Physical Activity and Glycemic Control Status in Chinese Patients with Type 2 Diabetes: A Secondary Analysis of a Randomized Controlled Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Subjects
2.2. Interventions
2.3. Data Collection and Assessment of PA Level
2.4. Reclassification of Subjects by Changes in PA Level
2.5. Statistical Methods
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Global Report on Diabetes; World Health Organization: Geneva, Switzerland, 2016. [Google Scholar]
- Shigaki, C.; Kruse, R.L.; Mehr, D.; Sheldon, K.M.; Ge, B.; Moore, C.; Lemaster, J. Motivation and diabetes self-management. Chronic Illn. 2010, 6, 202–214. [Google Scholar] [CrossRef] [PubMed]
- O’Hagan, C.; De Vito, G.; Boreham, C.A. Exercise prescription in the treatment of type 2 diabetes mellitus: Current practices, existing guidelines and future directions. Sports Med. 2013, 43, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Caspersen, C.J.; Powell, K.E.; Christenson, G.M. Physical activity, exercise, and physical fitness: Definitions and distinctions for health-related research. Public Health Rep. 1985, 100, 126–131. [Google Scholar]
- Dutton, G.R.; Lewis, C.E. The Look AHEAD Trial: Implications for lifestyle intervention in type 2 diabetes mellitus. Prog. Cardiovasc. Dis. 2015, 58, 69–75. [Google Scholar] [CrossRef]
- Blomster, J.I.; Chow, C.K.; Zoungas, S.; Woodward, M.; Patel, A.; Marre, M.; Harrap, S.; Chalmers, J.; Hilis, G.S. The influence of physical activity on vascular complications and mortality in patients with type 2 diabetes mellitus. Diabetes Obes. Metab. 2013, 15, 1008–1012. [Google Scholar] [CrossRef]
- 2018 Physical Activity Guidelines Advisory Comittee. The 2018 Physical Activity Guidelines Advisory Committee Scientific Report; U.S. Department of Health and Human Services: Washington, DC, USA, 2018; p. 779.
- Liu, H.; Liu, Z.; Wang, Y.; Stinchcombe, T.E.; Owzar, K.; Han, Y.; Hung, R.J.; Brhane, Y.; McLaughin, J.; Brennan, P.; et al. Functional variants in DCAF4 Associated with lung cancer risk in European populations. Carcinogenesis 2017, 38, 541–551. [Google Scholar] [CrossRef]
- Monda, K.L.; Adair, L.S.; Zhai, F.; Popkin, B.M. Longitudinal relationships between occupational and domestic physical activity patterns and body weight in China. Eur. J. Clin. Nutr. 2008, 62, 1318–1325. [Google Scholar] [CrossRef]
- Chao, D.; Foy, C.G.; Farmer, D. Exercise adherence among older adults: Challenges and strategies. Control. Clin. Trials 2000, 21, 212s–217s. [Google Scholar] [CrossRef]
- Byrne, H.; Caulfield, B.; De Vito, G. Effects of self-directed exercise programmes on individuals with type 2 diabetes mellitus: A systematic review evaluating their effect on HbA1c and other metabolic outcomes, physical characteristics, cardiorespiratory fitness and functional outcomes. Sports Med. 2017, 47, 717–733. [Google Scholar] [CrossRef]
