Contamination of Foods from Cameroon with Residues of 20 Halogenated Pesticides, and Health Risk of Adult Human Dietary Exposure
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Pesticide Residues Analysis
2.2.1. Chemicals
2.2.2. Extraction and Clean-Up of Pesticide Residues
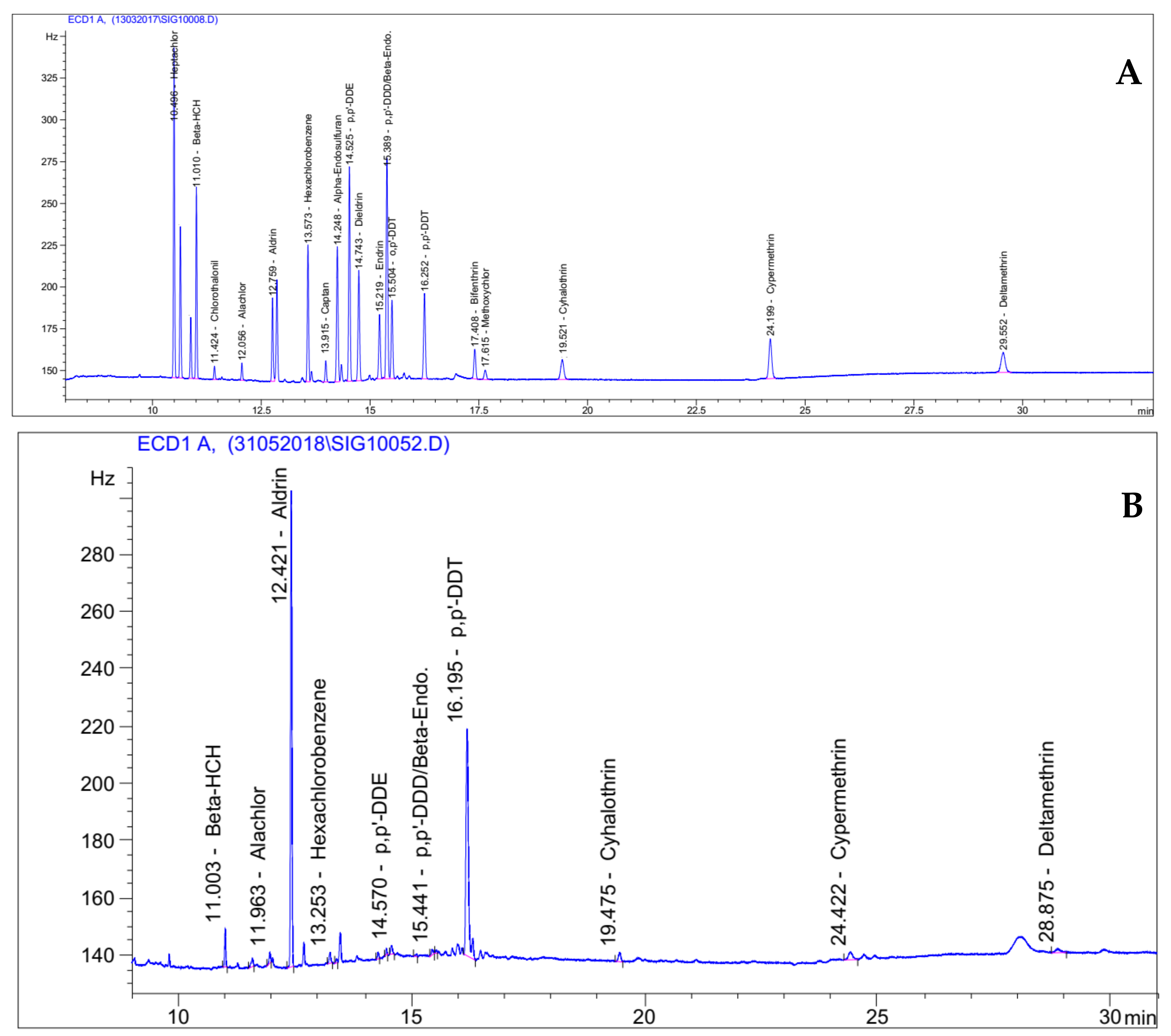
2.2.3. Residues Analysis by Gas Chromatography with Electron Capture Detection
2.2.4. Quality Control of Residues Analysis
2.3. Dietary Exposure and Health Risk Assessment
2.3.1. Deterministic Dietary Exposure
2.3.2. Non-Carcinogenic Dietary Health Risk Assessment
2.3.3. Carcinogenic Dietary Risk Assessment
2.4. Determination of the Source of DDT Residues in the Food Items
2.5. Data Analysis
3. Results and Discussion
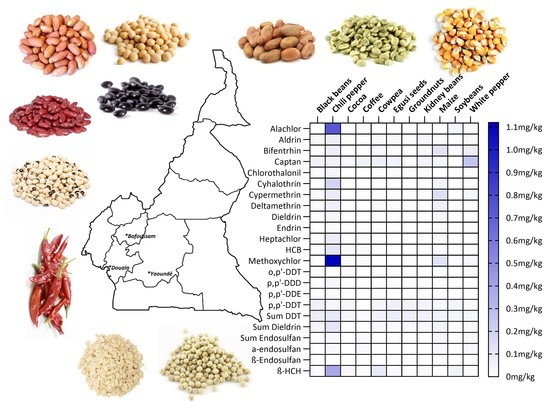
3.1. Concentration of Halogenated Pesticides in Foods from Cameroon
3.1.1. Distribution of Pesticide Residues Found in Food Samples
3.1.2. Compliance of the Quantified Pesticides with Regulation Limits
3.1.3. Source of DDT Contamination in Food Samples
3.1.4. Contamination of the Food Items with Pesticide Residues
3.1.5. Distribution of Pesticide Residues among the Sampling Locations
3.2. Carcinogenic and Non-Carcinogenic Health Risk Assessment
3.2.1. Dietary Chronic Risk Assessment of Pesticide Residues
3.2.2. Dietary Acute Risk Assessment of Pesticide Residues
3.2.3. Cumulative Dietary Risk Assessment of Multiple Pesticide Residues in Each Food Item
3.2.4. Major Contributor Pesticides to Cumulative Chronic Health Risk
3.2.5. Cancer Risk Assessment
4. Conclusions and Perspectives
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jeschke, P. Latest generation of halogen-containing pesticides. Pest Manag. Sci. 2017, 73, 1053–1066. [Google Scholar] [CrossRef] [PubMed]
- Dalla Valle, M.; Codato, E.; Marcomini, A. Climate change influence on POPs distribution and fate: A case study. Chemosphere 2007, 67, 1287–1295. [Google Scholar] [CrossRef]
- Gerber, R.; Smit, N.J.; Van Vuren, J.H.J.; Nakayama, S.M.M.; Yohannes, Y.B.; Ikenaka, Y.; Ishizuka, M.; Wepener, V. Bioaccumulation and human health risk assessment of DDT and other organochlorine pesticides in an apex aquatic predator from a premier conservation area. Sci. Total Environ. 2016, 550, 522–533. [Google Scholar] [CrossRef] [PubMed]
- Jayaraj, R.; Megha, P.; Sreedev, P. Organochlorine pesticides, their toxic effects on living organisms and their fate in the environment. Interdiscip. Toxicol. 2016, 9, 90–100. [Google Scholar] [CrossRef] [PubMed]
- Riana Bornman, M.S.; Bouwman, H. Environmental pollutants and diseases of sexual development in humans and wildlife in South Africa: Harbingers of impact on overall health? Reprod. Domest. Anim. 2012, 47, 327–332. [Google Scholar] [CrossRef]
- Ferguson, K.K.; O’Neill, M.S.; Meeker, J.D. Environmental contaminant exposures and preterm birth: A comprehensive review. J. Toxicol. Environ. Health Part B Crit. Rev. 2013, 16, 69–113. [Google Scholar] [CrossRef]
- Strong, A.L.; Shi, Z.; Strong, M.J.; Miller, D.F.B.; Rusch, D.B.; Buechlein, A.M.; Flemington, E.K.; McLachlan, J.A.; Nephew, K.P.; Burow, M.E.; et al. Effects of the endocrine-disrupting chemical DDT on self-renewal and differentiation of human Mesenchymal stem cells. Environ. Health Perspect. 2015, 123, 42–48. [Google Scholar] [CrossRef]
- IARC. IARC Monographs on the Identification of Carcinogenic Hazards to Humans. In Agents Classifed by the IARC Monographs; IARC: Lyon, France, 2019; Volume 1–125. [Google Scholar]
