Evaluating In Silico the Potential Health and Environmental Benefits of Houseplant Volatile Organic Compounds for an Emerging ‘Indoor Forest Bathing’ Approach
Abstract
:1. Introduction
2. Materials and Methods
2.1. Literature Analysis
2.2. Molecular Docking
2.3. Prediction of Pharmacokinetic Properties and Vapor Pressures
3. Results and Discussion
3.1. Houseplant-Emitted Volatile Organic Compounds
3.2. In Silico Analysis of the Main Houseplant Volatile Organic Compounds: Vapor Pressures, Chemico-Physical/Pharmacokinetic Properties, and SARS-CoV-2 Mpro Inhibitory Potential Activities
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Furuyashiki, A.; Tabuchi, K.; Norikoshi, K.; Kobayashi, T.; Oriyama, S. A comparative study of the physiological and psychological effects of forest bathing (Shinrin-yoku) on working age people with and without depressive tendencies. Environ. Health Prev. Med. 2019, 24, 46. [Google Scholar] [CrossRef] [PubMed]
- Bielinis, E.; Takayama, N.; Boiko, S.; Omelan, A.; Bielinis, L. The effect of winter forest bathing on psychological relaxation of young Polish adults. Urban For. Urban Green. 2018, 29, 276–283. [Google Scholar] [CrossRef]
- Peterfalvi, A.; Meggyes, M.; Makszin, L.; Farkas, N.; Miko, E.; Miseta, A.; Szereday, L. Forest Bathing Always Makes Sense: Blood Pressure-Lowering and Immune System-Balancing Effects in Late Spring and Winter in Central Europe. Int. J. Environ. Res. Public Health 2021, 18, 2067. [Google Scholar] [CrossRef] [PubMed]
- Van den Berg, A.E. From Green Space to Green Prescriptions: Challenges and Opportunities for Research and Practice. Front. Psychol. 2017, 8, 268. [Google Scholar] [CrossRef] [Green Version]
- Antonelli, M.; Donelli, D.; Barbieri, G.; Valussi, M.; Maggini, V.; Firenzuoli, F. Forest Volatile Organic Compounds and Their Effects on Human Health: A State-of-the-Art Review. Int. J. Environ. Res. Public Health 2020, 17, 6506. [Google Scholar] [CrossRef]
- Roviello, V.; Roviello, G.N. Less COVID-19 deaths in southern and insular Italy explained by forest bathing, Mediterranean environment, and antiviral plant volatile organic compounds. Environ. Chem. Lett. 2021, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Roviello, V.; Roviello, G.N. Lower COVID-19 mortality in Italian forested areas suggests immunoprotection by Mediterranean plants. Environ. Chem. Lett. 2021, 19, 699–710. [Google Scholar] [CrossRef]
