Disparity between Perceptual Fall Risk and Physiological Fall Risk in Older Cannabis Users: A Pilot Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Protocol
2.2. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cigolle, C.T.; Langa, K.M.; Kabeto, M.U.; Tian, Z.; Blaum, C.S. Geriatric conditions and disability: The health and retirement study. Ann. Intern. Med. 2007, 147, 156–164. [Google Scholar] [CrossRef]
- Tinetti, M.E.; Williams, C.S. The effect of falls and fall injuries on functioning in community-dwelling older persons. J. Gerontol. A Biol. Sci. Med. Sci. 1998, 53, M112–M119. [Google Scholar] [CrossRef]
- Montero-Odasso, M.; Verghese, J.; Beauchet, O.; Hausdorff, J.M. Gait and cognition: A complementary approach to understanding brain function and the risk of falling. J. Am. Geriatr. Soc. 2012, 60, 2127–2136. [Google Scholar] [CrossRef] [Green Version]
- Tinetti, M.E.; Speechley, M.; Ginter, S.F. Risk factors for falls among elderly persons living in the community. N. Engl. J. Med. 1988, 319, 1701–1707. [Google Scholar] [CrossRef]
- Solowij, N. Cannabis and Cognitive Functioning; Cambridge University Press: Cambridge, UK, 2006. [Google Scholar]
- Auer, R.; Vittinghoff, E.; Yaffe, K.; Künzi, A.; Kertesz, S.G.; Levine, D.A.; Albanese, E.; Whitmer, R.A.; Jacobs, D.R.; Sidney, S.; et al. Association between lifetime marijuana use and cognitive function in middle age: The coronary artery risk development in young adults (CARDIA) study. JAMA Intern. Med. 2016, 176, 352–361. [Google Scholar] [CrossRef] [Green Version]
- Grant, I.; Gonzalez, R.; Carey, C.L.; Natarajan, L.; Wolfson, T. Non-acute (residual) neurocognitive effects of cannabis use: A meta-analytic study. J. Int. Neuropsychol. Soc. 2003, 9, 679–689. [Google Scholar] [CrossRef]
- Meier, M.H.; Caspi, A.; Ambler, A.; Harrington, H.; Houts, R.; Keefe, R.S.E.; McDonald, K.; Ward, A.; Poulton, R.; Moffitt, T. Persistent cannabis users show neuropsychological decline from childhood to midlife. Proc. Natl. Acad. Sci. USA 2012, 109, E2657–E2664. [Google Scholar] [CrossRef] [Green Version]
- Solowij, N.; Stephens, R.S.; Roffman, R.A.; Babor, T.; Kadden, R.; Miller, M.; Christiansen, K.; McRee, B.; Vendetti, J. Cognitive functioning of long-term heavy cannabis users seeking treatment. JAMA 2002, 287, 1123–1131. [Google Scholar] [CrossRef] [Green Version]
- Sakurai, R.; Fujiwara, Y.; Yasunaga, M.; Suzuki, H.; Kanosue, K.; Montero-Odasso, M.; Ishii, K. Association between hypometabolism in the supplementary motor area and fear of falling in older adults. Front. Aging Neurosci. 2017, 9, 251. [Google Scholar] [CrossRef] [Green Version]
- Tuerk, C.; Zhang, H.; Sachdev, P.; Lord, S.R.; Brodaty, H.; Wen, W.; Delbaere, K. Regional gray matter volumes are related to concern about falling in older people: A voxel-based morphometric study. J. Gerontol. A Biol. Sci. Med. Sci. 2016, 71, 138–144. [Google Scholar] [CrossRef] [Green Version]
