The Burden of Dengue in Children by Calculating Spatial Temperature: A Methodological Approach Using Remote Sensing Techniques
Abstract
1. Introduction
2. Materials and Methods
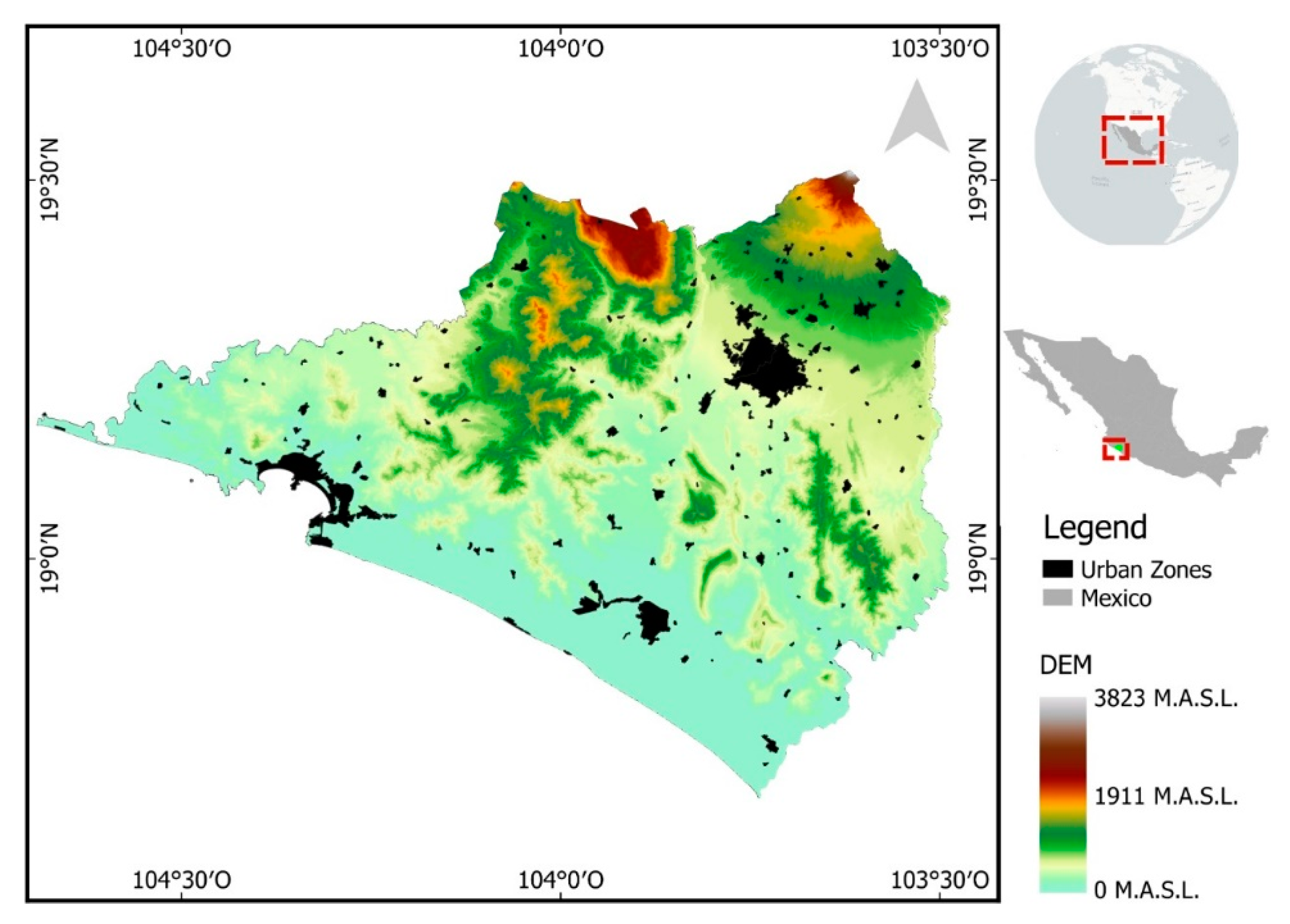
2.1. Study Site
2.2. Data Collection and Spatial Analysis
Spatial Surface Temperature Collection and Calculation
2.3. Health Economics Burden of Disease of Dengue
JointPoint Regression with IHME Metrics
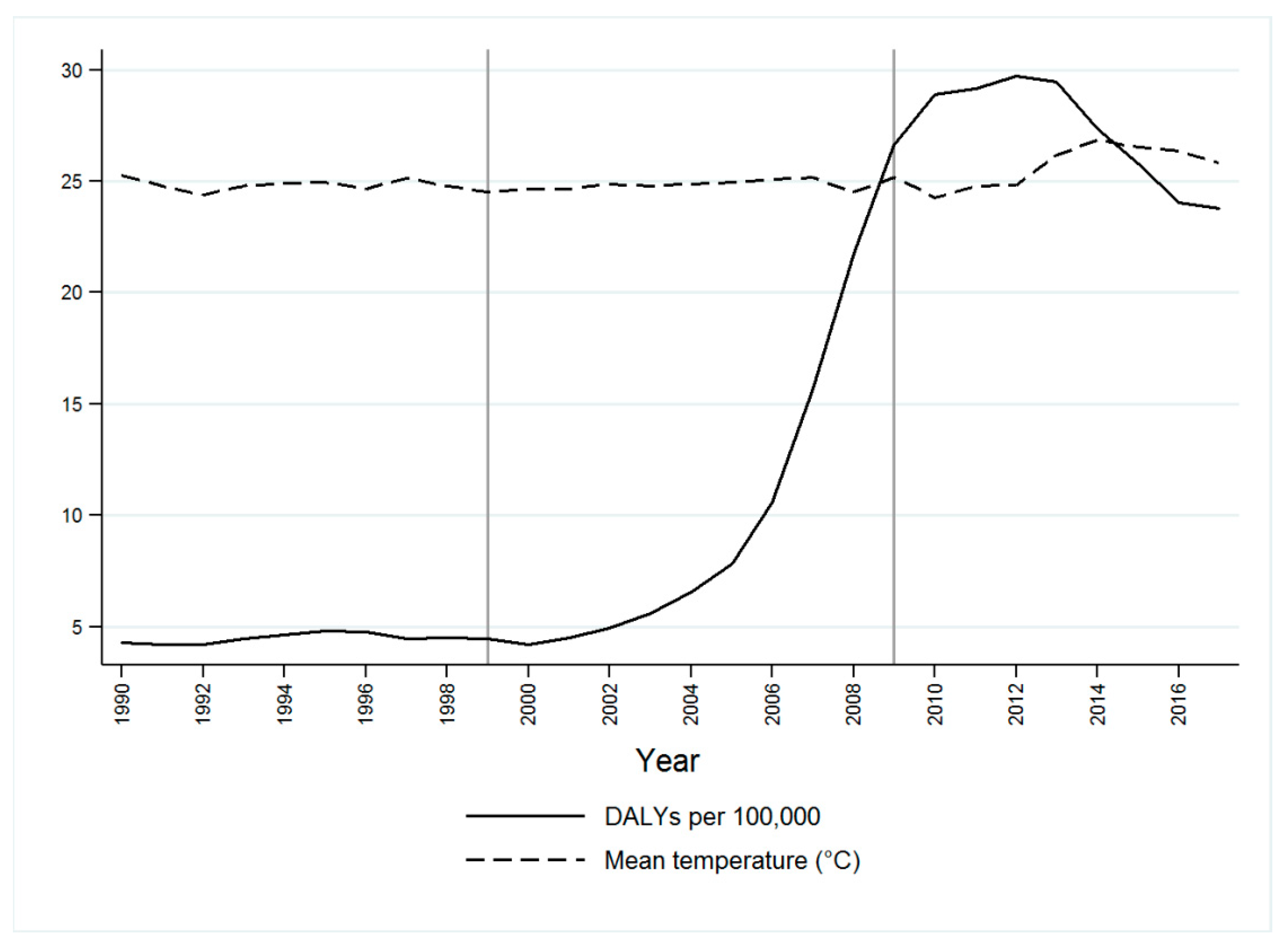
3. Results
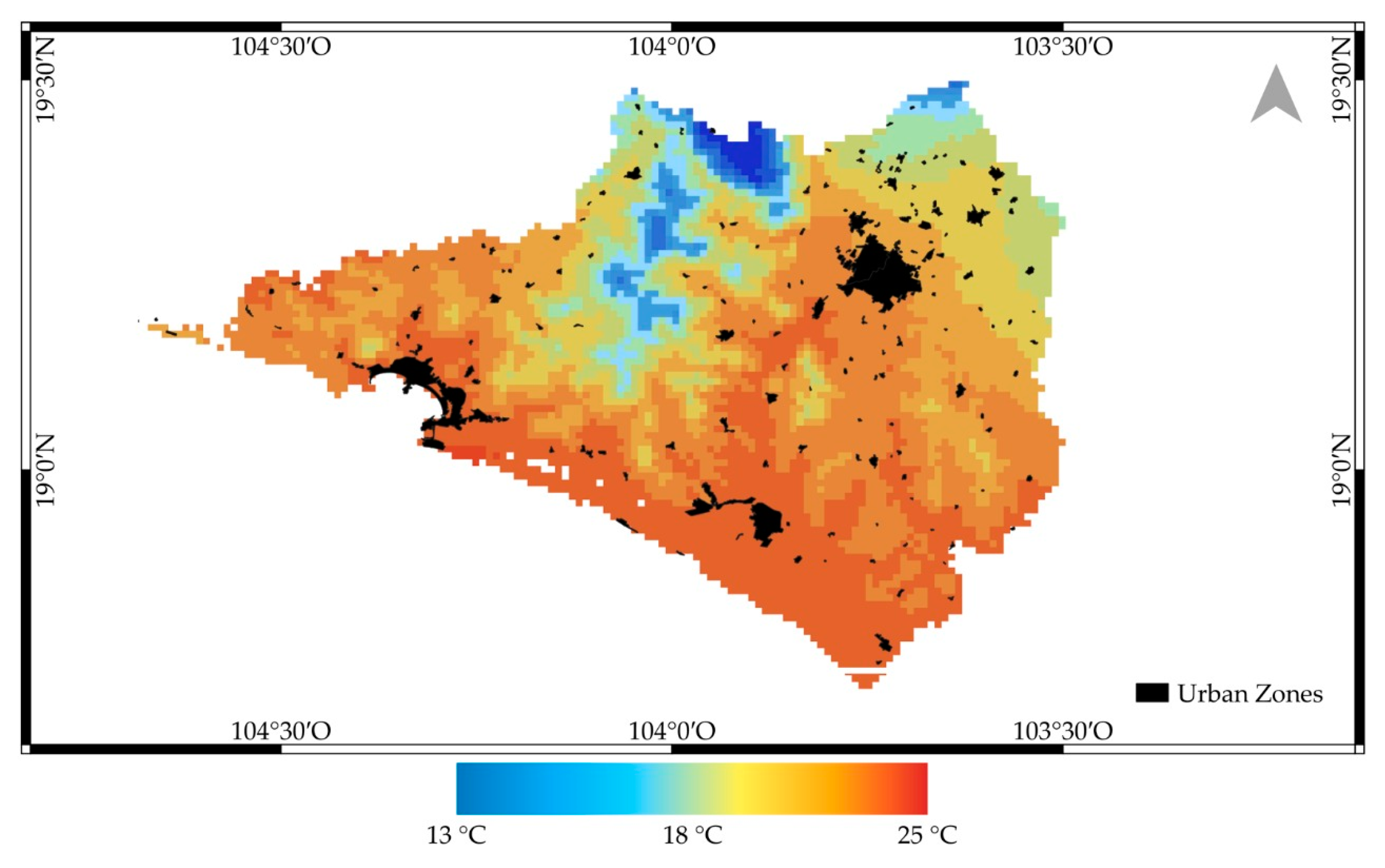
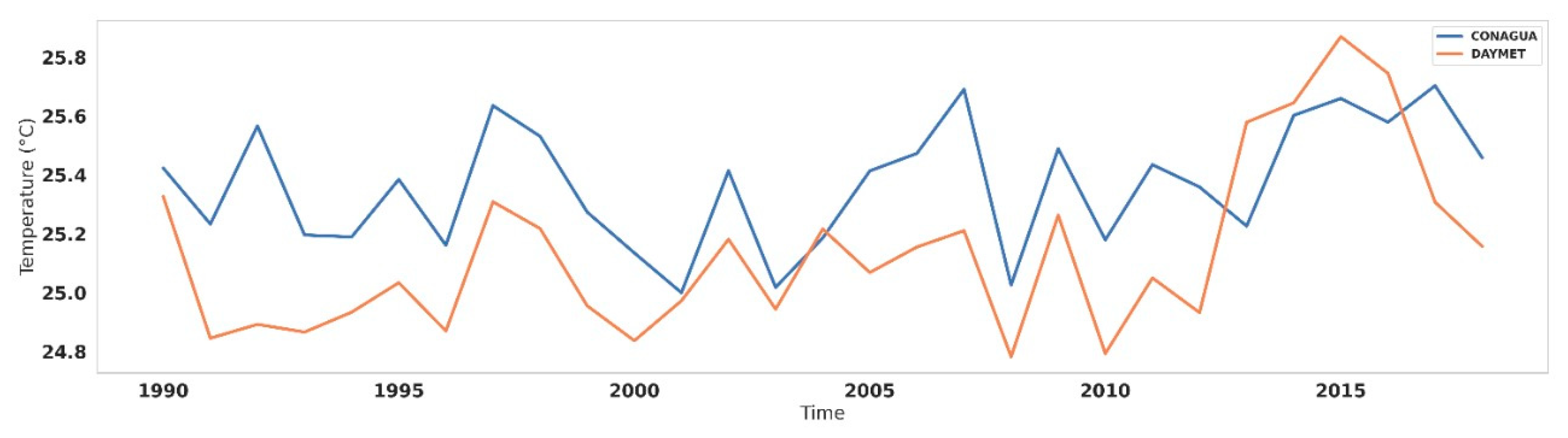
Spatial Surface Temperature Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Messina, J.P.; Brady, O.J.; Golding, N.; Kraemer, M.U.G.; Wint, G.R.W.; Ray, S.E.; Pigott, D.M.; Shearer, F.M.; Johnson, K.; Earl, L.; et al. The current and future global distribution and population at risk of dengue. Nat. Microbiol. 2019, 4, 1508–1515. [Google Scholar] [CrossRef]
- López, T.M.T.; Cordero, J.L.G.; Estrada, J.G.S. Dimensiones culturales del dengue que favorecen o dificultan su prevención en México. Rev. Panam. Salud Publica/Pan Am. J. Public Health 2012, 31, 197–203. [Google Scholar]