- Roviello, V.; Gilhen-Baker, M.; Vicidomini, C.; Roviello, G.N. Forest-bathing and physical activity as weapons against COVID-19: A review. Environ. Chem. Lett. 2021, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Nieuwenhuis, M.; Knight, C.; Postmes, T.; Haslam, S.A. The relative benefits of green versus lean office space: Three field experiments. J. Exp. Psychol. Appl. 2014, 20, 199–214. [Google Scholar] [CrossRef] [Green Version]
- Kalantzis, A. The Impact of Indoor Plants on Well-Being in the Workplace. Ph.D. Thesis, University of the Witwatersrand, Faculty of Humanities, School of Human and Community Development, Johannesburg, South Africa, 2016. [Google Scholar]
- Kim, H.-H.; Yang, J.-Y.; Lee, J.-Y.; Park, J.-W.; Kim, K.-J.; Lim, B.-S.; Lee, G.-W.; Lee, S.-E.; Shin, D.-C.; Lim, Y.-W. House-plant placement for indoor air purification and health benefits on asthmatics. Environ. Health Toxicol. 2014, 29, e2014014. [Google Scholar] [CrossRef]
- Zhang, H.; Pennisi, S.V.; Kays, S.J.; Habteselassie, M.Y. Isolation and Identification of Toluene-Metabolizing Bacteria from Rhizospheres of Two Indoor Plants. Water Air Soil Pollut. 2013, 224, 1648. [Google Scholar] [CrossRef]
- Yang, D.S.; Son, K.-C.; Kays, S.J. Volatile Organic Compounds Emanating from Indoor Ornamental Plants. HortScience 2009, 44, 396–400. [Google Scholar] [CrossRef] [Green Version]
- Gubb, C.; Blanusa, T.; Griffiths, A.; Pfrang, C. Can houseplants improve indoor air quality by removing CO2 and increasing relative humidity? Air Qual. Atmos. Health 2018, 11, 1191–1201. [Google Scholar] [CrossRef] [Green Version]
- Cummings, B.E.; Waring, M.S. Potted plants do not improve indoor air quality: A review and analysis of reported VOC removal efficiencies. J. Expo. Sci. Environ. Epidemiol. 2019, 30, 253–261. [Google Scholar] [CrossRef] [Green Version]
- Chang, L.-T.; Hong, G.-B.; Weng, S.-P.; Chuang, H.-C.; Chang, T.-Y.; Liu, C.-W.; Chuang, W.-Y.; Chuang, K.-J. Indoor ozone levels, houseplants and peak expiratory flow rates among healthy adults in Taipei, Taiwan. Environ. Int. 2019, 122, 231–236. [Google Scholar] [CrossRef]
- Abbass, O.A.; Sailor, D.J.; Gall, E.T. Effectiveness of indoor plants for passive removal of indoor ozone. Build. Environ. 2017, 119, 62–70. [Google Scholar] [CrossRef]