- Physical Activity Guidelines Advisory Committee. Physical Activity Guidelines Advisory Committee Report; U.S. Department of Health and Human Services: Washington, DC, USA, 2008. Available online: https://www.europarc.org/wp-content/uploads/2018/03/Physical-Activity-Guidelines-Advisory-Committee-Report-2008.pdf (accessed on 10 September 2012).
- Yu, R.B.; Hong, X.; Ding, W.-L.; Tan, Y.-F.; Zhang, Y.-X.; Sun, N.-X.; Wu, G.-L.; Zhan, S.-W.; Ge, D.-F. The association between the genetic polymorphism of HLA-DQA1, DQB1, and DRB1 and serum alanine aminotransferase levels in chronic hepatitis C in the Chinese population. J. Gastroenterol. Hepatol. 2008, 23, 1394–1402. [Google Scholar] [CrossRef]
- Nwasuruba, C.; Khan, M.; Egede, L.E. Racial/ethnic differences in multiple self-care behaviors in adults with diabetes. J. Gen. Intern. Med. 2007, 22, 115–120. [Google Scholar] [CrossRef]
- Thiel, D.M.; Sayah, F.A.; Vallance, J.K.; Johnson, S.T.; Johnson, J.A. Association between physical activity and health-related quality of life in adults with type 2 diabetes. Can. J. Diabetes 2017, 41, 58–63. [Google Scholar] [CrossRef]
- Xu, F.; Wang, Y.; Ware, R.S.; Tse, L.A.; Dunstan, D.W.; Liang, Y.; Wang, Z.; Hong, X.; Owen, N. Physical activity, family history of diabetes and risk of developing hyperglycaemia and diabetes among adults in mainland China. Diabet. Med. 2012, 29, 593–599. [Google Scholar] [CrossRef]
- Rossen, J.; Yngve, A.; Hagströmer, M.; Brismar, K.; Ainsworth, B.E.; Iskull, C.; Möller, P.; Johansson, U.-B. Physical activity promotion in the primary care setting in pre- and type 2 diabetes—The Sophia Step Study, an RCT. BMC Public Health 2015, 15, 647. [Google Scholar] [CrossRef]
- Nielsen-Bohlman, L.; Panzer, A.M.; Kindig, D.A. Health Literacy: A Prescription to End Confusion; National Academies Press (US): Washington, DC, USA, 2004. [Google Scholar]
- Lam, M.H.; Leung, A.Y. The effectiveness of health literacy oriented programs on physical activity behaviour in middle aged and older adults with type 2 diabetes: A systematic review. Health Psychol. Res. 2016, 4, 5595. [Google Scholar] [CrossRef]
- Rothman, R.L.; DeWalt, D.A.; Malone, R.; Bryant, B.; Shintani, A.; Crigler, B.; Weinberger, M.; Pignone, M. Influence of patient literacy on the effectiveness of a primary care-based diabetes disease management program. JAMA 2004, 292, 1711–1716. [Google Scholar] [CrossRef]
- Wang, L.; Fang, H.; Xia, Q.; Liu, X.; Chen, Y.; Zhou, P.; Yan, Y.; Yao, B.; Wei, Y.; Jiang, Y.; et al. Health literacy and exercise-focused interventions on clinical measurements in Chinese diabetes patients: A cluster randomized controlled trial. EClinicalMedicine 2019, 17, 100211. [Google Scholar] [CrossRef]
