1. Introduction
A year after the COVID-19 pandemic began its rapid geographic expansion across the globe, it is evident that the incidence rate in each country evolves in waves. In Europe, the typical pattern so far has been two waves, the first in the spring of 2020, and a second longer and stronger wave that started in the fall and is still ongoing at the time of writing, spring 2021 [
1]. The increased strength of the second wave is particularly prominent in the case notification rate, but part of this increase is due to increasing testing rate. Nevertheless, the tendency is clear also in the reported COVID-19 death rates, although many countries have seen lower death rates in the start of the second wave, because this wave began with infection spreading in the younger age groups.
Based on the experience from the first wave, countries should have been better prepared for the second wave than for the first, and mitigation should have been be more feasible. There is no apparent microbiological mechanisms that could have driven the strong second wave, even though new and more contagious mutants have started to make an impact as we enter the calendar year of 2021. Hence, the explanation seems to be associated to how our societies respond to the threats of the pandemic.
Some evidence suggest that the wavy pattern during the first year of the pandemic has been driven by an interaction between the pathogen’s natural tendency to reproduce and the non-pharmaceutical interventions (NPIs) implemented by governments [
2]. Even though the emergence of new and more contagious mutants of the SARS-CoV-2 virus with higher basic reproduction numbers
and the roll-out of vaccines will play an increasingly important role in reducing the effective reproduction numbers
, NPIs are expected to play an important role in regulating and controlling the incidence rate also in the upcoming year.
In this paper the term incidence rate will refer to the daily number of actual infections taking place in a country at the time t. It does not refer to the recorded incidence (case notification rate), and the time t is the time of infection, not the time the infection is detected. The instantaneous basic reproduction number refers to the average number of new infections transmitted by an infected individual at the time t in a population where all individuals are susceptible to the infection. The effective reproduction number is the reproduction rate in a population where only a fraction is susceptible at time t.
There is clear and strong correlation between case notification rate and NPIs in most countries, and the time lag between NPIs and changes in recorded incidence corresponds roughly to the sum of incubation period and time for testing, analysis and registration. Thus, it is reasonable to assume that the effect of NPI-induced changes of on the actual transmission of the infection is more or less instantaneous. The same is not the case with the effect of disease incidence on the NPIs. Here we would expect considerable delay between cause and effect, a delay we shall refer to as the social response time.
NPIs represent a great burden on society, and so far in the COVID-19 pandemic there are very few examples where interventions have been effectuated in a precautionary manner. Political pressure has forced policy makers to respond to the recorded disease burden, which is delayed by 1–2 weeks relative to the actual state, even though most governments have access to model projections that can inform them about the true present state of the epidemic and the likely development in the near future. The objective of this paper is to investigate whether or not this delay may have an important influence on the trajectory of the epidemic state.
It is intuitively evident that NPIs effectuated as responses to the true epidemic state will lead to oscillations in the disease incidence. This is because NPIs act as a restoring force counteracting the virus’ natural tendency to reproduce, while the disease activity level below or above a socially acceptable threshold will enhance or reduce the NPIs. In a recent paper [
2], we constructed a simple model that reduces to a damped harmonic oscillator in the small-amplitude (linearized) limit. In that paper we demonstrated that a weakening of the intervention response over time could counteract the damping and lead to stronger and longer secondary waves, but it was assumed that the intervention response is instantaneous. In the present paper, I explore a similar model, where the intervention fatigue is replaced by a delayed response.
In
Section 2, I formulate and explain the mathematical model, which takes the form of a system of first-order delay differential equations [
3], and we discuss briefly the nature of the equilibria and a possible limit cycle of the system and their relation to three model parameters expressing the reproductive ability of the pathogen, the intervention strength, and the response delay. I also introduce the effect of immunization through vaccination in the model. Then I explore the solutions of the system numerically in
Section 3, and demonstrate the existence of a tipping transition that transforms the solution from damped into growing oscillations, and I map the surface in the parameter space where this transition takes place. I explore the effect of vaccination programs on this behavior and also the necessity of establishing a post-pandemic normal with a lower permanent basic reproduction number to prevent future flare-ups of the pandemic. In
Section 4 I discuss the possible policy implications of these results.
3. Results
The delay-differential equation system (
6), with “initial condition” (
7), is an interesting object that warrants further mathematical study. The purpose of this paper, however, is not a mathematical exploration, but to extract those properties of the system that are of particular relevance to the delayed social response to changing reported incidence of the infection with the SARS-CoV-2 virus.