- Acharya, B.K.; Cao, C.; Xu, M.; Khanal, L.; Naeem, S.; Pandit, S. Present and Future of Dengue Fever in Nepal: Mapping Climatic Suitability by Ecological Niche Model. Int. J. Environ. Res. Public Health 2018, 15, 187. [Google Scholar] [CrossRef]
- Naish, S.; Dale, P.; Mackenzie, J.S.; McBride, J.; Mengersen, K.; Tong, S. Climate change and dengue: A critical and systematic review of quantitative modelling approaches. BMC Infect. Dis. 2014, 14, 167. [Google Scholar] [CrossRef]
- Morin, C.W.; Comrie, A.C.; Ernst, K. Climate and Dengue Transmission: Evidence and Implications. Environ. Health Perspect. 2013, 121, 1264–1272. [Google Scholar] [CrossRef]
- Fouque, F.; Reeder, J.C. Impact of past and on-going changes on climate and weather on vector-borne diseases transmission: A look at the evidence. Infect. Dis. Poverty 2019, 8, 51. [Google Scholar] [CrossRef]
- Campbell, K.M.; Lin, C.D.; Iamsirithaworn, S.; Scott, T.W. The Complex Relationship between Weather and Dengue Virus Transmission in Thailand. Am. J. Trop. Med. Hyg. 2013, 89, 1066–1080. [Google Scholar] [CrossRef]