- Xu, W.H.; Rothman, R.L.; Li, R.; Chen, Y.; Xia, Q.; Fang, H.; Gao, J.; Yan, Y.; Zhou, P.; Jiang, Y. Improved self-management skills in Chinese diabetes patients through a comprehensive health literacy strategy: study protocol of a cluster randomized controlled trial. Trials 2014, 15, 498. [Google Scholar] [CrossRef][Green Version]
- Turner, L.; Shamseer, L.; Altman, D.G.; Weeks, L.; Peters, J.; Kober, T.; Dias, S.; Schulz, K.F.; Plint, A.C.; Moher, D. Consolidated Standards of Reporting Trials (CONSORT) and the Completeness of Reporting of Randomised Controlled trials (RCTs) published in medical journals. Cochrane Database Syst. Rev. 2012, 11, MR000030. [Google Scholar] [CrossRef]
- Chinese Diabetes Society. Standards of care for type 2 diabetes in China (2013). Chin J Diabetes. 2014, 8, 2–42. (in Chinese). [Google Scholar]
- Borg, G. Psychophysical scaling with applications in physical work and the perception of exertion. Scand J. Work Environ. Health 1990, 16 (Suppl. 1), 55–58. [Google Scholar] [CrossRef] [PubMed]
- Wolff, K.; Chambers, L.; Bumol, S.; White, R.O.; Gregory, B.P.; Davis, D.; Rothman, R.L. The PRIDE (Partnership to Improve Diabetes Education) Toolkit: Development and evaluation of novel literacy and culturally sensitive diabetes education materials. Diabetes Educ. 2016, 42, 23–33. [Google Scholar] [CrossRef]
- Wolff, K.; Cavanaugh, K.; Malone, R.; Hawk, V.; Gregory, B.P.; Davis, D.; Wallston, K.; Rothman, R. The Diabetes Literacy and Numeracy Education Toolkit (DLNET): Materials to facilitate diabetes education and management in patients with low literacy and numeracy skills. Diabetes Educ. 2009, 35, 233–236, 238–241, 244–245. [Google Scholar] [CrossRef]
- Department of Noncommunicable Disease and Mental Health World Health Organization. Stepwise Surveillance for Chronic Non-Communicable Diseases (STEPS) I; World Health Organization: Geneva, Switzerland, 2010; Available online: https://www.who.int/ncds/surveillance/steps/GPAQ_CH.pdf (accessed on 10 September 2012). (in Chinese)
- Limb, E.S.; Ahmad, S.; Cook, D.G.; Kerry, S.M.; Ekelund, U.; Whincup, P.H.; Victor, C.R.; Iliffe, S.; Ussher, M.; Fox-Rushby, J.; et al. Measuring change in trials of physical activity interventions: A comparison of self-report questionnaire and accelerometry within the PACE-UP Trial. Int. J. Behav. Nutr. Phys. Act. 2019, 16, 10. [Google Scholar] [CrossRef] [PubMed]
- Ainsworth, B.E.; Haskell, W.L.; Leon, A.S.; Jacobs, D.R., Jr.; Montoye, H.J.; Sallis, J.F.; Paffenbarger, R.S. Compendium of physical activities: Classification of energy costs of human physical activities. Med. Sci. Sports Exerc. 1993, 25, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Chu, A.H.; Ng, S.H.X.; Koh, D.; Müller-Riemenschneider, F. Reliability and validity of the self- and interviewer-administered versions of the Global Physical Activity Questionnaire (GPAQ). PLoS ONE 2015, 10, e0136944. [Google Scholar]
- World Health Organization. Global Physical Activity Questionnaire (GPAQ) Analysis Guide; World Health Organization: Geneva, Switzerland, 2003. [Google Scholar]