- Xu, W.; Wang, X.; Cai, Z. Analytical chemistry of the persistent organic pollutants identified in the Stockholm Convention: A review. Anal. Chim. Acta 2013, 790, 1–13. [Google Scholar] [CrossRef]
- Bi, X.; Thomas, G.O.; Jones, K.C.; Qu, W.; Sheng, G.; Martin, F.L.; Fu, J. Exposure of electronics dismantling workers to polybrominated diphenyl ethers, polychlorinated biphenyls, and organochlorine pesticides in South China. Environ. Sci. Technol. 2007, 41, 5647–5653. [Google Scholar] [CrossRef]
- Ward, M.H.; Colt, J.S.; Metayer, C.; Gunier, R.B.; Lubin, J.; Crouse, V.; Nishioka, M.G.; Reynolds, P.; Buffler, P.A. Residential exposure to polychlorinated biphenyls and Organochlorine pesticides and risk of childhood leukemia. Environ. Health Perspect. 2009, 117, 1007–1013. [Google Scholar] [CrossRef]
- Safe, S.H. Development Validation and Problems with the Toxic Equivalency Factor Approach for Risk Assessment of Dioxins and Related Compounds. J. Anim. Sci. 1998, 76, 134–141. [Google Scholar] [CrossRef]
- Matthews, G.; Wiles, T.; Baleguel, P. A survey of pesticide application in Cameroon. Crop Prot. 2003, 22, 707–714. [Google Scholar] [CrossRef]
- Manfo, F.P.T.; Moundipa, P.F.; Déchaud, H.; Tchana, A.N.; Nantia, E.A.; Zabot, M.-T.; Pugeat, M. Effect of agropesticides use on male reproductive function: A study on farmers in Djutitsa (Cameroon). Environ. Toxicol. 2012, 27, 423–432. [Google Scholar] [CrossRef]
- Mahob, R.J.; Hoopen, G.M.T.E.N.; Dibog, L.; Nyassé, S.; Rutherford, M.; Mbenoun, M.; Babin, R.; Amang, J.A.; Bilong, C.F.B. Pesticides use in cocoa sector in Cameroon: Characterization of supply source, nature of actives ingredients, fashion and reasons for their utilization. Int. J. Biol. Chem. Sci. 2014, 8, 1976–1989. [Google Scholar] [CrossRef]
- Tarla, D.; Manu, I.N.; Tamedjouong, Z.T.; Kamga, A.; Fontem, D.A. Plight of pesticide applicators in Cameroon: Case of tomato (Lycopersicon esculentum Mill.) farmers in Foumbot. J. Agric. Environ. Sci. 2015, 4, 87–98. [Google Scholar] [CrossRef]
- Kenko, N.D.B.; Asanga, B.F.P.; Tchamadeu, N.N.; Mpoame, M. Environmental and human health assessment in relation to pesticide use by local farmers and the Cameroon development corporation (CDC), Fako division, South-West Cameroon. Eur. Sci. J. 2017, 13, 454–473. [Google Scholar] [CrossRef]
- Sopkoutie, N.; Abdulai, A.; Tarla, D.; Djeugap, F.J.; Galani, Y.J.H.; Ekengoue, C.; Tabang, W.; Nya, E.; Payne, V. Phytosanitary Practices and Evaluation of 17 Pesticides Residues in Tomatoes Fruits Produced in Foumbot District, Western Highland-Cameroon. Eur. Sci. J. 2021, 17, 30–50. [Google Scholar] [CrossRef]
- Carnevale, P.; Mouchet, J. La lutte antivectorielle au Cameroun. Passé-présent-avenir. Réflexions. Entomol. Méd. Bull. Soc. Pathol. Exot. 2001, 94, 202–209. [Google Scholar]
- Antonio-Nkondjio, C.; Sonhafouo-Chiana, N.; Ngadjeu, C.S.; Doumbe-Belisse, P.; Talipouo, A.; Djamouko-Djonkam, L.; Kopya, E.; Bamou, R.; Awono-Ambene, P.; Wondji, C.S. Review of the evolution of insecticide resistance in main malaria vectors in Cameroon from 1990 to 2017. Parasites Vectors 2017, 10, 472. [Google Scholar] [CrossRef]
- Gama, E.N.; Folorunso, S.T.; Adeola, S.S. Analysis of factors affecting the technical efficiency of cocoa producers in the south-west region of Cameroon. J. Agric. Res. Dev. 2016, 14, 89. [Google Scholar] [CrossRef]
- Nchare, A. Analysis of Factors Affecting the Technical Efficiency of Arabica Coffee Producers in Cameroon; Faculty of Agriculture, University of Ilorin: Ilorin, France, 2007; Volume 14. [Google Scholar]