- Mweya, C.N.; Kimera, S.I.; Kija, J.B.; Mboera, L.E.G. Predicting distribution of Aedes aegypti and Culex pipiens complex, potential vectors of Rift Valley fever virus in relation to disease epidemics in East Africa. Infect. Ecol. Epidemiol. 2013, 3, 21748. [Google Scholar] [CrossRef]
- Ochieng, A.O.; Nanyingi, M.; Kipruto, E.; Ondiba, I.M.; Amimo, F.A.; Oludhe, C.; Olago, D.O.; Nyamongo, I.K.; Estambale, B.B.A. Ecological niche modelling of Rift Valley fever virus vectors in Baringo, Kenya. Infect. Ecol. Epidemiol. 2016, 6, 32322. [Google Scholar] [CrossRef]
- Mweya, C.N.; Kimera, S.I.; Stanley, G.; Misinzo, G.; Mboera, L.E.G. Climate Change Influences Potential Distribution of Infected Aedes aegypti Co-Occurrence with Dengue Epidemics Risk Areas in Tanzania. PLoS ONE 2016, 11, e0162649. [Google Scholar] [CrossRef]
- Ren, Z.; Wang, D.; Ma, A.; Hwang, J.; Bennett, A.; Sturrock, H.J.W.; Fan, J.; Zhang, W.; Yang, D.; Feng, X.; et al. Predicting malaria vector distribution under climate change scenarios in China: Challenges for malaria elimination. Sci. Rep. 2016, 6, 20604. [Google Scholar] [CrossRef]
