178 |
| 5 | 92.0 | 8.80 | 10.3 | 17.0 | 60.0 | 32.0 |
| 6 | 40.0 | 29.0 | ND | ND | 8.20 | 18.0 |
| 7 | 401 | 21.0 | 33.0 | 19.0 | 29.0 | 59.0 |
| 8 | 6.30 | 5.50 | ND | ND | 92.0 | 21.0 |
| 9 | 199 | 14.0 | ND | ND | 47.0 | 48.0 |
| 10 | 110 | 11.0 | 18.0 | 22.0 | 58.0 | 39.0 |
| 11 | 11.0 | 7.50 | ND | ND | 74.0 | 29.0 |
| 12 | 3.20 | 11.0 | ND | ND | 159 | 42.0 |
| 14 | 61.0 | 49.0 | ND | ND | 25.0 | 33.0 |
| 15 | 6600 | 298 | ND | ND | 75.0 | 340 |
| 16 | 25.0 | 7.42 | ND | ND | 87.0 | 29.0 |
| 17 | 112 | 14.0 | 26.0 | 22.0 | 106 | 51.0 |
| 18 | 56.0 | 14.0 | ND | ND | 69.0 | 51.0 |
| 19 | 1.30 | 4.20 | 16.0 | 15.0 | 63.0 | 16.0 |
| 20 | 295 | 13.0 | ND | ND | 32.0 | 36.0 |
| 21 | 16.0 | 4.00 | ND | ND | 40.0 | 15.0 |
| 22 | 12.0 | 11.0 | ND | ND | 146 | 44.0 |
| 23 | 29.0 | 35.0 | ND | ND | 130 | 26.0 |
| 24 | 6.90 | 6.60 | 36.0 | 21.0 | 86.0 | 25.0 |
| 25 | 6.90 | 8.20 | ND | ND | 63.0 | 32.0 |
| 26 | 221 | 19.0 | ND | ND | 31.0 | 64.0 |
| 27 | 36,500 | 1376 | ND | ND | 11,400 | 735 |
| 28 | 49.0 | 29.0 | ND | ND | 73.0 | 19.0 |
| 29 | 218 | 19.0 | 20.0 | 27.0 | 170 | 65.0 |
| Metal/Metalloid | Soil Concentration Range mg kg−1 | Soil Regulatory Limits * mg kg−1 |
|---|---|---|
| As | 0–36,500 | 20 |
| Hg | 0–36 | 270 |
| Pb | 8–11,400 | 600 |
| Sample | As | Hg | Pb | |||
|---|---|---|---|---|---|---|
| Number | Concentrations mg kg−1 | RSD | Concentrations mg kg−1 | RSD | Concentrations mg kg−1 | RSD |
| 1 | 15,600 | 1.30 | 3.20 | 3.00 | 39.0 | 4.20 |
| 2 | 17,700 | 1.20 | 5.20 | 7.70 | 14.0 | 3.30 |
| 3 | 220 | 1.10 | 0.20 | 20.0 | 2.20 | 3.80 |
| 4 | 2810 | 5.50 | 0.90 | 5.60 | 22.0 | 4.80 |
| 5 | 570 | 1.60 | 0.40 | 4.10 | 0.30 | 1.20 |
| 6 | 5800 | 2.60 | 0.20 | 5.10 | 0.60 | 7.80 |
| 7 | 220 | 1.70 | 0.04 | 12.0 | 0.002 | 3.10 |
| 8 | 5800 | 0.50 | 0.30 | 21.0 | 4.10 | 2.20 |
| 9 | 14.0 | 0.90 | 0.01 | 10.0 | 0.002 | 1.40 |
| 10 | 32.0 | 18.0 | 0.20 | 11.0 | 0.90 | 1.50 |
| 11 | 2.50 | 2.10 | 0.60 | 5.00 | 0.10 | 3.00 |
| 12 | 570 | 1.60 | 0.40 | 4.00 | 3.60 | 1.20 |
| 13 | 77.0 | 0.40 | 0.45 | 7.00 | 0.50 | 1.60 |
| 14 | 32.0 | 0.70 | 0.02 | 24.0 | 0.03 | 5.00 |
| 15 | 690 | 1.50 | 0.80 | 3.00 | 0.90 | 1.10 |
| 16 | 160 | 0.90 | 0.20 | 4.00 | 0.53 | 1.50 |
| 17 | 2.80 | 1.10 | 0.01 | 17.0 | 0.02 | 1.20 |
| 18 | 140 | 1.30 | 0.04 | 7.10 | ND | ND |
| 19 | 39.0 | 0.90 | 0.14 | 19.0 | 0.90 | 1.70 |
| 20 | 69.0 | 0.80 | ND | ND | 0.70 | 2.50 |