- Dudareva, N.; Pichersky, E.; Gershenzon, J. Biochemistry of plant volatiles. Plant Physiol. 2004, 135, 1893–1902. [Google Scholar] [CrossRef] [Green Version]
- Pichersky, E.; Noel, J.P.; Dudareva, N. Biosynthesis of plant volatiles: Nature’s diversity and ingenuity. Science 2006, 311, 808–811. [Google Scholar] [CrossRef] [Green Version]
- Kays, S.J.; Hatch, J.; Yang, D.S. Volatile floral chemistry of Heliotropium arborescens L. ‘Marine’. HortScience 2005, 40, 1237–1238. [Google Scholar] [CrossRef]
- Ciotti, M.; Ciccozzi, M.; Terrinoni, A.; Jiang, W.-C.; Wang, C.-B.; Bernardini, S. The COVID-19 pandemic. Crit. Rev. Clin. Lab. Sci. 2020, 57, 365–388. [Google Scholar] [CrossRef] [PubMed]
- Berman, H.M.; Battistuz, T.; Bhat, T.N.; Bluhm, W.F.; Bourne, P.E.; Burkhardt, K.; Feng, Z.; Gilliland, G.L.; Iype, L.; Jain, S.; et al. The Protein Data Bank. Acta Crystallogr. Sect. D Biol. Crystallogr. 2002, 58, 899–907. [Google Scholar] [CrossRef]
- Potemkin, V.; Potemkin, A.; Grishina, M. Internet Resources for Drug Discovery and Design. Curr. Top. Med. Chem. 2019, 18, 1955–1975. [Google Scholar] [CrossRef] [PubMed]
- Fik-Jaskółka, M.A.; Mkrtchyan, A.F.; Saghyan, A.S.; Palumbo, R.; Belter, A.; Hayriyan, L.A.; Simonyan, H.; Roviello, V.; Roviello, G.N. Biological macromolecule binding and anticancer activity of synthetic alkyne-containing l-phenylalanine derivatives. Amino Acids 2020, 52, 755–769. [Google Scholar] [CrossRef] [PubMed]
- Fik-Jaskółka, M.A.; Mkrtchyan, A.F.; Saghyan, A.S.; Palumbo, R.; Belter, A.; Hayriyan, L.A.; Simonyan, H.; Roviello, V.; Roviello, G.N. Spectroscopic and SEM evidences for G4-DNA binding by a synthetic alkyne-containing amino acid with anticancer activity. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 229, 117884. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2009, 31, 455–461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Umesh; Kundu, D.; Selvaraj, C.; Singh, S.K.; Dubey, V.K. Identification of new anti-nCoV drug chemical compounds from Indian spices exploiting SARS-CoV-2 main protease as target. J. Biomol. Struct. Dyn. 2020, 39, 3428–3434. [Google Scholar]
- Adasme, M.F.; Linnemann, K.L.; Bolz, S.N.; Kaiser, F.; Salentin, S.; Haupt, V.J.; Schroeder, M. PLIP 2021: Expanding the scope of the protein–ligand interaction profiler to DNA and RNA. Nucleic Acids 2021, 49, W530–W534. [Google Scholar] [CrossRef]