- Sonchieu, J.; Benoit Ngassoum, M.; Bosco Tchatchueng, J.; Srivastava, A.K.; Srivastava, L.P. Survey of pesticide residues in maize, cowpea and millet from northern Cameroon: Part I. Food Addit. Contam. Part B 2010, 3, 178–184. [Google Scholar] [CrossRef] [PubMed]
- Tayoh, L.N.; Kiyo, M.L.I.; Nkemnyi, M.F. Chemical fertilizer application and farmers perception on food safety in Buea, Cameroon. Agric. Sci. Res. J. 2016, 6, 287–295. [Google Scholar]
- Tandi, T.E.; Wook, C.J.; Shendeh, T.T.; Eko, E.A.; Afoh, C.O. Small-scale tomato cultivators’ perception on pesticides usage and practices in Buea Cameroon. Health 2014, 6, 2945–2958. [Google Scholar] [CrossRef]
- Tarla, D.N.; Tchamba, N.M.; Baleguel, N.P.; Fontem, D.A.; Baleguel, P.D.; Hans, D. Inventory of obsolete pesticide stockpiles in Cameroon. Sch. J. Agric. Sci. 2014, 4, 43–50. [Google Scholar]
- Wodageneh, A. La prévention et l’élimination des stocks de pesticides périmés en Afrique et au Proche-Orient. In Proceedings of the InterOrganization Programme for the Sound Management of Chemicals (IOMC), Bamamko, Mali, 15–18 December 1997; pp. 58–61. [Google Scholar]
- Gimou, M.-M.; Charrondiere, U.R.; Leblanc, J.-C.; Pouillot, R. Dietary exposure to pesticide residues in Yaoundé: The Cameroonian total diet study. Food Addit. Contam. Part A 2008, 25, 458–471. [Google Scholar] [CrossRef]
- Sonchieu, J.; Srivastava, A.K.; Ngassoum, B.M.; Tchatchueng, J.B.; Srivastava, L.P. Contamination of cowpea (Vigna unguiculata (L.) Walp) and derived products by residues of banned pesticides in Cameroon: Health risk estimation. J. Pestic. 2017, 104, 147–160. [Google Scholar]
- Galani, J.H.Y.; Houbraken, M.; Wumbei, A.; Djeugap, F.J.; Fotio, D.; Spanoghe, P. Evaluation of 99 pesticide residues in major agricultural products from the western highlands zone of Cameroon using QuEChERS method extraction and LC-MS/MS and GC-ECD analyses. Foods 2018, 7, 184. [Google Scholar] [CrossRef]
- Sonchieu, J.; Ngassoum, M.B.; Tchatchueng, J.B.; Srivastava, A.K.; Srivastava, L.P. Contamination of cowpea and by-products by organophosphorous pesticide residues in Ngaoundere markets: Dietary risk estimation and degradation study. Afr. J. Food Sci. 2013, 7, 92–102. [Google Scholar] [CrossRef]
- Ingenbleek, L.; Hu, R.; Pereira, L.L.; Paineau, A.; Colet, I.; Koné, A.Z.; Adegboye, A.; Hossou, S.E.; Dembélé, Y.; Oyedele, A.D.; et al. Sub-Saharan Africa total diet study in Benin, Cameroon, Mali and Nigeria: Pesticides occurrence in foods. Food Chem. X 2019, 2, 100034. [Google Scholar] [CrossRef]
- INS. Chapitre 14: Agriculture. In Annuaire Statistique du Cameroun, Edition 2015; Institut National de la Statistique; INS: Yaounde, Cameroon, 2015; pp. 232–255. [Google Scholar]
- Seo, Y.H.; Cho, T.H.; Hong, C.K.; Kim, M.S.; Cho, S.J.; Park, W.H.; Hwang, I.S.; Kim, M.S. Monitoring and risk assessment of pesticide residues in commercially dried vegetables. Prev. Nutr. Food Sci. 2013, 18, 145–149. [Google Scholar] [CrossRef]
- Ingenbleek, L.; Jazet, E.; Dzossa, A.D.; Adebayo, S.B.; Ogungbangbe, J.; Dansou, S.; Diallo, Z.J.; Kouebou, C.; Adegboye, A.; Hossou, E.; et al. Methodology design of the regional Sub-Saharan Africa Total Diet Study in Benin, Cameroon, Mali and Nigeria. Food Chem. Toxicol. 2017, 109, 155–169. [Google Scholar] [CrossRef]
- MINADER. Liste des Pesticides Homologués au Cameroun au 31 Juillet 2013. Liste réservée au Grand Public; Commission Nationale d’Homologation des Pesticides à Usage Agricole; Ministère de l’Agriculture et du Développement Rural (MINADER): Yaoundé, Cameroun, 2013.