- Olson, E.A.; McAuley, E. Impact of a brief intervention on self-regulation, self-efficacy and physical activity in older adults with type 2 diabetes. J. Behav. Med. 2015, 38, 886–898. [Google Scholar] [CrossRef]
- Melkus, G.D. A 12-month intensive supervised exercise intervention and counselling reduces HbA1c, blood pressure and other modifiable cardiovascular risk factors in people with type 2 diabetes. Evid. Based Nurs. 2011, 14, 68–69. [Google Scholar] [CrossRef]
- Araiza, P.; Hewes, H.; Gashetewa, C.; Vella, C.A.; Burge, M.R. Efficacy of a pedometer-based physical activity program on parameters of diabetes control in type 2 diabetes mellitus. Metabolism 2006, 55, 1382–1387. [Google Scholar] [CrossRef]
- Rosal, M.C.; Ockene, I.S.; Restrepo, A.; White, M.J.; Borg, A.; Olendzki, B.; Scavron, J.; Candib, L.; Welch, G.; Reed, G. Randomized trial of a literacy-sensitive, culturally tailored diabetes self-management intervention for low-income Latinos: Latinos en control. Diabetes Care 2011, 34, 838–844. [Google Scholar] [CrossRef]
- Miquelon, P.; Castonguay, A. Motives for participation in physical activity and observance of physical activity recommendations among adults with type 2 diabetes. Can. J. Diabetes 2016, 40, 399–405. [Google Scholar] [CrossRef] [PubMed]
- Parajuli, J.; Saleh, F.; Thapa, N.; Ali, L. Factors associated with nonadherence to diet and physical activity among nepalese type 2 diabetes patients; a cross sectional study. BMC Res. Notes 2014, 7, 758. [Google Scholar] [CrossRef] [PubMed]
- Ng, S.W.; Popkin, B.M. Time use and physical activity: A shift away from movement across the globe. Obes. Rev. 2012, 13, 659–680. [Google Scholar] [CrossRef] [PubMed]
- Murano, I.; Asakawa, Y.; Mizukami, M.; Takihara, J.; Shimizu, K.; Imai, T. Factors increasing physical activity levels in diabetes mellitus: A survey of patients after an inpatient diabetes education program. J. Phys. Ther. Sci. 2014, 26, 695–699. [Google Scholar] [CrossRef][Green Version]
- Avery, L.; Flynn, D.; Dombrowski, S.U.; van Wersch, A.; Sniehotta, F.F.; Trenell, M.I. Successful behavioural strategies to increase physical activity and improve glucose control in adults with type 2 diabetes. Diabet. Med. 2015, 32, 1058–1062. [Google Scholar] [CrossRef]
- Mynarski, W.; Psurek, A.; Borek, Z.; Rozpara, M.; Grabara, M.; Strojek, K. Declared and real physical activity in patients with type 2 diabetes mellitus as assessed by the international physical activity questionnaire and caltrac accelerometer monitor: A potential tool for physical activity assessment in patients with type 2 diabetes mellitus. Diabetes Res. Clin. Pract. 2012, 98, 46–50. [Google Scholar]