- Campbell, L.P.; Luther, C.; Moo-Llanes, D.; Ramsey, J.M.; Danis-Lozano, R.; Peterson, A.T. Climate change influences on global distributions of dengue and chikungunya virus vectors. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2015, 370, 20140135. [Google Scholar] [CrossRef]
- Altizer, S.; Ostfeld, R.S.; Johnson, P.T.J.; Kutz, S.; Harvell, C.D. Climate Change and Infectious Diseases: From Evidence to a Predictive Framework. Science 2013, 341, 514–519. [Google Scholar] [CrossRef]
- Ebi, K.L.; Nealon, J. Dengue in a changing climate. Environ. Res. 2016, 151, 115–123. [Google Scholar] [CrossRef]
- Marinho, R.A.; Beserra, E.B.; Bezerra-Gusmão, M.A.; Porto, V.d.S.; Olinda, R.A.; dos Santos, C.A.C. Effects of temperature on the life cycle, expansion, and dispersion of Aedes aegypti (Diptera: Culicidae) in three cities in Paraiba, Brazil. J. Vector Ecol. 2016, 41, 1–10. [Google Scholar] [CrossRef]
- Eisen, L.; Monaghan, A.J.; Lozano-Fuentes, S.; Steinhoff, D.F.; Hayden, M.H.; Bieringer, P.E. The impact of temperature on the bionomics of Aedes (Stegomyia) aegypti, with special reference to the cool geographic range margins. J. Med. Entomol. 2014, 51, 496–516. [Google Scholar] [CrossRef]