- Mechoulam, R.; Gaoni, Y. Recent advances in the chemistry of hashish. Fortschr. Chem. Org. Naturst. 1967, 25, 175–213. [Google Scholar] [CrossRef]
- Bhattacharyya, S.; Atakan, Z.; Martin-Santos, R.; Crippa, J.A.; McGuire, P.K. Neural mechanisms for the cannabinoid modulation of cognition and affect in man: A critical review of neuroimaging studies. Curr. Pharm. Des. 2012, 18, 5045–5054. [Google Scholar] [CrossRef] [PubMed]
- Bloomfield, M.A.P.; Hindocha, C.; Green, S.F.; Wall, M.B.; Lees, R.; Petrilli, K.; Costello, H.; Ogunbiyi, M.O.; Bossong, M.G.; Freeman, T.P. The neuropsychopharmaogy of cannabis: A review of human imaging studies. Pharmacol. Ther. 2019, 195, 132–161. [Google Scholar] [CrossRef] [PubMed]
- Colizzi, M.; Bhattacharyya, S. Does cannabis composition matter? Differential effects of delta-9-tetrahydrocannabinol and cannabidiol on human cognition. Curr. Addict. Rep. 2017, 4, 62–74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ebbert, J.O.; Scharf, E.L.; Hurt, R.T. Medical cannabis. Mayo Clin. Proc. 2018, 93, 1842–1847. [Google Scholar] [CrossRef] [Green Version]
- Han, B.H.; Sherman, S.; Mauro, P.M.; Martins, S.S.; Rotenberg, J.; Palamar, J.J. Demographic trends among older cannabis users in the United States, 2006–2013. Addiction 2017, 112, 516–525. [Google Scholar] [CrossRef]
- Romero-Sandoval, E.A.; Kolano, A.L.; Alvarado-Vázquez, P.A. Cannabis and cannabinoids for chronic pain. Curr. Rheumatol. Rep. 2017, 19, 67. [Google Scholar] [CrossRef] [PubMed]
- Delbaere, K.; Close, J.C.; Brodaty, H.; Sachdev, P.; Lord, S.R. Determinants of disparities between perceived and physiological risk of falling among elderly people: Cohort study. BMJ 2010, 341, c4165. [Google Scholar] [CrossRef] [PubMed]
- Workman, C.D.; Fietsam, A.C.; Sosnoff, J.; Rudroff, T. Increased likelihood of falling in older cannabis users vs. non-users. Brain Sci. 2021, 11, 134. [Google Scholar] [CrossRef]
- Goodman, S.; Wadsworth, E.; Schauer, G.; Hammond, D. Use and perceptions of cannabidiol products in Canada and in the United States. Cannabis Cannabinoid Res. 2020. [Google Scholar] [CrossRef]
- Kaufmann, C.N.; Kim, A.; Miyoshi, M.; Han, B.H. Patterns of medical cannabis use among older adults from a cannabis dispensary in New York state. Cannabis Cannabinoid Res. 2020. [Google Scholar] [CrossRef] [PubMed]
- Lajoie, Y.; Gallagher, S.P. Predicting falls within the elderly community: Comparison of postural sway, reaction time, the Berg Balance Scale and the Activities-specific Balance Confidence (ABC) scale for comparing fallers and non-fallers. Arch. Gerontol. Geriatr. 2004, 38, 11–26. [Google Scholar] [CrossRef]