The introduction of dimensionless, independent and dependent variables has revealed that the epidemic evolution depends on the response time delay
measured in units of the effective infectious time
. For COVID-19, a reasonable estimate of
is about one week [
2], which means that time in the plots in this paper is measured in weeks. It is reasonable to expect delays of the order of one week, so delay times in a range around
are explored.
The interpretation of the dimensionless intervention strength
can be seen from the first equation in (
6), if we consider a state with very low incidence
, such that the first term on the right hand side can be neglected. In that case, we can write
, which is the relative rate of change of
measured in the time unit of a week. From experience with the first wave of the COVID-19 epidemic, we have seen that a characteristic time scale of change of
varies from a few weeks to a few months, which is included in the
-range we explore below;
.
3.1. Solutions without Vaccination
Without vaccination,
, and hence
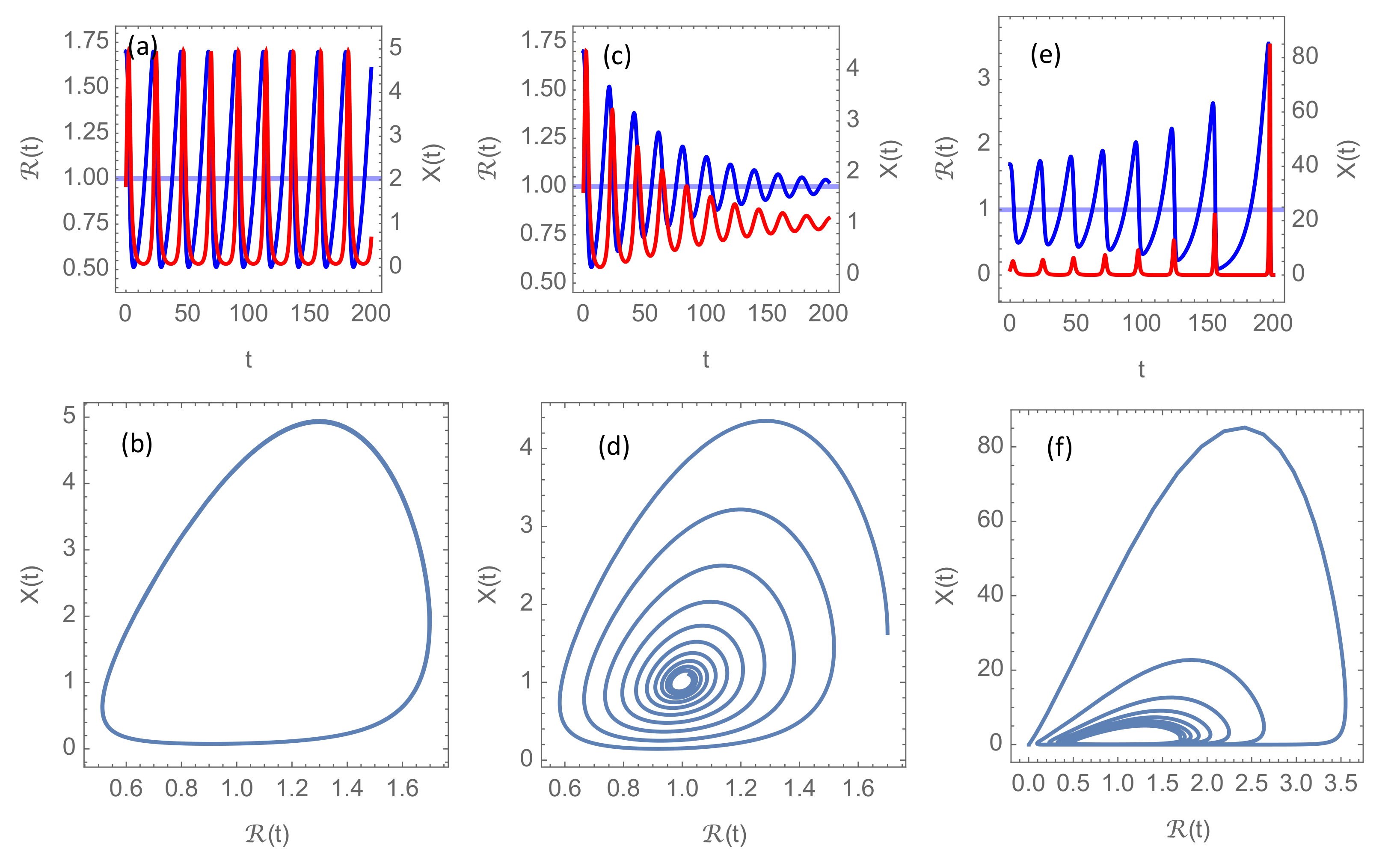
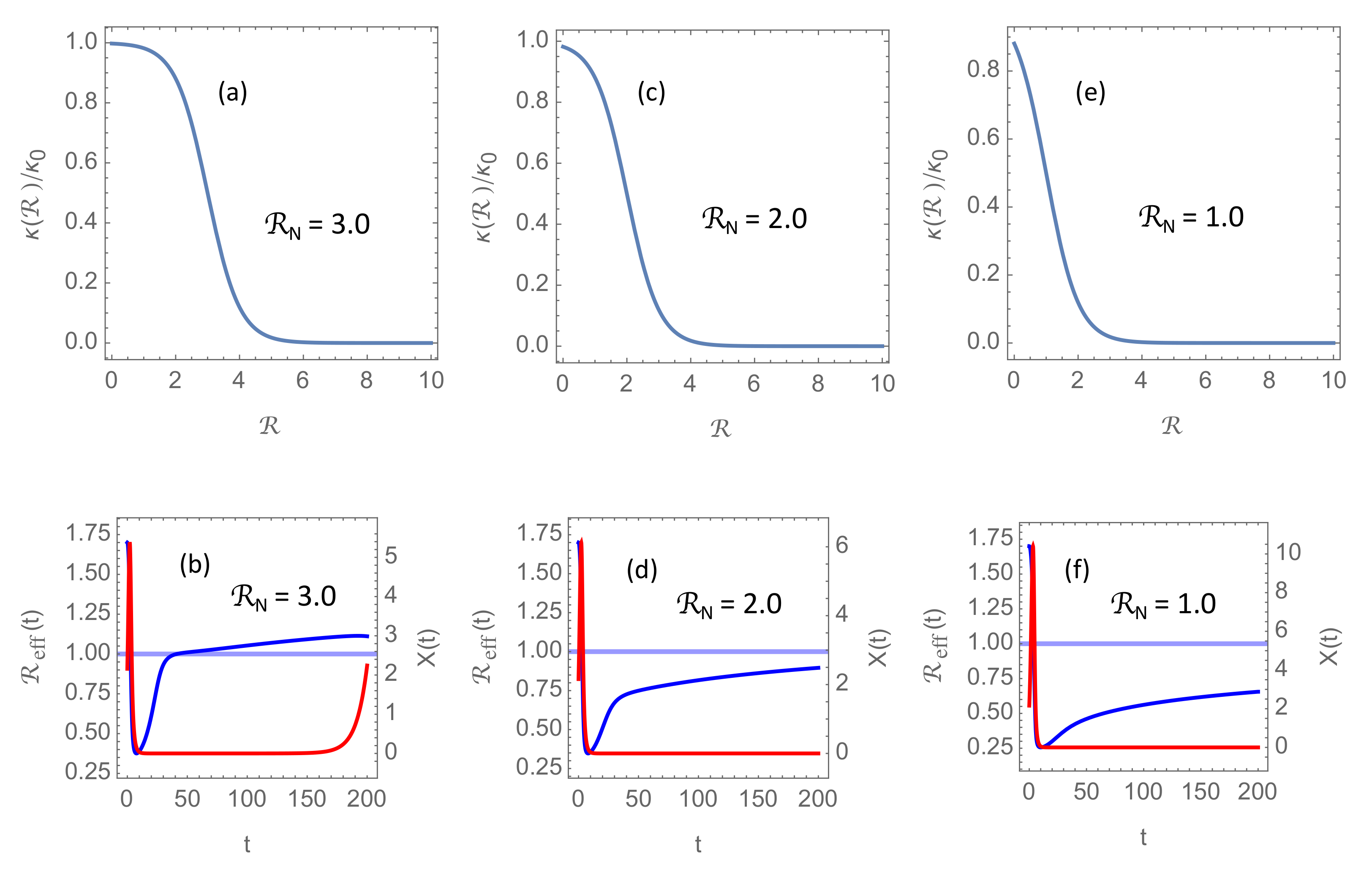
. The three characteristic modes of epidemic development are shown in
Figure 2. On a “transition surface” in the
parameter space, the solution to the system (
6) is a nonlinear oscillation (limit cycle), as shown in
Figure 2a,b, but this oscillation is parametrically unstable. This means that an infinitesimal perturbation of the parameters could lead either to a damped oscillation, as in
Figure 2c,d, or to a growing oscillation, as in
Figure 2e,f, depending on which side of the transition surface the perturbed parameter point is located.