- Feng, Q.; Gao, Q.-Y.; Zhang, R.-M.; Gao, Y.; Hou, P. Constituent analysis of volatile organic compounds in three Liliaceae. J. Zhejiang AF Univ. 2011, 28, 513–518. [Google Scholar]
- A-jun, G.; Wang, Z.-Y. Inhibition of 9 Indoor Plants Against 4 Microorganism. North. Hortic. 2007, 8, 128–130. [Google Scholar]
- Lipinski, C.A. Lead- and drug-like compounds: The rule-of-five revolution. Drug Discov. Today Technol. 2004, 1, 337–341. [Google Scholar] [CrossRef]
- Rizzuti, B.; Ceballos-Laita, L.; Ortega-Alarcon, D.; Jimenez-Alesanco, A.; Vega, S.; Grande, F.; Conforti, F.; Abian, O.; Velazquez-Campoy, A. Sub-Micromolar Inhibition of SARS-CoV-2 3CLpro by Natural Compounds. Pharmaceuticals 2021, 14, 892. [Google Scholar] [CrossRef] [PubMed]
- Mancini, E.; De Martino, L.; Marandino, A.; Scognamiglio, M.R.; De Feo, V. Chemical Composition and Possible in Vitro Phytotoxic Activity of Helichrsyum italicum (Roth) Don ssp. italicum. Molecules 2011, 16, 7725–7735. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dhinakaran, D.I.; Lipton, A.P. Bioactive compounds from Holothuria atra of Indian ocean. SpringerPlus 2014, 3, 673. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharbidre, A.; Dhage, P.; Duggal, H.; Meshram, R. In silico Investigation of Tridax procumbens Phyto-Constituents Against SARS-CoV-2 Infection. Biointerface Res. Appl. Chem. 2021, 11, 12120–12148. [Google Scholar]
- Hiremath, S.; Kumar, H.D.V.; Nandan, M.; Mantesh, M.; Shankarappa, K.S.; Venkataravanappa, V.; Basha, C.R.J.; Reddy, C.N.L. In silico docking analysis revealed the potential of phytochemicals present in Phyllanthus amarus and Andrographis paniculata, used in Ayurveda medicine in inhibiting SARS-CoV-2. 3 Biotech 2021, 11, 44. [Google Scholar] [CrossRef]
- Lambert, H.; Mohan, N.; Lee, T.-C. Ultrahigh binding affinity of a hydrocarbon guest inside cucurbit[7]uril enhanced by strong host–guest charge matching. Phys. Chem. Chem. Phys. 2019, 21, 14521–14529. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- My, T.T.A.; Loan, H.T.P.; Hai, N.T.T.; Hieu, L.T.; Hoa, T.T.; Thuy, B.T.P.; Quang, D.T.; Triet, N.T.; Anh, T.T.V.; Dieu, N.T.X.; et al. Evaluation of the Inhibitory Activities of COVID-19 of Melaleuca cajuputi Oil Using Docking Simulation. ChemistrySelect 2020, 5, 6312–6320. [Google Scholar] [CrossRef]