- European Commission. Guidance Document on Analytical Quality Control and Method Validation Procedures for Pesticides Residues Analysis in Food and Feed; European Commission: Luxembourg, 2015. [Google Scholar]
- Chawla, S.; Patel, H.K.; Gor, H.N.; Vaghela, K.M.; Solanki, P.P.; Shah, P.G. Evaluation of matrix effects in multiresidue analysis of pesticide residues in vegetables and spices by LC-MS/MS. J. AOAC Int. 2017, 100, 616–623. [Google Scholar] [CrossRef]
- De Sousa, F.A.; Guido Costa, A.I.; De Queiroz, M.E.L.R.; Teófilo, R.F.; Neves, A.A.; De Pinho, G.P. Evaluation of matrix effect on the GC response of eleven pesticides by PCA. Food Chem. 2012, 135, 179–185. [Google Scholar] [CrossRef]
- Kim, N.H.; Lee, J.S.; Park, K.A.; Kim, Y.H.; Lee, S.R.; Lee, J.M.; Yu, I.S.; Jung, K.; Lee, Y.K. Determination of matrix effects occurred during the analysis of organochlorine pesticides in agricultural products using GC-ECD. Food Sci. Biotechnol. 2016, 25, 33–40. [Google Scholar] [CrossRef]
- Hernández Torres, M.E.; Egea González, F.J.; Cuadros-Rodríguez, L.; Almansa López, E.; Martínez Vidal, J.L. Assessment of matrix effects in gas chromatography electron capture pesticide-residue analysis. Chromatographia 2003, 57, 657–664. [Google Scholar] [CrossRef]
- Sonchieu, J.; Azouline, M.; Ngassoum, B.M. Investigation on Five Years (2010–2014) Food Poisonings Recorded in Bamenda and NDOP Public Hospitals in Cameroon. Insights Biomed. 2018, 3, 9–16. [Google Scholar] [CrossRef]
- Pouokam, G.B.; Album, W.L.; Ndikontar, A.S.; Sidatt, M.E.H. A pilot study in cameroon to understand safe uses of pesticides in agriculture, risk factors for farmers’ exposure and management of accidental cases. Toxics 2017, 5, 30. [Google Scholar] [CrossRef]
- Reffstrup, T.K.; Larsen, J.C.; Meyer, O. Risk assessment of mixtures of pesticides. Current approaches and future strategies. Regul. Toxicol. Pharmacol. 2010, 56, 174–192. [Google Scholar] [CrossRef]
- El Hawari, K.; Mokh, S.; Al Iskandarani, M.; Halloum, W.; Jaber, F. Pesticide residues in Lebanese apples and health risk assessment. Food Addit. Contam. Part B Surveill. 2019, 12, 81–89. [Google Scholar] [CrossRef]
- Liu, Y.; Shen, D.; Li, S.; Ni, Z.; Ding, M.; Ye, C.; Tang, F. Residue levels and risk assessment of pesticides in nuts of China. Chemosphere 2016, 144, 645–651. [Google Scholar] [CrossRef]
- European Commission. Regulation (EC) No 396/2005 of the European Parliament and of the Council of 23 February 2005 on Maximum Residue Levels of Pesticides in or on Food and Feed of Plant and Animal Origin and Amending Council Directive 91/414/EEC, Official Journal of the Euro; European Commission: Luxembourg, 2005. [Google Scholar]
- WHO/FAO. Dietary Exposure Assessment of Chemicals in Food; Report of a joint FAO/WHO Consultation, 2–6 May 2005; WHO/FAO; Annapolis, MD, USA, 2008. Available online: https://www.who.int/foodsafety/chem/dietary_exposure.pdf (accessed on 5 April 2021).
- EFSA. Guidance of the scientific committee on a request from EFSA related to uncertainties in dietary exposure assessment. EFSA J. 2006, 438, 1–54. [Google Scholar]
- Walpole, S.C.; Prieto-Merino, D.; Edwards, P.; Cleland, J.; Stevens, G.; Roberts, I. The weight of nations: An estimation of adult human biomass. BMC Public Health 2012, 12, 439. [Google Scholar] [CrossRef]
- EFSA. Management of left-censored data in dietary exposure assessment of chemical substances. EFSA J. 2010, 8, 1557–1653. [Google Scholar] [CrossRef]
- European Commission EU Pesticides Database. Available online: https://ec.europa.eu/food/plant/pesticides/eu-pesticides-db_en (accessed on 1 February 2020).