| 21 | 39.0 | 2.00 | ND | ND | 0.04 | 1.10 |
| 22 | 71.0 | 3.10 | 0.10 | 8.20 | 0.30 | 2.40 |
| 23 | 3.10 | 0.70 | 0.01 | 7.20 | 0.004 | 3.10 |
| 24 | 8.30 | 2.00 | 0.01 | 6.50 | ND | ND |
| 25 | 47.0 | 1.90 | 0.00 | 0.79 | 4.80 | 26.0 |
| 26 | 1.30 | 1.33 | 0.02 | 14.2 | 0.03 | 1.30 |
| 27 | 130 | 0.90 | ND | ND | 0.70 | 0.90 |
| 28 | 95.0 | 3.10 | 0.20 | 5.89 | 3.80 | 1.80 |
| 29 | 8.80 | 0.80 | 0.10 | 6.87 | 0.20 | 8.00 |
| 30 | 5.40 | 0.90 | 0.01 | 15.5 | ND | 2.20 |
| 31 | 7.00 | 0.70 | 0.01 | 18.3 | 0.01 | 3.90 |
| 32 | 8.20 | 4.00 | 0.70 | 26.3 | 0.10 | 6.10 |
| 33 | 6.40 | 1.30 | 0.02 | 5.60 | ND | 2.40 |
| 34 | 95.0 | 0.90 | 0.20 | 3.90 | 3.82 | 2.00 |
| 35 | 34.0 | 1.20 | ND | ND | 0.10 | 17.0 |
| 36 | 153 | 0.80 | ND | ND | 0.90 | 1.40 |
| 37 | 2.10 | 1.20 | 0.01 | 15.0 | ND | 6.40 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gafur, N.A.; Sakakibara, M.; Komatsu, S.; Sano, S.; Sera, K. Environmental Survey of the Distribution and Metal Contents of Pteris vittata in Arsenic–Lead–Mercury-Contaminated Gold Mining Areas along the Bone River in Gorontalo Province, Indonesia. Int. J. Environ. Res. Public Health 2022, 19, 530. https://doi.org/10.3390/ijerph19010530
Gafur NA, Sakakibara M, Komatsu S, Sano S, Sera K. Environmental Survey of the Distribution and Metal Contents of Pteris vittata in Arsenic–Lead–Mercury-Contaminated Gold Mining Areas along the Bone River in Gorontalo Province, Indonesia. International Journal of Environmental Research and Public Health. 2022; 19(1):530. https://doi.org/10.3390/ijerph19010530
Chicago/Turabian StyleGafur, Nurfitri Abdul, Masayuki Sakakibara, Satoru Komatsu, Sakae Sano, and Koichiro Sera. 2022. "Environmental Survey of the Distribution and Metal Contents of Pteris vittata in Arsenic–Lead–Mercury-Contaminated Gold Mining Areas along the Bone River in Gorontalo Province, Indonesia" International Journal of Environmental Research and Public Health 19, no. 1: 530. https://doi.org/10.3390/ijerph19010530
APA StyleGafur, N. A., Sakakibara, M., Komatsu, S., Sano, S., & Sera, K. (2022). Environmental Survey of the Distribution and Metal Contents of Pteris vittata in Arsenic–Lead–Mercury-Contaminated Gold Mining Areas along the Bone River in Gorontalo Province, Indonesia. International Journal of Environmental Research and Public Health, 19(1), 530. https://doi.org/10.3390/ijerph19010530