The simulations shown in
Figure 2 have been made with
, which is a reasonable basic reproduction number in the absence of NPIs for the more aggressive mutants circulating in the first months of 2021. However, since
in these simulations always stays well below
, there there is no significant effect of introducing the cut-off at
through Equation (
5). In
Section 3.2 and
Section 3.3, however, it will become clear that this cap
on the basic reproduction number will play an important rôle when vaccination is introduced.
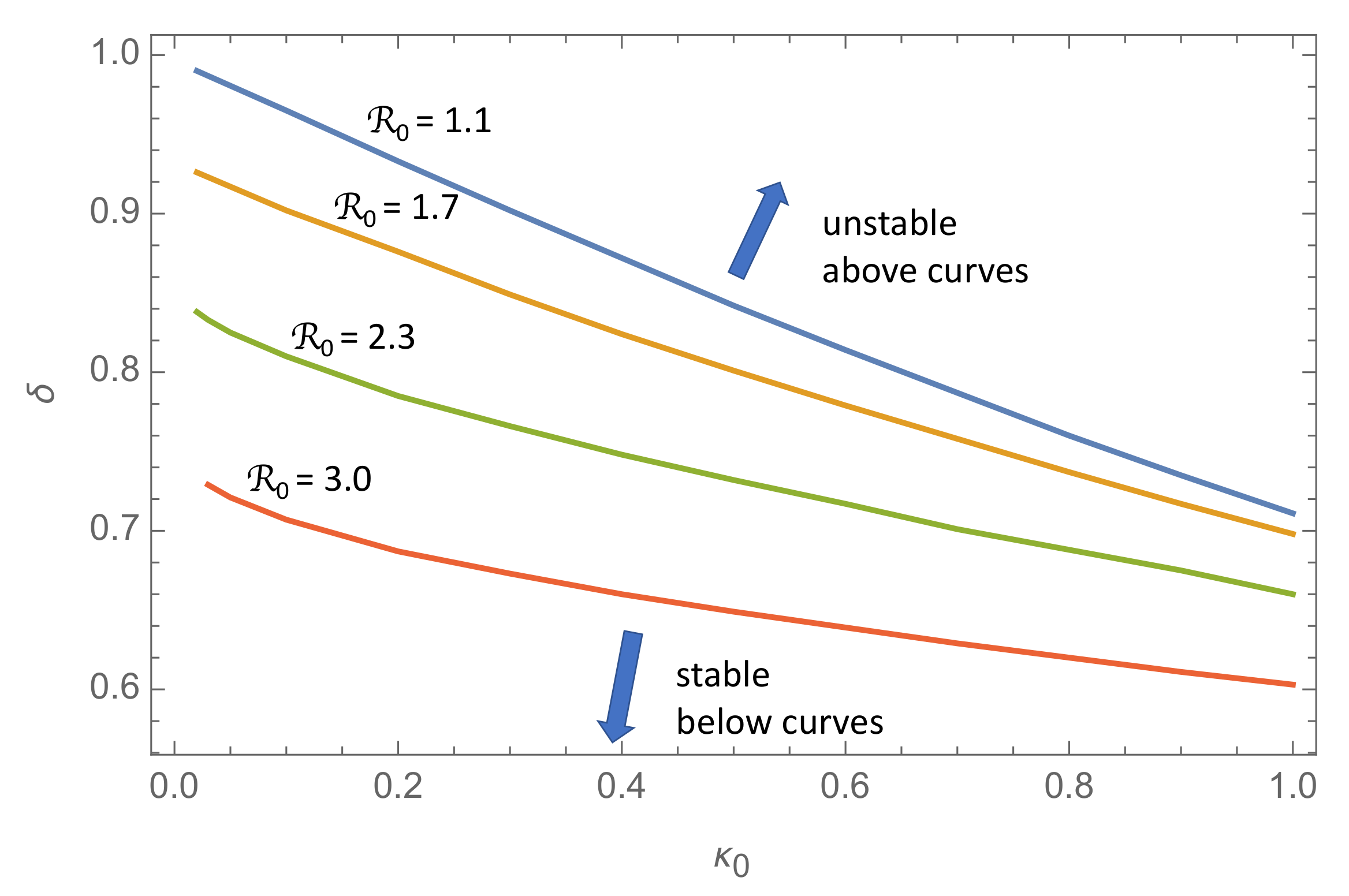
Figure 3 shows isolines (curves of constant
) in the
-plane for
. For any value of
in this range and
in the range
, the solution is a growing oscillation if the
-point is located above the isoline in the (
)-plane, and a damped oscillation below this line.