- Lewis, K.A.; Tzilivakis, J.; Warner, D.J.; Green, A. An international database for pesticide risk assessments and management. Hum. Ecol. Risk Assess. 2016, 22, 1050–1064. [Google Scholar] [CrossRef]
- Boobis, A.R.; Ossendorp, B.C.; Banasiak, U.; Hamey, P.Y.; Sebestyen, I.; Moretto, A. Cumulative risk assessment of pesticide residues in food. Toxicol. Lett. 2008, 180, 137–150. [Google Scholar] [CrossRef]
- Dougherty, C.P.; Holtz, S.H.; Reinert, J.C.; Panyacosit, L.; Axelrad, D.A.; Woodruff, T.J. Dietary exposures to food contaminants across the United States. In Proceedings of the Environmental Research; Academic Press Inc.: Cambridge, MA, USA, 2000; Volume 84, pp. 170–185. [Google Scholar]
- EPA Integrated Risk Information System (IRIS). United States Environmental Protection Agency. Available online: http://www.epa.gov/IRIS/ (accessed on 10 April 2020).
- Doong, R.A.; Peng, C.K.; Sun, Y.C.; Liao, P.L. Composition and distribution of organochlorine pesticide residues in surface sediments from the Wu-Shi River estuary, Taiwan. Mar. Pollut. Bull. 2002, 45, 246–253. [Google Scholar] [CrossRef]
- Chourasiya, S.; Khillare, P.S.; Jyethi, D.S. Health risk assessment of organochlorine pesticide exposure through dietary intake of vegetables grown in the periurban sites of Delhi, India. Environ. Sci. Pollut. Res. 2015, 22, 5793–5806. [Google Scholar] [CrossRef]
- Mekonen, S.; Ambelu, A.; Negassa, B.; Spanoghe, P. Exposure to DDT and its metabolites from khat (Catha edulis) chewing: Consumers risk assessment from southwestern Ethiopia. Regul. Toxicol. Pharmacol. 2017, 87, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Tsakiris, I.N.; Goumenou, M.; Tzatzarakis, M.N.; Alegakis, A.K.; Tsitsimpikou, C.; Ozcagli, E.; Vynias, D.; Tsatsakis, A.M. Risk assessment for children exposed to DDT residues in various milk types from the Greek market. Food Chem. Toxicol. 2015, 75, 156–165. [Google Scholar] [CrossRef]
- Thompson, L.A.; Ikenaka, Y.; Yohannes, Y.B.; van Vuren, J.J.; Wepener, V.; Smit, N.J.; Darwish, W.S.; Nakayama, S.M.M.; Mizukawa, H.; Ishizuka, M. Concentrations and human health risk assessment of DDT and its metabolites in free-range and commercial chicken products from KwaZulu-Natal, South Africa. Food Addit. Contam. Part A Chem. Anal. Control. Expo. Risk Assess. 2017, 34, 1959–1969. [Google Scholar] [CrossRef]
- Ferreira, V.B.; Estrella, L.F.; Alves, M.G.R.; Gallistl, C.; Vetter, W.; Silva, T.T.C.; Malm, O.; Torres, J.P.M.; Abadio Finco, F.D.B. Residues of legacy organochlorine pesticides and DDT metabolites in highly consumed fish from the polluted Guanabara Bay, Brazil: Distribution and assessment of human health risk. J. Environ. Sci. Health Part B Pestic. Food Contam. Agric. Wastes 2020, 55, 30–41. [Google Scholar] [CrossRef] [PubMed]
- Ogah, C.O.; Coker, H.B. Quantification of Organophosphate and Carbamate Pesticide Residues in Maize. J. Appl. Pharm. Sci. 2012, 2, 093–097. [Google Scholar] [CrossRef]
- Oyeyiola, A.O.; Fatunsin, O.T.; Akanbi, L.M.; Fadahunsi, D.E.; Moshood, M.O. Human health risk of organochlorine pesticides in foods grown in Nigeria. J. Health Pollut. 2017, 7, 63–70. [Google Scholar] [CrossRef]
- Forkuoh, F.; Boadi, N.O.; Borquaye, L.S.; Afful, S. Risk Of Human Dietary Exposure To Organochlorine Pesticide Residues In Fruits From Ghana. Sci. Rep. 2018, 8, 1–5. [Google Scholar] [CrossRef]
- Bempah, C.K.; Donkor, A.K. Pesticide residues in fruits at the market level in Accra Metropolis, Ghana, a preliminary study. Environ. Monit. Assess. 2011, 175, 551–561. [Google Scholar] [CrossRef]
- Mekonen, S.; Lachat, C.; Ambelu, A.; Steurbaut, W.; Kolsteren, P.; Jacxsens, L.; Wondafrash, M.; Houbraken, M.; Spanoghe, P. Risk of DDT residue in maize consumed by infants as complementary diet in southwest Ethiopia. Sci. Total Environ. 2015, 511, 454–460. [Google Scholar] [CrossRef]