- Llivisaca-Contreras, S.A.; Naranjo-Morán, J.; Pino-Acosta, A.; Pieters, L.; Vanden Berghe, W.; Manzano, P.; Vargas-Pérez, J.; León-Tamariz, F.; Cevallos-Cevallos, J.M. Plants and Natural Products with Activity against Various Types of Coronaviruses: A Review with Focus on SARS-CoV-2. Molecules 2021, 26, 4099. [Google Scholar] [CrossRef]
- Panikar, S.; Shoba, G.; Arun, M.; Sahayarayan, J.J.; Usha Raja Nanthini, A.; Chinnathambi, A.; Alharbi, S.A.; Nasif, O.; Kim, H.-J. Essential oils as an effective alternative for the treatment of COVID-19: Molecular interaction analysis of protease (Mpro) with pharmacokinetics and toxicological properties. J. Infect. Public Health 2021, 14, 601–610. [Google Scholar] [CrossRef] [PubMed]
- Asif, M.; Saleem, M.; Saadullah, M.; Yaseen, H.S.; Al Zarzour, R. COVID-19 and therapy with essential oils having antiviral, anti-inflammatory, and immunomodulatory properties. Inflammopharmacology 2020, 28, 1153–1161. [Google Scholar] [CrossRef]
- Li, Q. Effect of forest bathing trips on human immune function. Environ. Health Prev. Med. 2009, 15, 9–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Erickson, M.A.; Rhea, E.M.; Knopp, R.C.; Banks, W.A. Interactions of SARS-CoV-2 with the Blood–Brain Barrier. Int. J. Mol. Sci. 2021, 22, 2681. [Google Scholar] [CrossRef] [PubMed]

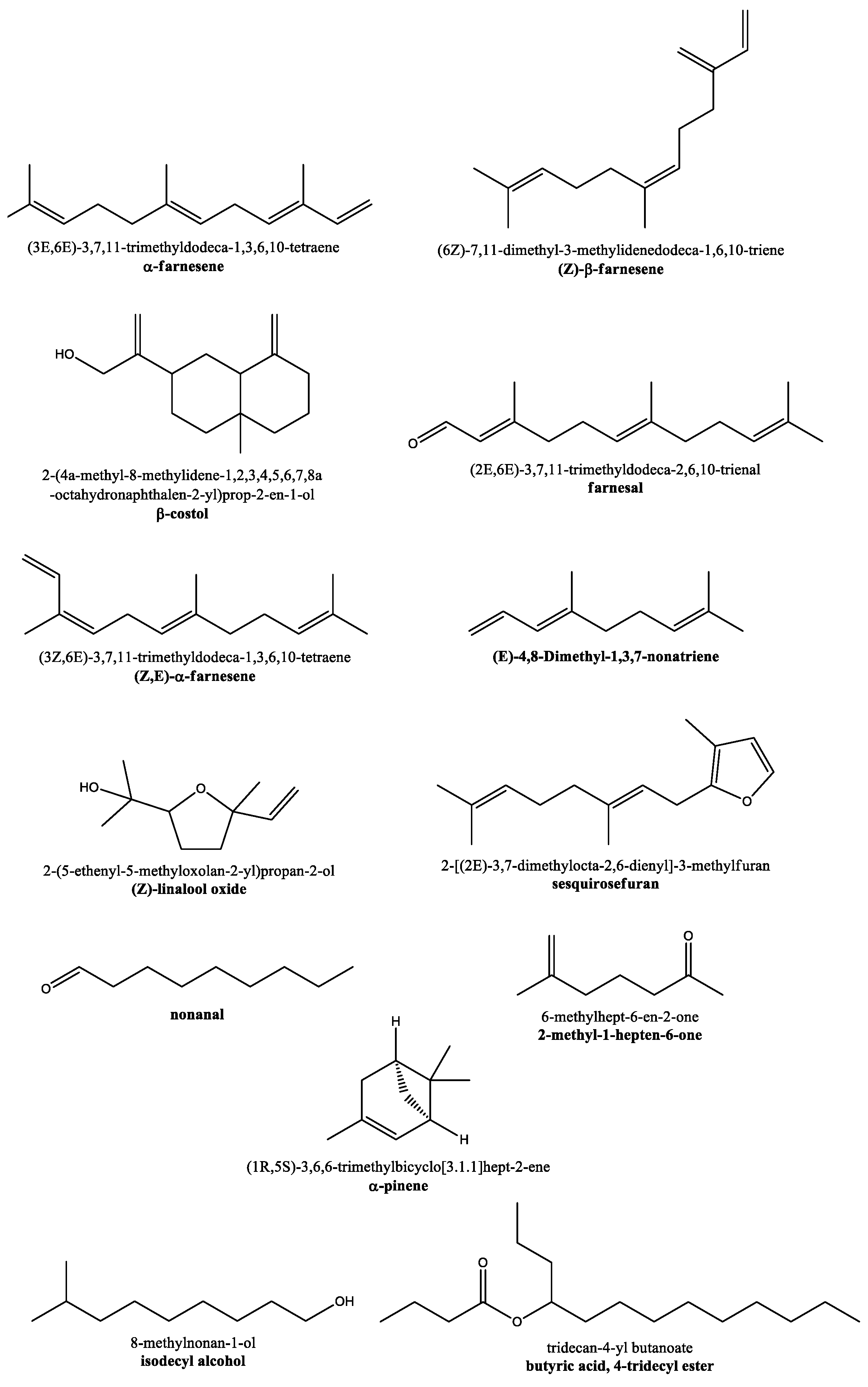
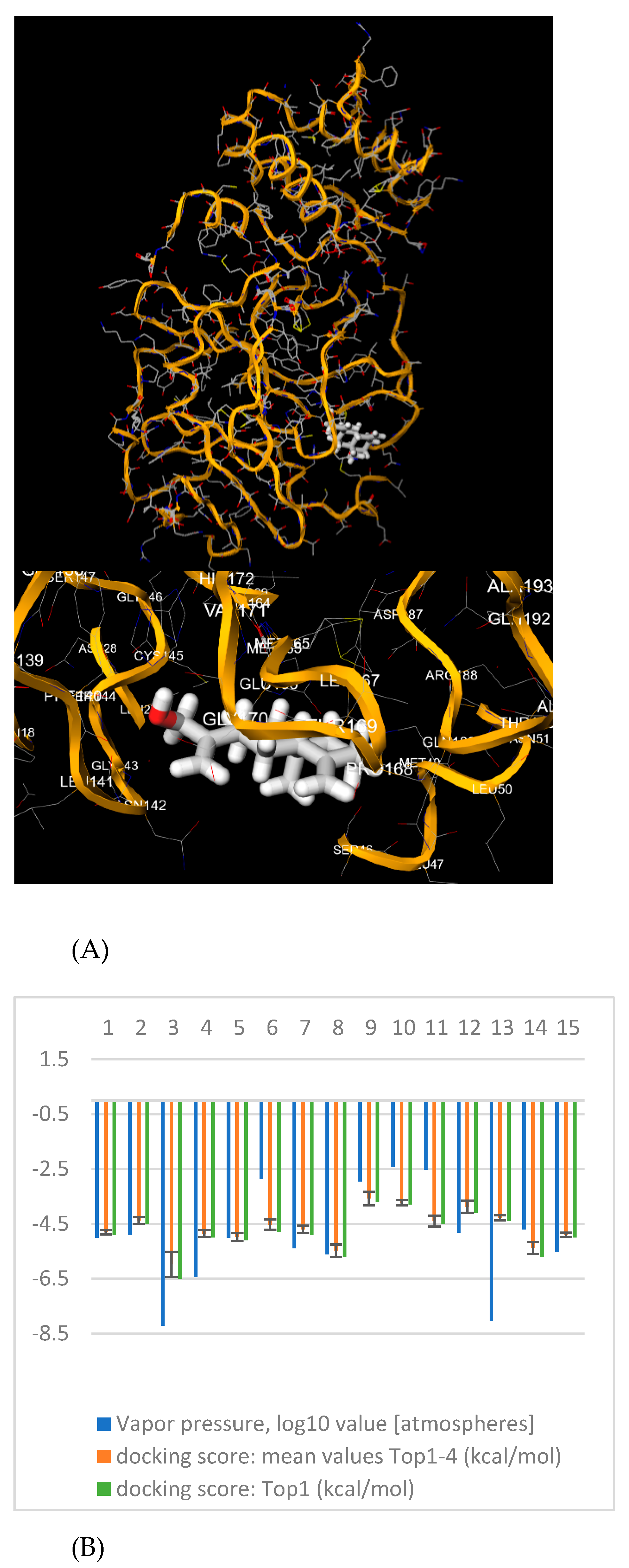

| Comp. | SMILES | Vapor Pressure, log10 at 25 °C * | cLogP | BBB Perm. | Druglikeness (Lipinski—n. Violations) | PAINS | Docking Score: Top-1 Ranked Pose (kcal/mol) | Docking Score: Mean Value ± S.D. Top 1–4 Poses (kcal/mol) |
|---|---|---|---|---|---|---|---|---|
| α-farnesene (1) | CC(=CCC/C(=C/C/C=C(\C)/C=C)/C)C | −5.008 | 4.96 | N | Y (1) | N | −4.9 | −4.80 ± 0.08 |
| (Z)-β-farnesene (2) | CC(=CCC/C(=C\CCC(=C)C=C)/C)C | −4.885 | 4.97 | N | Y (1) | N | −4.5 | −4.38 ± 0.13 |
| β-costol (3) | CC12CCCC(=C)C1CC(CC2)C(=C)CO | −8.215 | 3.66 | Y | Y (0) | N | −6.5 | −5.98 ± 0.46 |
| farnesal (4) | CC(=CCC/C(=C/CC/C(=C/C=O)/C)/C)C | −6.437 | 3.66 | Y | Y (0) | N | −5.0 | −4.85 ± 0.13 |
| (Z,E)-α-farnesene (5) | CC(=CCC/C(=C/C/C=C(/C)\C=C)/C)C | −5.008 | 3.66 | Y | Y (0) | N | −5.1 | −4.98 ± 0.15 |
| (E)-4,8-dimethyl-1,3,7-nonatriene (6) | CC(=CCC/C(=C/C=C)/C)C | −2.864 | 3.75 | Y | Y (0) | N | −4.8 | −4.52 ± 0.19 |
| (Z)-linalool oxide (7) | C[C@]1(CC[C@H](O1)C(C)(C)O)C=C | −5.389 | 2.05 | Y | Y (0) | N | −4.9 | −4.70 ± 0.14 |