- Wisse, W.; Rookhuizen, M.B.; de Kruif, M.D.; van Rossum, J.; Jordans, I.; ten Cate, H.; van Loon, L.J.C.; Meesters, E.W. Prescription of physical activity is not sufficient to change sedentary behavior and improve glycemic control in type 2 diabetes patients. Diabetes Res. Clin. Pract. 2010, 88, e10-3. [Google Scholar] [CrossRef]
- Van Dyck, D.; De Greef, K.; Deforche, B.; Ruige, J.; Bouckaert, J.; Tudor-Locke, C.E.; Kaufman, J.-M.; De Bourdeaudhuij, I. The relationship between changes in steps/day and health outcomes after a pedometer-based physical activity intervention with telephone support in type 2 diabetes patients. Health Educ. Res. 2013, 28, 539–545. [Google Scholar] [CrossRef]
- Li, L.; Yin, X.; Yu, D.; Li, H. Impact of physical activity on glycemic control and insulin resistance: A study of community-dwelling diabetic patients in eastern China. Intern. Med. 2016, 55, 1055–1060. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nicaise, V.; Crespo, S.; Marshall, S. Agreement between the IPAQ and accelerometer for detecting intervention-related changes in physical activity in a sample of Latina women. J. Phys. Act. Health 2014, 11, 846–852. [Google Scholar] [CrossRef]
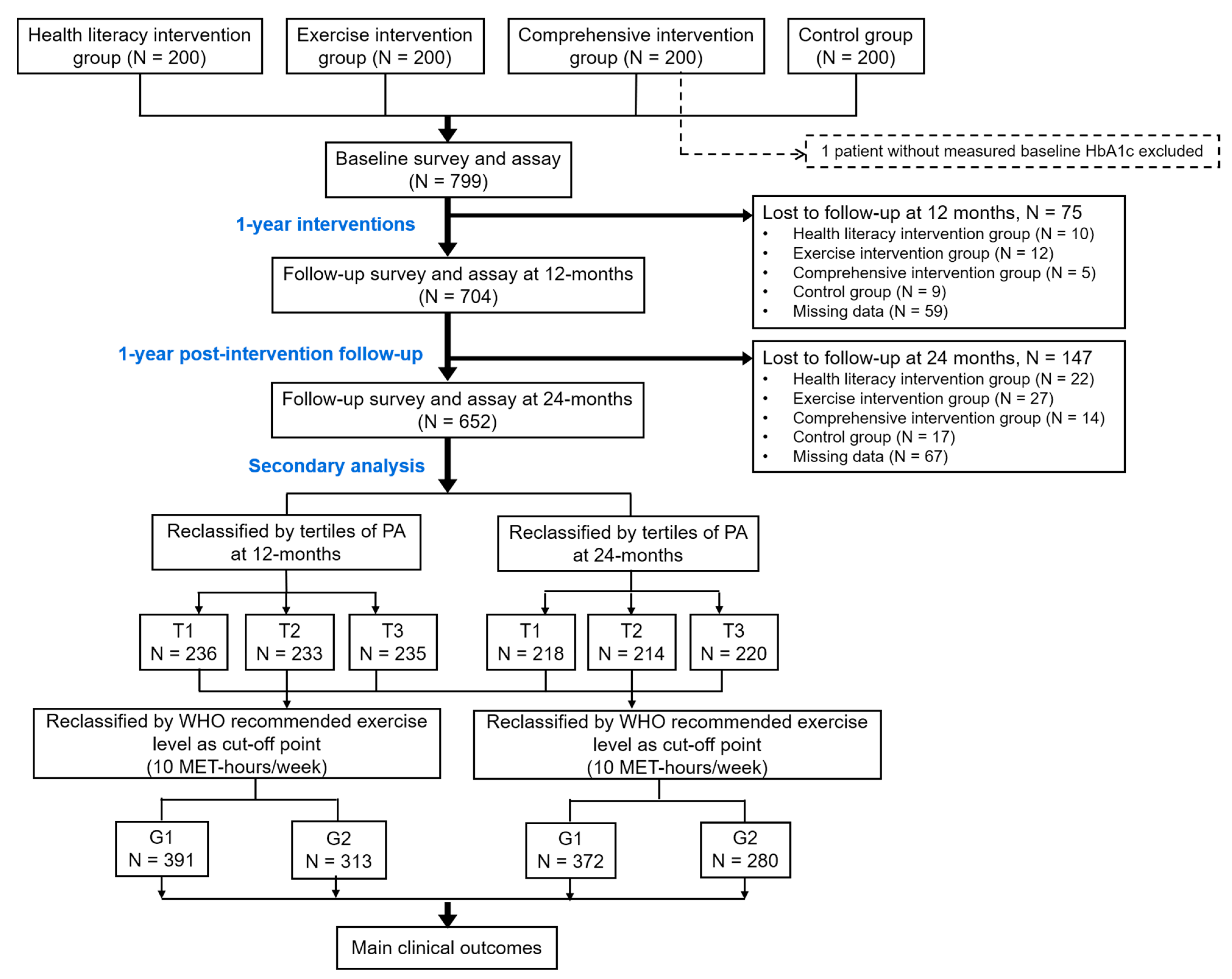
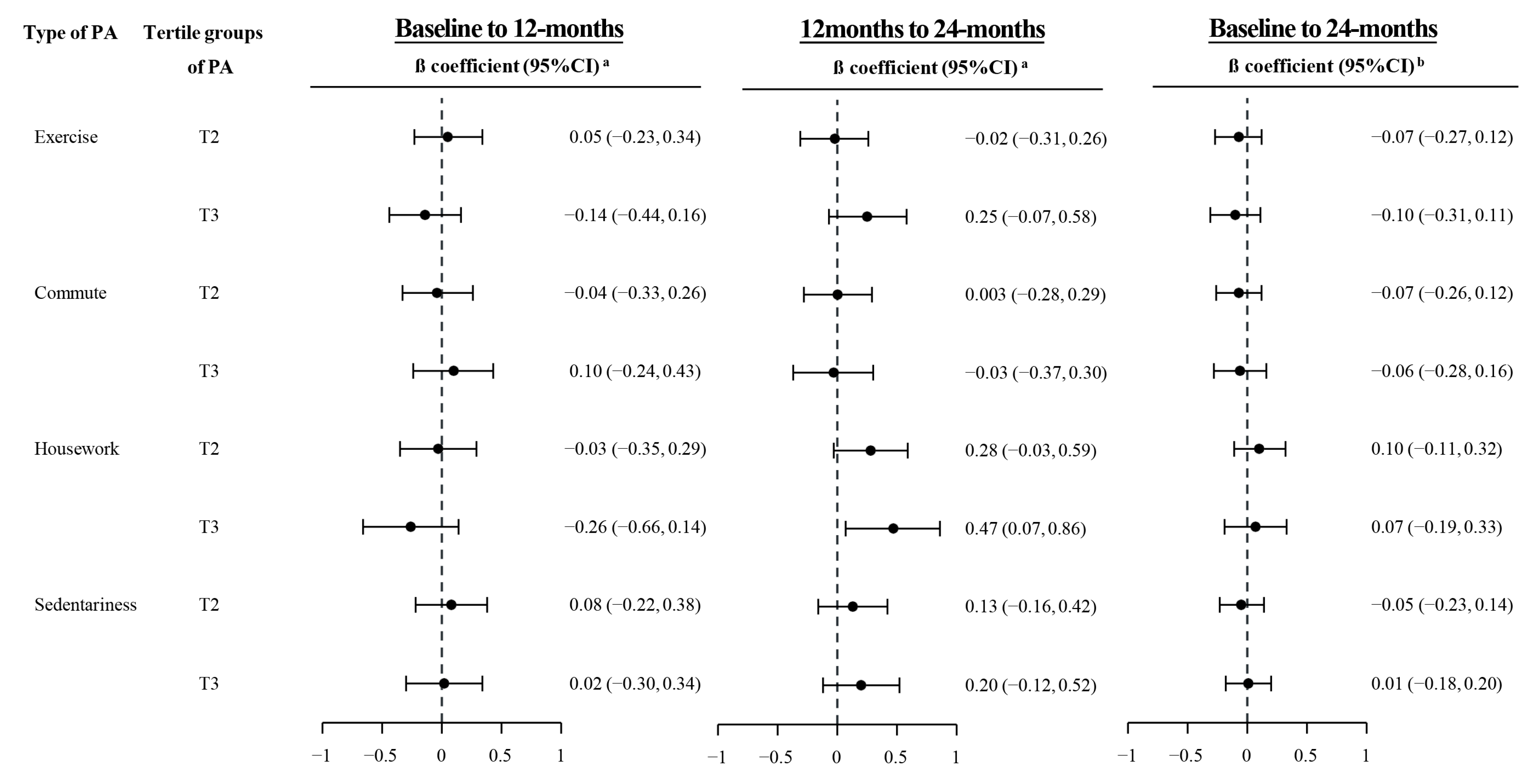
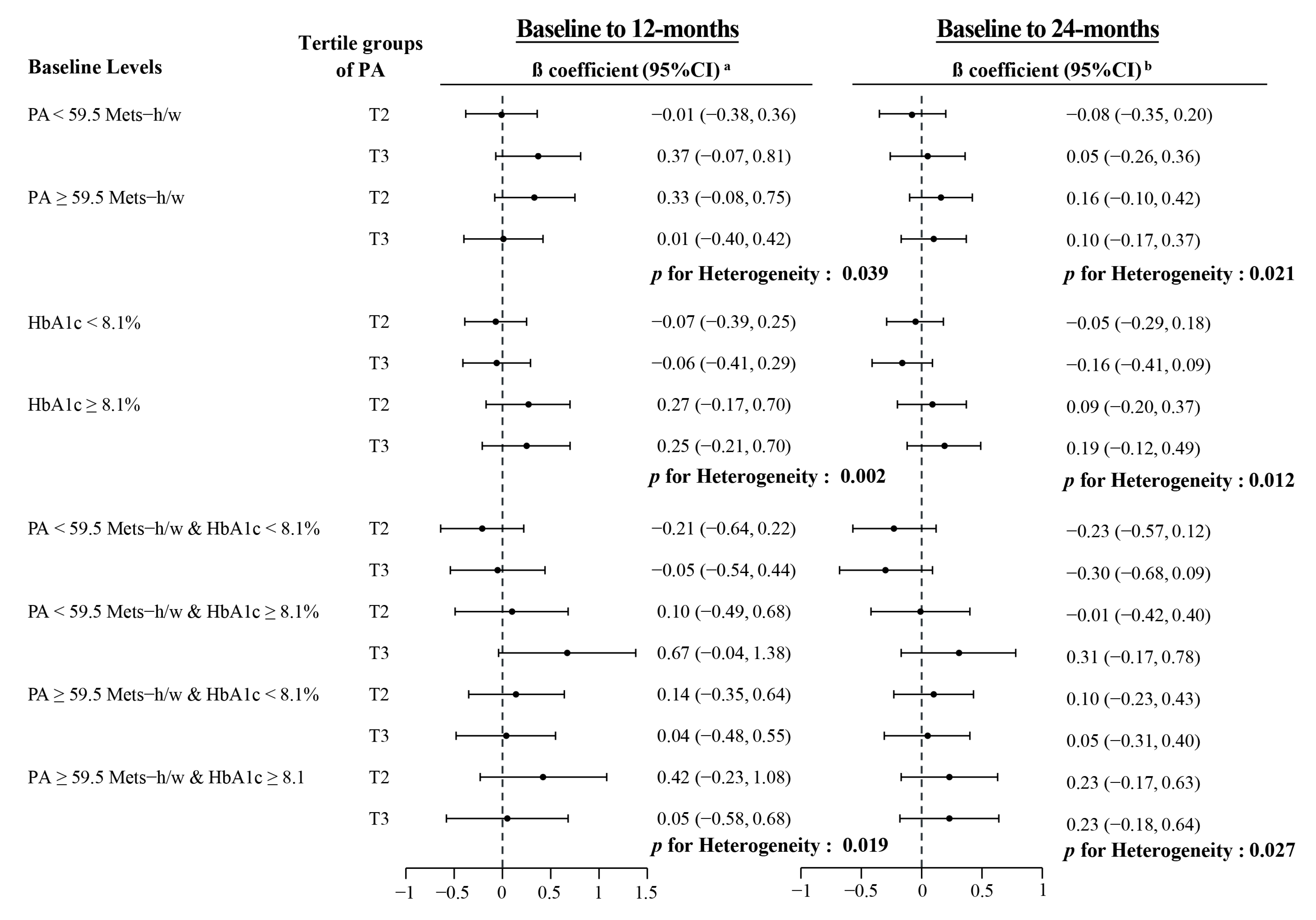
| Median (IQR) of PA (METs-h/w) | Intervention Groups | Control Arm | p-Value a | ||
|---|---|---|---|---|---|
| Health Literacy | Exercise | Comprehensive | |||
| At baseline | |||||
| Overall | 56.0 (30.0, 84.0) | 50.5 (33.5, 80.7) | 50.0 (28.0, 90.0) | 71.5 (53.0, 108.0) | <0.001 |
| Exercise | 0 (0, 14.0) | 4.0 (0, 20.0) | 0 (0, 20.0) | 7.5 (0, 28.0) | 0.01 |
| Commute | 18.7 (0, 30.2) | 16.0 (8.0, 28.0) | 12.0 (0, 28.0) | 28.0 (14.0, 29.0) | <0.001 |
| Housework | 21.0 (4.5, 42.0) | 21.0 (10.5, 42.0) | 21.0 (10.5, 42.0) | 42.0 (21.0, 52.5) | <0.001 |
| Sedentariness | 35.0 (28.0, 49.0) | 31.5 (21.0, 49.0) | 28.0 (14.1, 35.0) | 24.5 (14.0, 31.5) | <0.001 |
| At 12 months | |||||
| Overall | 41.8 (16.9, 66.1) * | 70.0 (41.5, 97.4) * | 55.0 (28.0, 81.0) | 73.7 (44.3, 108.3) | <0.001 |
| Exercise | 0 (0, 10.0) | 12.0 (0, 22.7) | 8.2 (0, 28.0) | 7.3 (0, 28.0) | <0.001 |
| Commute | 10.0 (0, 28.0) * | 23.3 (10.0, 38.5) * | 12.0 (0, 28.0) | 28.0 (6.3, 42.0) | <0.001 |
| Housework | 18.0 (5.0, 42.0) * | 30.8 (9.0, 42.0) | 21.0 (7.5, 42.0) * | 31.5 (10.5, 42.0) * | 0.001 |
| Sedentariness | 28.0 (21.0, 35.0) * | 28.0 (21.6, 35.0) * | 28.0 (21.0, 35.0) | 28.0 (21.0, 42.0) * | 0.94 |
| At 24 months | |||||
| Overall | 39.5 (17.8, 69.4) * | 57.0 (29.0, 91.0) + | 64.8 (30.8, 104.5) + | 70.0 (36.0, 110.0) | <0.001 |
| Exercise | 0 (0, 4.7) *+ | 4.0 (0, 20.0) + | 17.0 (0, 38.0) *,+ | 8.0 (0, 24.0) | <0.001 |
| Commute | 10.0 (0, 28.0) * | 18.7 (8.0, 42.0) * | 13.7 (0, 30.0) + | 14.0 (0, 30.7) | <0.001 |
| Housework | 21.0 (6.0, 42.0) + | 21.0 (9.0, 42.0) + | 21.0 (9.0, 42.0) * | 31.5 (10.5, 49.0) * | 0.03 |
| Sedentariness | 35.0 (21.0, 42.0) *+ | 31.5 (21.0, 45.5) + | 28.0 (21.0, 42.0) * | 28.0 (17.5, 35.0) * | <0.001 |