- Mekonen, S.; Ambelu, A.; Spanoghe, P. Pesticide residue evaluation in major staple food items of Ethiopia using the QuEChERS method: A case study from the Jimma zone. Environ. Toxicol. Chem. 2014, 33, 1294–1302. [Google Scholar] [CrossRef]
- Mekonen, S.; Argaw, R.; Simanesew, A.; Houbraken, M.; Senaeve, D.; Ambelu, A.; Spanoghe, P. Pesticide residues in drinking water and associated risk to consumers in Ethiopia. Chemosphere 2016, 162, 252–260. [Google Scholar] [CrossRef]
- Zhou, P.; Zhao, Y.; Li, J.; Wu, G.; Zhang, L.; Liu, Q.; Fan, S.; Yang, X.; Li, X.; Wu, Y. Dietary exposure to persistent organochlorine pesticides in 2007 Chinese total diet study. Environ. Int. 2012, 42, 152–159. [Google Scholar] [CrossRef]
- Darko, G.; Acquaah, S.O. Levels of organochlorine pesticides residues in dairy products in Kumasi, Ghana. Chemosphere 2008, 71, 294–298. [Google Scholar] [CrossRef]
- Nougadère, A.; Sirot, V.; Kadar, A.; Fastier, A.; Truchot, E.; Vergnet, C.; Hommet, F.; Baylé, J.; Gros, P.; Leblanc, J.C. Total diet study on pesticide residues in France: Levels in food as consumed and chronic dietary risk to consumers. Environ. Int. 2012, 45, 135–150. [Google Scholar] [CrossRef]
- Golge, O.; Hepsag, F.; Kabak, B. Health risk assessment of selected pesticide residues in green pepper and cucumber. Food Chem. Toxicol. 2018, 121, 51–64. [Google Scholar] [CrossRef] [PubMed]
- Tsakiris, N.I.; Toutoudaki, M.; Kokkinakis, M.; Paraskevi, M.; Tsakiris, M.A. A risk assessment study of Greek population dietary chronic exposure to pesticide residues in fruits, vegetables and olive oil. In Pesticides Formulations, Effects, Fate; Stoytcheva, M., Ed.; IntechOpen: London, UK, 2011; pp. 253–268. ISBN 978-953-307-532-7. [Google Scholar]
- Kouebou, C.P.; Achu, M.; Nzali, S.; Chelea, M.; Bonglaisin, J.; Kamda, A.; Djiele, P.; Yadang, G.; Ponka, R.; Ngoh Newilah, G.; et al. A review of composition studies of Cameroon traditional dishes: Macronutrients and minerals. Food Chem. 2013, 140, 483–494. [Google Scholar] [CrossRef]
- Sop, M.M.K.; Gouado, I.; Mofor, C.T.; Smriga, M.; Fotso, M.; Ekoe, T. Mineral content in some Cameroonian household foods eaten in Douala. Afr. J. Biotechnol. 2008, 7, 3085–3091. [Google Scholar] [CrossRef]
- Ponka, R.; Fokou, E.; Fotso, M.; Tchouanguep, F.M.; Leke, R.; Souopgui, J.; Bih, M.A. Composition of dishes consumed in Cameroon. Int. J. Food Sci. Technol. 2006, 41, 361–365. [Google Scholar] [CrossRef]
- Sharma, S.; Mbanya, J.C.; Cruickshank, K.; Cade, J.; Tanya, A.K.N.; Cao, X.; Hurbos, M.; Wong, M.R.K.M. Nutritional composition of commonly consumed composite dishes from the Central Province of Cameroon. Int. J. Food Sci. Nutr. 2007, 58, 475–485. [Google Scholar] [CrossRef]
- Taiwo, A.M. A review of environmental and health effects of organochlorine pesticide residues in Africa. Chemosphere 2019, 220, 1126–1140. [Google Scholar] [CrossRef]
- Akan, J.C.; Mohammed, Z.; Jafiya, L.; Ogugbuaja, V.O. Organochlorine Pesticide Residues in Fish Samples from Alau Dam, Borno State, North Eastern Nigeria. J. Environ. Anal. Toxicol. 2013, 3, 1–17. [Google Scholar] [CrossRef]
- Mbah, N.L.J.; Rabia, H.; Ngondi, J.L.; Shah, S.T.A.; Valis, M.; Kuca, K.; Nurulain, S.M. Chronic exposure to organophosphates pesticides and risk of metabolic disorder in cohort from pakistan and cameroon. Int. J. Environ. Res. Public Health 2021, 18, 2310. [Google Scholar] [CrossRef]




| Sr. No. | Pesticide Residue | Chemical Class | IARC Group | Application | Banned in Cameroon? | Number of Positive Samples | Percent of Positive Samples (%) | Number of Positive Food Items | Lowest Residue Value (mg/kg) | Highest Residue Value (mg/kg) | Mean Residue Value (mg/kg) | Median Residue Value (mg/kg) | 95th Percentile (mg/kg) | Number of Samples >MRLs | Percent of Positive Samples > MRLs (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Alachlor | Phenylamides | Herbicide | Yes | 35 | 21.9 | 6 | 0.0240 | 5.8116 | 0.3532 | 0.0684 | 1.0893 | 35 | 100.0 | |