The take-home message from this figure is that there is a transition from a series of damped epidemic waves to a series of growing waves as the response delay exceeds a critical transition threshold ∼1, or in dimensional units, about one week. Thus, this simple model suggests that a policy responding blindly to the actual incidence rate delayed by more than approximately a week may lead to a succession of epidemic waves of increasing amplitude.
3.2. Solutions with Vaccination
At the time of writing, April 2021, a very low fraction of the world population has achieved immunity due to past infections, and even though vaccines are being rolled out at accelerating pace, there is growing concerns that herd immunity may never be attained [
5]. There are several reasons for this concern. It is not known how long natural immunity from past infections will last, in particular considering the pace at which new mutants of the virus appears. It is not known how well, and how long, the vaccines protect against transmission. And it is not known whether children will be vaccinated at large scale and how large fraction of the population that will reject to take the vaccine. For these reasons, it is difficult to make precise and reliable estimates of the evolution of community immunity in the years to come. Nevertheless, it seems reasonable to assume that natural immunity due to past infections will play a minor rôle. Even if a majority of the population finally may have contracted the virus, waning immunity against new mutants will make such immunity insignificant compared to immunity obtained by new vaccines tailored to combat these new variants. In order to illustrate the possible effect of the vaccination programmes, I shall employ the model Equation (
9) for the fraction of susceptible individuals
, which is shown in
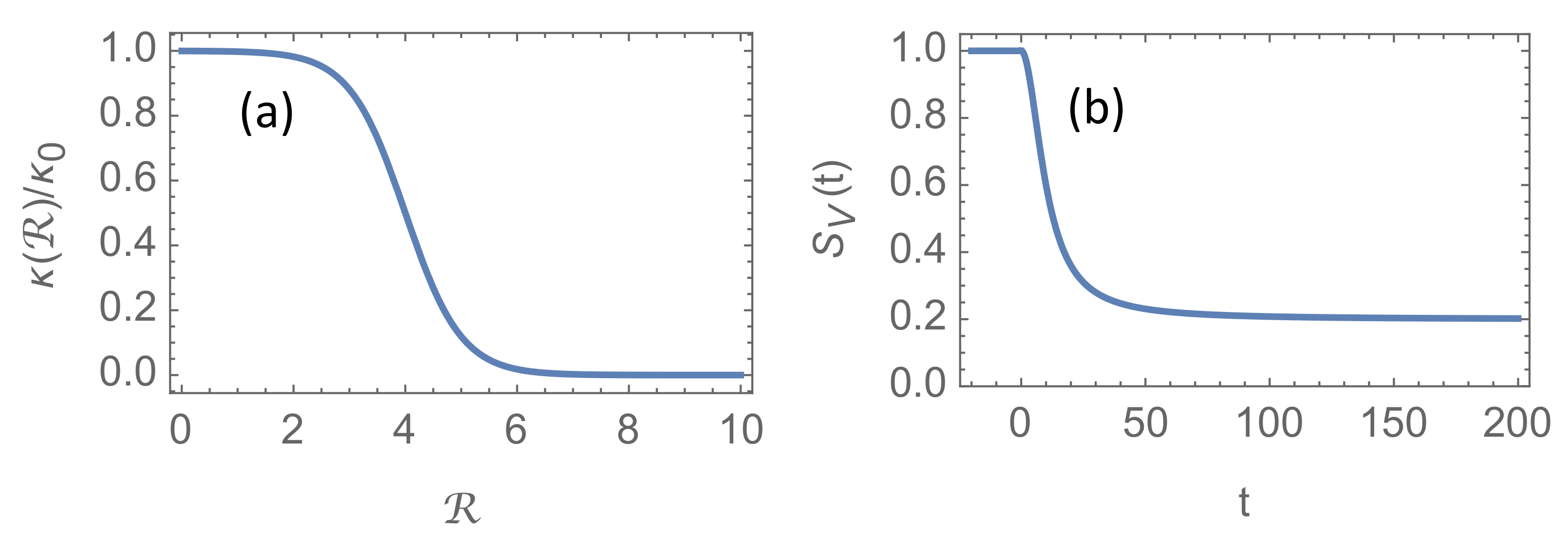
Figure 1b. The results for parameters that are otherwise similar to those in
Figure 2 are shown in
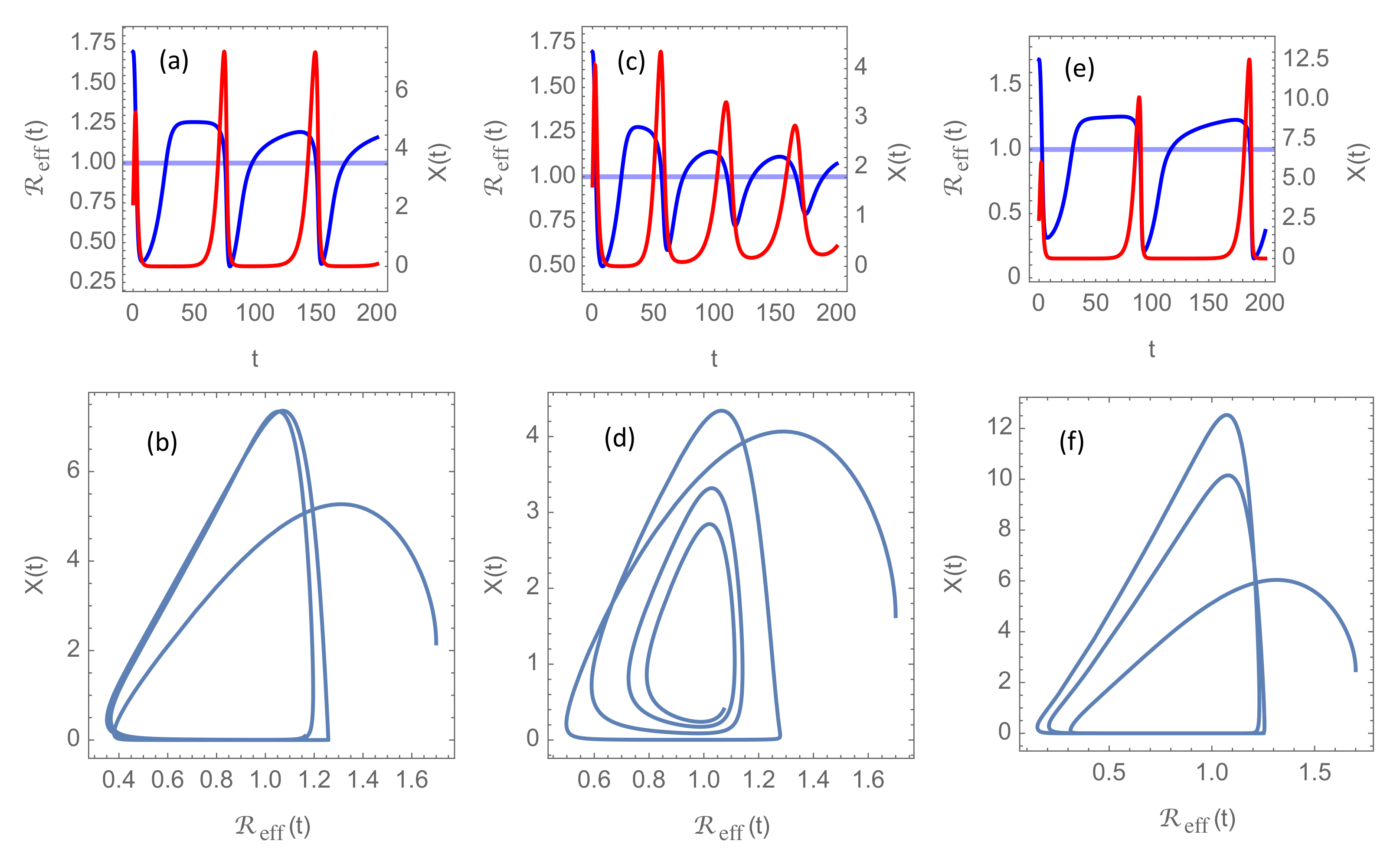
Figure 4. In
Figure 4a,b, I have chosen
to illustrate the convergence towards a stationary cycle, analogous to
Figure 2a,b.