| Baseline Characteristics | HbA1c Level (%, Median, IQR) | β Coefficients (SE) | |||
|---|---|---|---|---|---|
| At baseline | At 12-months | At 24-months | Unadjusted | Adjusted a | |
| Age (years) | |||||
| <65 | 8.1 (7.5, 9.0) | 8.0 (6.9, 9.1) | 7.9 (7.0, 9.1) | 0 (ref) | 0 (ref) |
| ≥65 | 8.1 (7.6, 9.2) | 8.0 (7.1, 9.1) | 7.9 (6.9, 9.1) | 0.05 (0.10) | 0.06 (0.14) |
| Sex (%) | |||||
| Men | 8.1 (7.6, 9.1) | 8.1 (7.1, 9.2) | 7.9 (7.0, 9.1) | 0 (ref) | 0 (ref) |
| Women | 8.1 (7.5, 9.1) | 8.0 (7.1, 9.1) | 7.8 (6.9, 9.1) | −0.11 (0.10) | −0.11 (0.09) |
| Educational level (%) | |||||
| Primary school or below | 8.1 (7.5, 8.9) | 8.1 (7.2, 9.3) | 8.1 (7.2, 9.7) | 0 (ref) | 0 (ref) |
| Junior high school | 8.2 (7.6, 9.3) | 8.1 (7.1, 9.2) | 7.9 (7.0, 9.1) | −0.13 (0.13) | −0.23 (0.13) |
| Senior high school | 8.1 (7.6, 8.9) | 8.0 (7.1, 8.8) | 7.9 (7.0, 8.9) | −0.20 (0.14) | −0.25 (0.13) |
| College and above | 7.9 (7.4, 8.7) | 7.6 (6.7, 9.1) | 7.5 (6.7, 8.6) | −0.31 (0.17) | −0.29 (0.16) |
| Monthly income per capita (USD, %) | |||||
| <308 | 8.1 (7.5, 8.8) | 8.3 (7.5, 9.5) | 8.3 (7.4, 9.7) | 0 (ref) | 0 (ref) |
| 308–769 | 8.1 (7.6, 9.2) | 7.9 (6.9, 9.0) | 7.8 (7.0, 9.0) | −0.32 (0.14) * | −0.40 (0.13) ** |
| ≥769 | 8.0 (7.5, 9.2) | 8.0 (7.2, 9.1) | 7.7 (6.9, 8.8) | −0.31 (0.16) * | −0.34 (0.15) * |
| Tobacco use (%) | |||||
| Never | 8.1 (7.6, 9.1) | 8.0 (7.1, 9.1) | 7.9 (7.0, 9.0) | 0 (ref) | 0 (ref) |
| Ever | 8.0 (7.5, 9.2) | 8.2 (7.1, 9.3) | 8.2 (7.1, 9.4) | 0.09 (0.14) | -0.01 (0.14) |
| Alcohol drinking (%) | |||||
| Never | 8.1 (7.6, 9.1) | 8.0 (7.0, 9.1) | 7.9 (6.9, 9.1) | 0 (ref) | 0 (ref) |
| Ever | 8.3 (7.5, 9.2) | 8.3 (7.2, 9.3) | 8.3 (7.4, 9.2) | 0.08 (0.15) | -0.01 (0.15) |
| Years of diabetes | |||||
| <10 | 8.0 (7.5, 8.9) | 7.7 (6.9, 9.0) | 7.7 (6.8, 8.9) | 0 (ref) | 0 (ref) |
| ≥10 | 8.2 (7.7, 9.4) | 8.1 (7.4, 9.3) | 8.0 (7.2, 9.2) | 0.37 (0.09) ** | 0.31 (0.09) ** |
| Medications (%) | |||||
| Diabetes pills only | 8.1 (7.5, 9.1) | 7.9 (7.0, 9.1) | 7.9 (6.9, 9.1) | 0.09 (0.18) | 0.02 (0.17) |
| Insulin shot only | 8.1 (7.6, 9.5) | 8.4 (7.3, 9.0) | 8.0 (7.3, 9.2) | 0.10 (0.12) | 0.06 (0.11) |
| Neither | 8.0 (7.5, 8.9) | 7.7 (6.8, 9.5) | 7.3 (6.7, 8.2) | 0 (ref) | 0 (ref) |
| Both | 8.3 (7.8, 9.2) | 8.3 (7.4, 9.4) | 8.0 (7.1, 9.4) | −0.19 (0.21) | −0.10 (0.20) |
| c-HeLMS score | |||||
| <116 | 8.1 (7.6, 9.2) | 8.0 (7.1, 9.1) | 7.9 (6.8, 9.1) | 0 (ref) | 0 (ref) |
| ≥116 | 8.1 (7.6, 9.1) | 7.9 (7.0, 9.1) | 7.8 (7.1, 9.0) | 0.04 (0.10) | 0.04 (0.09) |
| Correct rate of c-DNT-5 | |||||
| <80 | 8.3 (7.7, 9.4) | 8.0 (7.1, 9.1) | 7.7 (6.8, 9.2) | 0 (ref) | 0 (ref) |
| ≥80 | 8.1 (7.5, 9.0) | 8.0 (7.1, 9.1) | 7.9 (7.0, 9.0) | −0.01 (0.11) | 0.11 (0.10) |
| PA level (Mets, by tertile) | |||||
| <42 | 8.2 (7.7, 9.0) | 8.0 (7.1, 9.0) | 8.0 (6.9, 9.1) | 0 (ref) | 0 (ref) |