| 2 | Aldrin | Organochlorines | 2A | Insecticide | Yes | 136 | 85.0 | 11 | 0.0051 | 0.3240 | 0.0291 | 0.0144 | 0.1131 | 48 | 35.3 |
| 3 | Bifenthrin | Pyrethroid | Insecticide | No | 83 | 51.9 | 11 | 0.0072 | 0.5691 | 0.0674 | 0.0246 | 0.2457 | 30 | 36.1 | |
| 4 | Captan | Phtalimides | 3 | Fungicide | No | 110 | 68.8 | 10 | 0.0345 | 0.4818 | 0.0897 | 0.0522 | 0.3309 | 26 | 23.6 |
| 5 | Chlorothalonil | Chloronitrile | 2B | Fungicide | No | 106 | 66.3 | 10 | 0.0057 | 0.2550 | 0.0165 | 0.0101 | 0.0469 | 13 | 12.3 |
| 6 | Cyhalothrin | Pyrethroids | Insecticide | No | 31 | 19.4 | 7 | 0.0111 | 0.8235 | 0.1258 | 0.0756 | 0.4931 | 14 | 45.2 | |
| 7 | Cypermethrin | Pyrethroids | Insecticide | No | 53 | 33.1 | 7 | 0.0114 | 1.8439 | 0.0885 | 0.0312 | 0.2053 | 3 | 5.7 | |
| 8 | Deltamethrin | Pyrethroids | 3 | Insecticide | No | 29 | 18.1 | 7 | 0.0117 | 0.5979 | 0.0964 | 0.0370 | 0.3343 | 2 | 6.9 |
| 9 | Dieldrin | Organochlorines | 2A | Insecticide | Yes | 54 | 33.8 | 7 | 0.0033 | 0.2871 | 0.0247 | 0.0083 | 0.0986 | NA | NA |
| 10 | Endrin | Organochlorines | 3 | Insecticide | No | 42 | 26.3 | 8 | 0.0039 | 0.0540 | 0.0190 | 0.0134 | 0.0458 | NA | NA |
| 11 | Heptachlor | Organochlorines | 2B | Insecticide | Yes | 40 | 25.0 | 9 | 0.0048 | 0.3993 | 0.0544 | 0.0117 | 0.2810 | 22 | 55.0 |
| 12 | HCB | Organochlorines | 2B | Fungicide | No | 99 | 61.9 | 10 | 0.0051 | 0.8904 | 0.0330 | 0.0090 | 0.1162 | 40 | 40.4 |
| 13 | Methoxychlor | Organochlorines | 3 | Insecticide | No | 34 | 21.3 | 8 | 0.0351 | 7.2504 | 0.5823 | 0.2064 | 1.8143 | 30 | 88.2 |
| 14 | o,p’-DDT | Organochlorines | 2A | Insecticide | Yes | 89 | 55.6 | 10 | 0.0078 | 0.0366 | 0.0143 | 0.0120 | 0.0329 | NA | NA |
| 15 | p,p’-DDD | Organochlorines | Insecticide | Yes | 24 | 15.0 | 7 | 0.0032 | 0.2193 | 0.0322 | 0.0105 | 0.1818 | NA | NA | |
| 16 | p,p’-DDE | Organochlorines | Insecticide | Yes | 80 | 50.0 | 8 | 0.0039 | 0.1392 | 0.0113 | 0.0060 | 0.0276 | NA | NA | |
| 17 | p,p’-DDT | Organochlorines | 2A | Insecticide | Yes | 131 | 81.9 | 10 | 0.0087 | 0.1770 | 0.0507 | 0.0486 | 0.0964 | NA | NA |
| 18 | α-endosulfan | Organochlorines | Insecticide | Yes | 63 | 39.4 | 10 | 0.0060 | 0.1617 | 0.0287 | 0.0233 | 0.0641 | NA | NA | |
| 19 | β-Endosulfan | Organochlorines | Insecticide | Yes | 9 | 5.6 | 5 | 0.0045 | 0.0408 | 0.0178 | 0.0141 | 0.0370 | NA | NA | |
| 20 | β-HCH | Organochlorines | 2A | Insecticide | Yes | 116 | 72.5 | 11 | 0.0048 | 1.5567 | 0.0864 | 0.0372 | 0.2921 | 91 | 78.4 |
| 21 | Ʃdieldrin | Organochlorines | Insecticide | Yes | 139 | 86.9 | 11 | 0.0045 | 0.3552 | 0.0381 | 0.0198 | 0.1442 | 61 | 43.9 | |
| 22 | ƩDDT | Organochlorines | Insecticide | Yes | 153 | 95.6 | 11 | 0.0063 | 0.3364 | 0.0627 | 0.0573 | 0.1251 | 74 | 48.4 | |
| 23 | ƩEndosulfan | Organochlorines | Insecticide | Yes | 67 | 41.9 | 10 | 0.006 | 0.185 | 0.030 | 0.024 | 0.064 | 4 | 6.0 |
| Sr.No | Food Item | Hazard Index | |||||
|---|---|---|---|---|---|---|---|
| Average Exposure | 95th Percentile Exposure | ||||||
| Upper Bound | Medium Bound | Lower Bound | Upper Bound | Medium Bound | Lower Bound | ||
| 1 | Black beans | 0.7958 | 0.7433 | 0.6908 | 2.6025 | 2.5918 | 2.5811 |
| 2 | Chili pepper | 0.1052 | 0.0966 | 0.0880 | 0.3053 | 0.3053 | 0.3053 |
| 3 | Cocoa | 0.0068 | 0.0040 | 0.0011 | 0.0095 | 0.0072 | 0.0050 |
| 4 | Coffee | 0.0005 | 0.0004 | 0.0003 | 0.0012 | 0.0012 | 0.0011 |
| 5 | Cowpea | 0.0109 | 0.0102 | 0.0096 | 0.0324 | 0.0322 | 0.0319 |
| 6 | Egusi seeds | 0.0103 | 0.0090 | 0.0078 | 0.0171 | 0.0163 | 0.0156 |
| 7 | Groundnuts | 0.1946 | 0.1656 | 0.1366 | 0.3069 | 0.2827 | 0.2585 |
| 8 | Kidney beans | 0.5696 | 0.5143 | 0.4589 | 1.7234 | 1.7121 | 1.7007 |
| 9 | Maize | 5.7491 | 5.2668 | 4.7846 | 19.4587 | 19.4409 | 19.4232 |
| 10 | Soybeans | 0.0046 | 0.0031 | 0.0016 | 0.0068 | 0.0056 | 0.0044 |