Figure 4c,d, for
, show an example of a decaying oscillation that will finally end up in the equilibrium point
, and
Figure 4e,f, for
, display growing oscillations. Hence, for these model parameters, and specifically for
, we see the same qualitative features as without vaccination. For delay times
well below 1, new waves come at weaker amplitudes, while for
well above 1, the amplitudes are growing with time.
A pronounced difference, though, is the longer intervals of very low incidence between the waves in the vaccination scenarios. Assuming the time unit is approximately one week, there are about three wave peaks per year in the unvaccinated scenarios. In contrast, there is less than one wave per year if the vaccination program is implemented. The reason is that the decreasing fraction of susceptible individuals reduces the effective reproduction number, and this reduces the growth rate of the basic reproduction number during the quiet phases when the incidence is extremely low. The incidence does not start increasing again until exceeds 1, and even if it then grows faster than exponential, it takes considerable time until it attains the threshold for intervention.
Another noticeable difference is that the effective reproduction number in
Figure 4 remains quite low (
) for
. This is because
when the vaccination program is completed and
. The
basic reproduction number
is almost five times larger and is limited by the cap
.
3.3. The Post-Pandemic Normal—A Permanent Cap on the Basic Reproduction Number
An additional message one can take home from
Figure 4 is that, even when vaccination seems to have eradicated the disease, the existence of the virus in small pockets of the population is sufficient to cause strong new outbreaks if society is allowed to return to the pre-pandemic state with a large “normal” basic reproduction number
. In
Figure 4, it is assumed
, which is a reasonable basic reproduction number for the most aggressive mutants circulating in early 2021 in high-income countries with no NPIs implemented.
The key to prevent this kind kind of repeated flare-up of the epidemic could be that policy makers decide to introduce some permanent NPIs that effectively introduce a long term cap
on the the basic reproduction number which is lower than expected in a society with no NPIs. In practice, this would imply retaining on a permanent basis some of the most effective, but least annoying, NPIs, or invention of new NPIs. This idea, that fundamental long-term changes will have to be made in order to prevent new global outbreaks of COVID-19 or other contagious diseases, is often referred to as the new post-pandemic normal [
6,
7].
Figure 5 shows the results of implementing permanent NPIs giving rise to lower
in the model. Specifically, it shows solutions with
3, 2, and 1, respectively. Otherwise, the parameters are the same as in
Figure 4a, where
and
. It is seen that in scenarios where delayed response tend to give repeated flare-ups, reduction of
can slow the rise of
and push the second wave several years into the future. With such a long quiet period with almost no transmission of the virus one could realistically expect that a sufficient fraction of the population could be immunized from new and efficient vaccines for herd immunity to be established, or at least that the disease could enter an endemic state comparable to present day’s seasonal influenzas.
4. Discussion
Our model system (
6) is an extremely simplified representation of a complex reality, although the second equation is probably far easier to accept than the first, since it just describes the balance between new infections produced in a population where the instantaneous reproduction number is
and the reduction of the number of infections due to recovery or death.The first equation, on the other hand, aspires to encapsulate the complex social dynamics that determines the evolution of
in one single delay differential equation. Such a simple representation of a complex reality is certainly wrong, but may still be a valuable supplement to purely qualitative reasoning over the socio-political process that determines the response to a changing disease burden.
The potentially disastrous effect of delayed NPI response was recognized by Pei, Shandula, and Shaman (2020), employing a metapopulation transmission model and data on infections, deaths and human mobility in the United States [
8]. Their findings indicate that if control measures and reductions of
had been implemented just 1 to 2 weeks earlier, substantial cases and deaths could have been avoided. It is concluded that rapid detection of increasing case numbers and fast reimplementation of control measures are needed to control repeated outbreaks. They run a considerably more complex transmission model than our Equation (
6), but make no attempt to model the social NPI response. They rather project the disease spread under factual and counterfactual NPI scenarios, and hence, the possibility that NPI delays may lead to a succession of epidemic waves of increasing amplitude is not explored.