| Sesquirosefuran (8) | CC1=C(OC=C1)C/C=C(\C)/CCC=C(C)C | −5.610 | 4.36 | Y | Y (0) | N | −5.7 | −5.48 ± 0.22 |
| Nonanal (9) | CCCCCCCCC=O | −2.957 | 2.78 | Y | Y (0) | N | −3.7 | −3.58 ± 0.25 |
| 2-methyl-1-hepten-6-one (10) | CC(=C)CCCC(=O)C | −2.436 | 2.11 | Y | Y (0) | N | −3.8 | −3.73 ± 0.10 |
| α-pinene (11) | CC1=C[C@H]2C[C@@H](C1)C2(C)C | −2.523 | 3.06 | Y | Y (1) | N | −4.5 | −4.40 ± 0.20 |
| isodecyl alcohol (12) | CC(C)CCCCCCCO | −4.821 | 3.44 | Y | Y (0) | N | −4.1 | −3.88 ± 0.22 |
| butyric acid, 4-tridecyl ester (13) | CCCCCCCCCCCC(C)OC(=O)CCC | −8.032 | 5.42 | Y | Y (1) | N | −4.4 | −4.28 ± 0.10 |
| umbelliferone (14) | C1=CC(=CC2=C1C=CC(=O)O2)O | −4.705 | 1.51 | Y | Y (0) | N | −5.7 | −5.38 ± 0.22 |
| eugenol (15) | COC1=C(C=CC(=C1)CC=C)O | −5.531 | 2.25 | Y | Y (0) | N | −5.0 | −4.90 ± 0.08 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roviello, V.; Scognamiglio, P.L.; Caruso, U.; Vicidomini, C.; Roviello, G.N. Evaluating In Silico the Potential Health and Environmental Benefits of Houseplant Volatile Organic Compounds for an Emerging ‘Indoor Forest Bathing’ Approach. Int. J. Environ. Res. Public Health 2022, 19, 273. https://doi.org/10.3390/ijerph19010273
Roviello V, Scognamiglio PL, Caruso U, Vicidomini C, Roviello GN. Evaluating In Silico the Potential Health and Environmental Benefits of Houseplant Volatile Organic Compounds for an Emerging ‘Indoor Forest Bathing’ Approach. International Journal of Environmental Research and Public Health. 2022; 19(1):273. https://doi.org/10.3390/ijerph19010273
Chicago/Turabian StyleRoviello, Valentina, Pasqualina Liana Scognamiglio, Ugo Caruso, Caterina Vicidomini, and Giovanni N. Roviello. 2022. "Evaluating In Silico the Potential Health and Environmental Benefits of Houseplant Volatile Organic Compounds for an Emerging ‘Indoor Forest Bathing’ Approach" International Journal of Environmental Research and Public Health 19, no. 1: 273. https://doi.org/10.3390/ijerph19010273
APA StyleRoviello, V., Scognamiglio, P. L., Caruso, U., Vicidomini, C., & Roviello, G. N. (2022). Evaluating In Silico the Potential Health and Environmental Benefits of Houseplant Volatile Organic Compounds for an Emerging ‘Indoor Forest Bathing’ Approach. International Journal of Environmental Research and Public Health, 19(1), 273. https://doi.org/10.3390/ijerph19010273