| 11 | White pepper | 0.0131 | 0.0098 | 0.0066 | 0.0160 | 0.0133 | 0.0105 |
| Residue | OSF | Food Item | Carcinogenic Hazard Ratio > 1 | |||
|---|---|---|---|---|---|---|
| Average Exposure | 95th Percentile Exposure | |||||
| Upper Bound | Medium Bound | Lower Bound | Upper Bound | |||
| Aldrin | 17 | Maize | 12,312.94 | 12,312.94 | 12,312.94 | 38,883.96 |
| Black beans | 1041.83 | 1039.51 | 1037.19 | 3653.47 | ||
| Kidney beans | 830.28 | 830.28 | 830.28 | 3104.32 | ||
| Groundnuts | 96.18 | 94.93 | 93.68 | 209.97 | ||
| Chili pepper | 2.11 | 2.09 | 2.07 | 9.06 | ||
| Dieldrin | 16 | Maize | 16,038.25 | 15,944.25 | 15,850.25 | 58,395.59 |
| Black beans | 242.15 | 171.52 | 100.90 | 363.23 | ||
| Kidney beans | 215.41 | 116.54 | 17.66 | 227.77 | ||
| Groundnuts | 26.70 | 25.35 | 24.01 | 83.68 | ||
| Chili pepper | 1.20 | 1.15 | 1.10 | 6.68 | ||
| HCB | 1.6 | Maize | 1525.43 | 1363.57 | 1201.70 | 4453.17 |
| Black beans | 32.69 | 32.69 | 32.69 | 71.74 | ||
| Kidney beans | 18.82 | 18.82 | 18.82 | 31.10 | ||
| Groundnuts | 8.32 | 4.62 | 0.93 | 9.72 | ||
| Chili pepper | 0.31 | 0.31 | 0.31 | 1.21 | ||
| Heptachlor | 4.5 | Maize | 5041.98 | 4086.56 | 3131.15 | 15,137.05 |
| Black beans | 92.75 | 81.63 | 70.52 | 328.18 | ||
| Kidney beans | 45.55 | 32.21 | 18.87 | 90.45 | ||
| Groundnuts | 11.35 | 5.67 | 0.00 | 11.35 | ||
| Chili pepper | 0.89 | 0.75 | 0.61 | 2.61 | ||
| o,p’-DDT | 0.34 | Maize | 155.77 | 98.72 | 41.68 | 218.23 |
| Black beans | 5.36 | 5.10 | 4.83 | 10.39 | ||
| Kidney beans | 4.19 | 3.93 | 3.67 | 4.82 | ||
| Groundnuts | 1.53 | 1.49 | 1.46 | 1.79 | ||
| p,p’-DDD | 0.24 | Maize | 116.73 | 116.73 | 116.73 | 205.11 |
| Black beans | 3.21 | 2.08 | 0.95 | 4.89 | ||
| Kidney beans | 2.97 | 2.08 | 1.18 | 3.68 | ||
| p,p’-DDE | 0.34 | Maize | 110.23 | 101.44 | 92.65 | 347.31 |
| Black beans | 3.09 | 3.09 | 3.09 | 4.12 | ||
| Kidney beans | 2.72 | 2.65 | 2.58 | 3.75 | ||
| p,p’-DDT | 0.34 | Maize | 532.30 | 477.21 | 422.12 | 990.81 |
| Black beans | 33.55 | 33.42 | 33.29 | 69.81 | ||
| Kidney beans | 26.36 | 25.96 | 25.56 | 57.50 | ||
| Groundnuts | 5.62 | 5.54 | 5.46 | 8.25 | ||
| β-HCH | 1.8 | Maize | 961.89 | 563.23 | 164.57 | 1145.22 |
| Black beans | 78.43 | 78.43 | 78.43 | 157.83 | ||
| Kidney beans | 56.41 | 56.41 | 56.41 | 135.44 | ||
| Groundnuts | 7.82 | 5.62 | 3.42 | 18.18 | ||
| Chili pepper | 1.22 | 1.21 | 1.20 | 3.38 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Galani, Y.J.H.; Houbraken, M.; Wumbei, A.; Djeugap, J.F.; Fotio, D.; Gong, Y.Y.; Spanoghe, P. Contamination of Foods from Cameroon with Residues of 20 Halogenated Pesticides, and Health Risk of Adult Human Dietary Exposure. Int. J. Environ. Res. Public Health 2021, 18, 5043. https://doi.org/10.3390/ijerph18095043
Galani YJH, Houbraken M, Wumbei A, Djeugap JF, Fotio D, Gong YY, Spanoghe P. Contamination of Foods from Cameroon with Residues of 20 Halogenated Pesticides, and Health Risk of Adult Human Dietary Exposure. International Journal of Environmental Research and Public Health. 2021; 18(9):5043. https://doi.org/10.3390/ijerph18095043
Chicago/Turabian StyleGalani, Yamdeu Joseph Hubert, Michael Houbraken, Abukari Wumbei, Joseph Fovo Djeugap, Daniel Fotio, Yun Yun Gong, and Pieter Spanoghe. 2021. "Contamination of Foods from Cameroon with Residues of 20 Halogenated Pesticides, and Health Risk of Adult Human Dietary Exposure" International Journal of Environmental Research and Public Health 18, no. 9: 5043. https://doi.org/10.3390/ijerph18095043
APA StyleGalani, Y. J. H., Houbraken, M., Wumbei, A., Djeugap, J. F., Fotio, D., Gong, Y. Y., & Spanoghe, P. (2021). Contamination of Foods from Cameroon with Residues of 20 Halogenated Pesticides, and Health Risk of Adult Human Dietary Exposure. International Journal of Environmental Research and Public Health, 18(9), 5043. https://doi.org/10.3390/ijerph18095043