| 42–78 | 8.1 (7.5, 9.2) | 7.9 (7.0, 9.2) | 7.8 (7.0, 9.0) | 0.04 (0.12) | 0.07 (0.11) |
| ≥78 | 8.1 (7.5, 9.2) | 8.1 (7.1, 9.3) | 7.9 (7.0, 9.0) | −0.05 (0.12) | −0.02 (0.12) |
| Characteristics at Baseline | PA Level at the 12-Month Survey (METs-h/w) | p-Values | PA Level at the 24-Month Survey (METs-h/w) | p-Values | ||||
|---|---|---|---|---|---|---|---|---|
| Tertile Group 1 (<38.7, n = 236) | Tertile Group 2 (38.7 to 77.0, n = 233) | Tertile Group 3 (≥77.0, n = 235) | Tertile Group 1 (<38.8, n = 218) | Tertile Group 2 (38.8 to 82.0, n = 214) | Tertile Group 3 (≥82.0, n = 220) | |||
| Age (years, median, IQR) a | 67 (60, 74) | 67 (60, 72) | 64 (58, 69) | <0.001 | 68 (60, 74) | 66 (59, 70) | 64 (59, 70) | 0.002 |
| Sex of men (%) b | 53.8 | 46.8 | 37.0 | <0.001 | 53.7 | 36.5 | 44.1 | 0.045 |
| Educational level (%) b | 0.118 | 0.432 | ||||||
| Primary school or below | 25.1 | 26.6 | 15.7 | 27.2 | 18.7 | 20.9 | ||
| Junior high school | 35.3 | 39.1 | 40.9 | 34.5 | 39.7 | 40.0 | ||
| Senior high school | 26.4 | 19.3 | 29.4 | 24.9 | 25.2 | 25.5 | ||
| College and above | 12.2 | 15.0 | 14.0 | 13.4 | 16.4 | 13.6 | ||
| Monthly income per capita (USD, %) b | 0.482 | 0.838 | ||||||
| <308 | 13.5 | 18.7 | 11.5 | 20.0 | 11.7 | 12.9 | ||
| 308–769 | 53.0 | 59.6 | 61.3 | 49.8 | 58.7 | 62.7 | ||
| >769 | 33.5 | 21.7 | 27.2 | 30.2 | 29.6 | 24.4 | ||
| Tobacco smoking (%) b | 18.8 | 13.4 | 14.6 | 0.210 | 15.3 | 14.6 | 14.3 | 0.771 |
| Alcohol drinking (%) b | 14.2 | 11.9 | 8.3 | 0.045 | 16.6 | 10.1 | 7.6 | 0.003 |
| Years of diabetes (median, IQR) a | 10.0 (5.3, 16.4) | 9.8 (5.0, 14.8) | 9.4 (4.8, 15.1) | 0.362 | 11.0 (5.5, 17.0) | 8.9 (4.8, 14.9) | 9.7 (5.0, 15.0) | 0.026 |
| Use of anti-diabetes agents and insulin (%) b | 0.024 | 0.660 | ||||||
| Diabetes pills only | 61.9 | 67.2 | 52.1 | 62.2 | 64.4 | 61.5 | ||
| Insulin shot only | 10.2 | 4.9 | 9.3 | 10.1 | 7.3 | 8.9 | ||
| Both | 24.8 | 23.0 | 49.4 | 22.0 | 23.4 | 22.1 | ||
| Neither | 3.1 | 4.9 | 9.2 | 5.7 | 4.9 | 7.5 | ||
| PA level (MET/h-w, median, IQR) a | 49.0 (26.6, 73.0) | 56.0 (33.0, 84.0) | 77.0 (48.7, 112.0) | <0.0001 | 50.5 (24.0, 80.7) | 63.0 (41.0, 90.0) | 69.3 (44.4, 104.0) | <0.0001 |
| HbA1c level (%) b | 8.2 (7.6, 9.2) | 8.1 (7.5, 9.0) | 8.2 (7.5, 9.3) | 0.655 | 8.2 (7.6, 9.2) | 8.2 (7.5, 9.2) | 8.1 (7.6, 8.9) | 0.567 |
| HbA1c < 7.0% (%) b | 22.5 | 22.3 | 21.7 | 0.844 | 26.2 | 24.3 | 25.9 | 0.956 |
| Energy Intake (Kcal, median, IQR) a | 1448 (1116, 1793) | 1522 (1197, 1826) | 1380 (1130, 1691) | 0.120 | 1467 (1134, 1821) | 1433 (1132, 1720) | 1410 (1141, 1711) | 0.384 |
| Associations of PA level with HbA1c | Tertile Groups by PA Level (METs-h/w) a | p-Values | Achievement of Recommended Exercise Level | p-Values | |||
|---|---|---|---|---|---|---|---|
| Lowest | Medium | Highest | No | Yes | |||
| At 12 months | |||||||
| Number of subjects | 236 | 233 | 235 | 391 | 313 | ||