- Powell, L.E.; Myers, A.M. The Activities-specific Balance Confidence (ABC) scale. J. Gerontol. A Biol. Sci. Med. Sci. 1995, 50, M28–M34. [Google Scholar] [CrossRef] [PubMed]
- Berg, K.O.; Wood-Dauphinee, S.L.; Williams, J.I.; Maki, B. Measuring balance in the elderly: Validation of an instrument. Can. J. Public Health 1992, 83 (Suppl. 2), S7–S11. [Google Scholar]
- Landers, M.R.; Durand, C.; Powell, D.S.; Dibble, L.E.; Young, D.L. Development of a scale to assess avoidance behavior due to a fear of falling: The fear of falling avoidance behavior questionnaire. Phys. Ther. 2011, 91, 1253–1265. [Google Scholar] [CrossRef] [Green Version]
- Hatch, J.; Gill-Body, K.M.; Portney, L.G. Determinants of balance confidence in community-dwelling elderly people. Phys. Ther. 2003, 83, 1072–1079. [Google Scholar] [CrossRef] [Green Version]
- McGraw, K.O.; Wong, S.P. A common language effect size statistic. Psychol. Bull. 1992, 111, 361–365. [Google Scholar] [CrossRef]
- Ruscio, J. A probability-based measure of effect size: Robustness to base rates and other factors. Psychol. Methods 2008, 13, 19–30. [Google Scholar] [CrossRef] [Green Version]
- Schober, P.; Boer, C.; Schwarte, L.A. Correlation coefficients: Appropriate use and interpretation. Anesth. Analg. 2018, 126, 1763–1768. [Google Scholar] [CrossRef]
- Hindocha, C.; Freeman, T.; Schafer, G.; Gardener, C.; Das, R.K.; Morgan, C.J.; Curran, H.V. Acute effects of delta-9-tetrahydrocannabinol, cannabidiol and their combination on facial emotion recognition: A randomised, double-blind, placebo-controlled study in cannabis users. Eur. Neuropsychopharmacol. 2015, 25, 325–334. [Google Scholar] [CrossRef] [Green Version]
- Bhattacharyya, S.; Morrison, P.D.; Fusar-Poli, P.; Martin-Santos, R.; Borgwardt, S.; Wintonbrown, T.T.; Nosarti, C.; Carroll, C.M.O.; Seal, M.L.; Allen, P.; et al. Opposite effects of delta-9-tetrahydrocannabinol and cannabidiol on human brain function and psychopathology. Neuropsychopharmacology 2010, 35, 764–774. [Google Scholar] [CrossRef]
- Martin-Santos, R.; Crippa, J.A.; Batalla, A.; Bhattacharyya, S.; Atakan, Z.; Borgwardt, S.; Allen, P.; Seal, M.; Langohr, K.; Farre, M.; et al. Acute effects of a single, oral dose of d9-tetrahydrocannabinol (THC) and cannabidiol (CBD) administration in healthy volunteers. Curr. Pharm. Des. 2012, 18, 4966–4979. [Google Scholar] [CrossRef]
- Russo, E.; Guy, G.W. A tale of two cannabinoids: The therapeutic rationale for combining tetrahydrocannabinol and cannabidiol. Med. Hypotheses 2006, 66, 234–246. [Google Scholar] [CrossRef]