The idea of NPIs trigged as incidence rates exceed a certain threshold is not new either. In a paper on strategies for mitigation and suppression of COVID-19 in countries of different income level, Walker et al. (2020) [
9] modeled an oscillatory pattern of occupancy in intensive care units (ICUs) by assuming NPIs resulting in instantaneous reduction of 75% in
from a basic level 3.0 each time the threshold is exceeded, and a duration of the NPIs of 1 month. Thus, in that model
flips between 0.75 and a value that starts at 3.0 but is slightly reduced in successive flips as herd immunity starts to emerge. By construction, this model will not allow the oscillations of disease incidence rate to grow in amplitude; the incidence threshold cannot be exceeded since the reproduction number drops instantaneously below 1 once the threshold is attained. The model presented here differs from [
9] in two important respects:
- (1)
The effect on
of crossing the incidence threshold is not an instantaneous shift, but an effect on the rate of change
which is proportional to the deviation from the threshold. In this way one allows for an inertia in the response, which gives rise to a damped oscillation around the threshold incidence. This can be seen from introducing the perturbations
,
around the equilibrium
, and linearizing the model system (
6) for
, which yields the damped harmonic oscillator equation;
where the “friction coefficient” and the “spring constant” both are the same and given by
.
- (2)
We allow for a delay in the NPI-response with respect to the time the incidence threshold is crossed, and demonstrate that a sufficiently large delay may lead to a transition from a damped oscillation to a growing oscillation.
The only damping taking place in [
9] is a very slow reduction in effective reproduction number arising from emerging herd immunity, i.e., reduction of the fraction of susceptible individuals,
S. This damping effect is not taken into account in our model, since it assumed to be a negligible effect until vaccines effective against disease transmission have been rolled out in all adult age groups. On the other hand, the present work takes immunity due to vaccination into account and shows that it can prolong the quiet periods between outbreaks, but may not alone be sufficient to prevent new outbreaks altogether. Effective prevention of repeated outbreaks may require a permanent reduction of the basic reproduction number, or in other words, a societal transformation to a new post-pandemic normal. Some countries, like China, New-Zealand, and Australia, have had some success in adopting such a new normal by implementing varying approaches to reduce
. Common to all, though, is that this has been achieved without extensive vaccination.
The absence in [
9] of delay in the social response and the absence of a reduction in the response strength due to intervention fatigue (as explored in [
2]), also preclude the possibility of repeated pandemic waves of growing amplitude. Thus, the value of the scenarios depicted in [
9] is primarily to quantify the fraction of the time societies will have to spend in lockdown in order to keep ICU occupancy below a given threshold, under the rather unrealistic assumption of full societal control of the reproduction number.
Under the assumption of an infectious period of days, and reasonable values of the parameters and , our model predicts that the transition from damped to growing oscillation occurs for a response delay of the order of one week. This number should of course be taken with a grain of salt, considering the simplicity of the response model. Nevertheless, it is disturbing that this critical response time turns out to be so short that it may be impossible, even under ideal circumstances, to avoid this regime of recurrent waves of growing amplitudes, provided we assume that NPIs are implemented by only using information about the most recent available case notification rate.
A valid objection to this assumption, and hence to the model system (
6), is that governments have access to far more information when they lift on existing NPIs and decide on new ones. They can consider existing trends in the incidence rates, not just the the most recent reported recordings, and model projections are available. On the other hand, it is clear for anyone who follow the public debate on the necessity of interventions, that it is extremely difficult for policy makers to gain public acceptance for precautionary interventions, even in cases when a pattern has been repeated several times in the past. The common belief that the present wave is the last one seems to be a major hurdle to successful control of the epidemic.
The tendency of repeated and stronger epidemic waves has been ubiquitous across the world’s countries as we move into the second year of the pandemic. In [
2], which was a precursor to the present paper, we demonstrated this by analyzing incidence data for 150 countries for the first eight months of the pandemic. Patterns of decaying as well as growing waves were observed and attempted explained by a model similar to the one presented here, but without the effects of response delay, vaccination, and a post-pandemic normal with lower
. In particular, repeated waves of growing amplitude were explained as a result of intervention fatigue, which was introduced in the model. Here, we show that this unstable growth may alternatively be a result of delays in the social response which only can be avoided if governments are able to take precautionary action. The key message from the present work is that relatively small changes in governments’ ability to respond in a more precautionary manner may have profound effects on controlling and mitigating new outbreaks. Modest, permanent changes towards a new post-pandemic normal can also be effective. The main challenge seems to be convey this insight to policy makers, the media, and others who shape the public opinion.