| HbA1c (%, median, IQR) | 8.0 (7.1, 9.0) | 7.9 (7.0, 9.2) | 8.1 (7.1, 9.3) | 0.561 | 8.0 (7.1, 9.2) | 7.9 (7.0, 9.0) | 0.276 |
| HbA1c < 7.0% (n, %) | 53 (22.5) | 52 (22.3) | 51 (21.7) | 0.844 | 87 (22.3) | 69 (22.0) | 0.948 |
| β1 (95%CI) | 0 (ref) | 0.12 (−0.16, 0.40) | 0.15 (−0.15, 0.45) | 0 (ref) | −0.15 (−0.39, 0.09) | ||
| β2 (95%CI) | 0 (ref) | 0.15 (−0.12, 0.42) | 0.06 (−0.23, 0.35) | 0 (ref) | −0.05 (−0.29, 0.18) | ||
| β3 (95%CI) a | 0 (ref) | −3.47 (−5.33, −1.60) | −0.85 (−2.58, 0.88) | 0 (ref) | −0.28 (−0.54, −0.02) | ||
| At 24 months | |||||||
| Number of subjects | 218 | 214 | 220 | 372 | 280 | ||
| HbA1c (%, median, IQR) | 8.0 (6.9, 9.1) | 7.9 (7.0, 8.9) | 7.8 (6.9, 9.0) | 0.794 | 7.8 (6.3, 9.0) | 7.9 (6.9, 9.1) | 0.816 |
| HbA1c < 7.0% (n, %) | 57 (26.2) | 52 (24.3) | 57 (25.9) | 0.956 | 94 (25.3) | 72 (25.7) | 0.897 |
| β1 (95%CI) | 0 (ref) | −0.01 (−0.31, 0.29) | −0.17 (−0.47, 0.14) | 0 (ref) | −0.07 (−0.33, 0.18) | ||
| β2 (95%CI) | 0 (ref) | 0.03 (−0.25, 0.31) | −0.10 (−0.39, 0.19) | 0 (ref) | −0.01 (−0.25, 0.23) | ||
| β3 (95%CI) | 0 (ref) | −0.04 (−0.49, 0.41) | −0.50 (−1.00, −0.01) | 0 (ref) | −1.65 (−3.12, −0.18) | ||
| From baseline to 24 months | |||||||
| β (95%CI) | 0 (ref) | −1.29 (−3.33, 0.75) | −3.49 (−5.87, −1.11) | 0 (ref) | −0.20 (−0.38, −0.02) | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yao, W.-Y.; Han, M.-G.; De Vito, G.; Fang, H.; Xia, Q.; Chen, Y.; Liu, X.; Wei, Y.; Rothman, R.L.; Xu, W.-H. Physical Activity and Glycemic Control Status in Chinese Patients with Type 2 Diabetes: A Secondary Analysis of a Randomized Controlled Trial. Int. J. Environ. Res. Public Health 2021, 18, 4292. https://doi.org/10.3390/ijerph18084292
Yao W-Y, Han M-G, De Vito G, Fang H, Xia Q, Chen Y, Liu X, Wei Y, Rothman RL, Xu W-H. Physical Activity and Glycemic Control Status in Chinese Patients with Type 2 Diabetes: A Secondary Analysis of a Randomized Controlled Trial. International Journal of Environmental Research and Public Health. 2021; 18(8):4292. https://doi.org/10.3390/ijerph18084292
Chicago/Turabian StyleYao, Wei-Yuan, Meng-Ge Han, Giuseppe De Vito, Hong Fang, Qinghua Xia, Yingyao Chen, Xiaona Liu, Yan Wei, Russell L. Rothman, and Wang-Hong Xu. 2021. "Physical Activity and Glycemic Control Status in Chinese Patients with Type 2 Diabetes: A Secondary Analysis of a Randomized Controlled Trial" International Journal of Environmental Research and Public Health 18, no. 8: 4292. https://doi.org/10.3390/ijerph18084292
APA StyleYao, W.-Y., Han, M.-G., De Vito, G., Fang, H., Xia, Q., Chen, Y., Liu, X., Wei, Y., Rothman, R. L., & Xu, W.-H. (2021). Physical Activity and Glycemic Control Status in Chinese Patients with Type 2 Diabetes: A Secondary Analysis of a Randomized Controlled Trial. International Journal of Environmental Research and Public Health, 18(8), 4292. https://doi.org/10.3390/ijerph18084292