- Arkell, T.R.; Lintzeris, N.; Kevin, R.C.; Ramaekers, J.G.; Vandrey, R.; Irwin, C.; Haber, P.S.; McGregor, I.S. Cannabidiol (CBD) content in vaporized cannabis does not prevent tetrahydrocannabinol (THC)-induced impairment of driving and cognition. Psychopharmacology 2019, 236, 2713–2724. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caserta, M.T.; Bannon, Y.; Fernandez, F.; Giunta, B.; Schoenberg, M.R.; Tan, J. Normal brain aging clinical, immunological, neuropsychological, and neuroimaging features. Int. Rev. Neurobiol. 2009, 84, 1–19. [Google Scholar] [CrossRef]
- Nordstrom, B.R.; Hart, C.L. Assessing cognitive functioning in cannabis users: Cannabis use history an important consideration. Neuropsychopharmacology 2006, 31, 2798–2799. [Google Scholar] [CrossRef]
- Ramaekers, J.; Moeller, M.; Van Ruitenbeek, P.; Theunissen, E.; Schneider, E.; Kauert, G. Cognition and motor control as a function of delta9-THC concentration in serum and oral fluid: Limits of impairment. Drug Alcohol Depend. 2006, 85, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Perez-Reyes, M.; Di Guiseppi, S.; Davis, K.H.; Schindler, V.H.; Cook, C.E. Comparison of effects of marihuana cigarettes to three different potencies. Clin. Pharmacol. Ther. 1982, 31, 617–624. [Google Scholar] [CrossRef]
- Huestis, M.A. Human cannabinoid pharmacokinetics. Chem. Biodivers. 2007, 4, 1770–1804. [Google Scholar] [CrossRef] [Green Version]
- Velayudhan, L.; McGoohan, K.L.; Bhattacharyya, S. Evaluation of THC-related neuropsychiatric symptoms among adults aged 50 years and older: A systematic review and metaregression analysis. JAMA Netw. Open 2021, 4, e2035913. [Google Scholar] [CrossRef] [PubMed]
- Maki, B.E.; Holliday, P.J.; Topper, A.K. Fear of falling and postural performance in the elderly. J. Gerontol. 1991, 46, M123–M131. [Google Scholar] [CrossRef] [PubMed]
- Montesinos, L.; Castaldo, R.; Pecchia, L. On the use of approximate entropy and sample entropy with centre of pressure time-series. J. Neuroeng. Rehabil. 2018, 15, 116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yardley, L.; Beyer, N.; Hauer, K.; Kempen, G.; Piot-Ziegler, C.; Todd, C. Development and initial validation of the Falls Efficacy Scale-International (FES-I). Age Ageing 2005, 34, 614–619. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nasreddine, Z.S.; Phillips, N.A.; Bédirian, V.; Charbonneau, S.; Whitehead, V.; Collin, I.; Cummings, J.L.; Chertkow, H. The Montreal Cognitive Assessment, MoCA: A brief screening tool for mild cognitive impairment. J. Am. Geriatr. Soc. 2005, 53, 695–699. [Google Scholar] [CrossRef]
- van Haastregt, J.C.; Zijlstra, G.A.; van Rossum, E.; van Eijk, J.T.; Kempen, G.I. Feelings of anxiety and symptoms of depression in community-living older persons who avoid activity for fear of falling. Am. J. Geriatr Psychiatry. 2008, 16, 186–193. [Google Scholar] [CrossRef]
- Mann, R.; Birks, Y.; Hall, J.; Torgerson, D.; Watt, I. Exploring the relationship between fear of falling and neuroticism: A cross-sectional study in community-dwelling women over 70. Age Ageing 2006, 35, 143–147. [Google Scholar] [CrossRef] [Green Version]
- Holtzer, R.; Friedman, R.; Lipton, R.B.; Katz, M.; Xue, X.; Verghese, J. The relationship between specific cognitive functions and falls in aging. Neuropsychology 2007, 21, 540–548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Demographics | Non-Users | Users |
|---|---|---|
| Sex (M/F) | 3/5 | 3/5 |
| Age (years) | 61.0 (53.0–66.0) | 60.0 (52.0–66.0) |
| Height (cm) | 167.6 (157.5–185.4) | 167.6 (152.4–185.4) |
| Weight (kg) | 80.5 (59–124.7) | 94.1 (60.1–127) |
| Duration of Cannabis Use (years) | n/a | 4.5 (0.6–30) |
| Uses per week (days) | n/a | 5.5 (1.0–7.0) |
| Uses per day (times) | n/a | 1.0 (1.0–3.0) |
| THC Dominant (n) | n/a | 4 |
| THC = CBD (n) | n/a | 2 |
| CBD Dominant (n) | n/a | 1 |
| Multiple Types (n) | n/a | 1 |
| Medical reasons for use (n) | n/a | Pain (7), PD (1) |
| Variable Name | Users | Non-Users | p-Value | Effect Size |
|---|---|---|---|---|
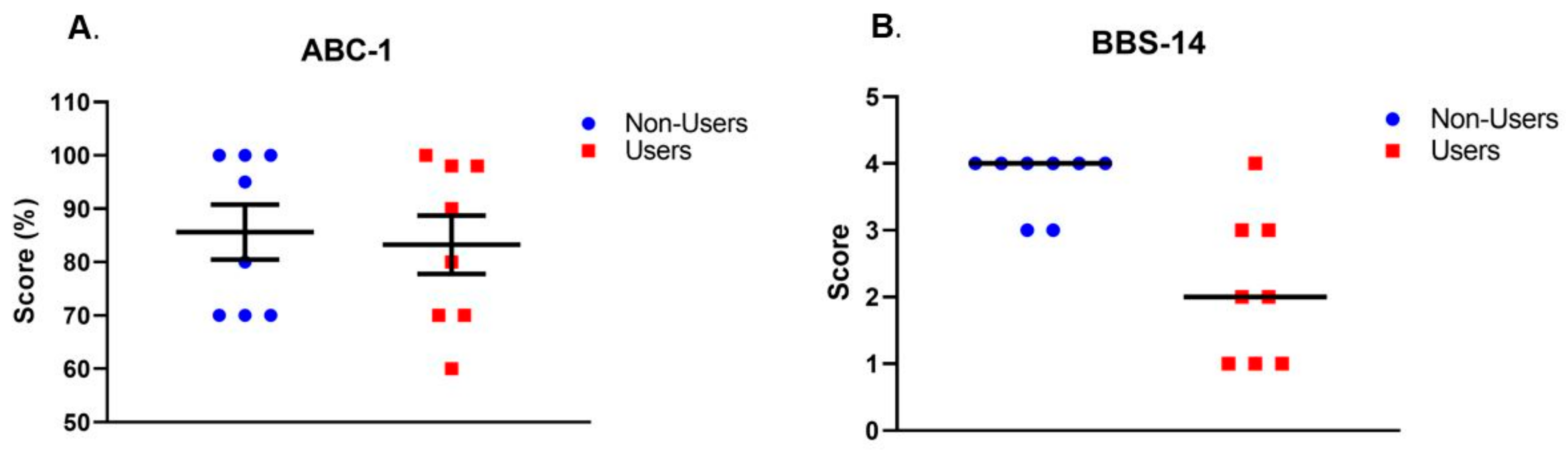
| ABC-1 (%) | 83.3 ± 15.4 | 85.6 ± 14.5 | 0.76 | d = 0.2 |
| BBS-14 (score) | 2 (1–4) | 4 (3–4) | 0.008 | A = 0.89 |
| AP-Pathlength (cm) | 2.5 ± 0.8 | 2.2 ± 0.6 | 0.47 | d = 0.4 |
| ML-Pathlength (cm) | 1.1 ± 0.4 | 0.9 ± 0.3 | 0.28 | d = 0.6 |
| COParea (cm2) | 1.6 (0.9–3.8) | 1.1 (0.4–6.6) | 0.38 | A = 0.36 |
| Users | p-Value | Non-Users | p-Value | |
|---|---|---|---|---|
| BBS-14 (score) | 0.17 | 0.70 | 0.66 | 0.21 |
| AP-Pathlength (cm) | 0.28 | 0.51 | −0.18 | 0.58 |
| ML-Pathlength (cm) | 0.15 | 0.74 | −0.50 | 0.20 |
| COParea (cm2) | 0.17 | 0.70 | −0.23 | 0.70 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Workman, C.D.; Sosnoff, J.J.; Rudroff, T. Disparity between Perceptual Fall Risk and Physiological Fall Risk in Older Cannabis Users: A Pilot Study. Int. J. Environ. Res. Public Health 2022, 19, 109. https://doi.org/10.3390/ijerph19010109
Workman CD, Sosnoff JJ, Rudroff T. Disparity between Perceptual Fall Risk and Physiological Fall Risk in Older Cannabis Users: A Pilot Study. International Journal of Environmental Research and Public Health. 2022; 19(1):109. https://doi.org/10.3390/ijerph19010109
Chicago/Turabian StyleWorkman, Craig D., Jacob J. Sosnoff, and Thorsten Rudroff. 2022. "Disparity between Perceptual Fall Risk and Physiological Fall Risk in Older Cannabis Users: A Pilot Study" International Journal of Environmental Research and Public Health 19, no. 1: 109. https://doi.org/10.3390/ijerph19010109
APA StyleWorkman, C. D., Sosnoff, J. J., & Rudroff, T. (2022). Disparity between Perceptual Fall Risk and Physiological Fall Risk in Older Cannabis Users: A Pilot Study. International Journal of Environmental Research and Public Health, 19(1), 109. https://doi.org/10.3390/ijerph19010109