- Kraemer, M.U.G.; Sinka, M.E.; Duda, K.A.; Mylne, A.Q.N.; Shearer, F.M.; Barker, C.M.; Moore, C.G.; Carvalho, R.G.; Coelho, G.E.; Van Bortel, W.; et al. The global distribution of the arbovirus vectors Aedes aegypti and Ae. albopictus. eLife 2015, 4, e08347. [Google Scholar] [CrossRef]
- Mweya, C.N.; Kimera, S.I.; Mellau, L.S.B.; Mboera, L.E.G. Inter-epidemic abundance and distribution of potential mosquito vectors for Rift Valley fever virus in Ngorongoro district, Tanzania. Glob. Health Action 2015, 8, 25929. [Google Scholar] [CrossRef][Green Version]
- Alvarado-Castro, V.M.; Ramírez-Hernández, E.; Paredes-Solís, S.; Legorreta-Soberanis, J.; Saldaña-Herrera, V.G.; Salas-Franco, L.S.; del Castillo-Medina, J.A.; Andersson, N. Clinical profile of dengue and predictive severity variables among children at a secondary care hospital of Chilpancingo, Guerrero, Mexico: Case series. Boletín Médico Del Hosp. Infant. México (Engl. Ed.) 2016, 73, 237–242. [Google Scholar] [CrossRef]
- Hernández-Suárez, C.M.; Mendoza-Cano, O. Empirical evidence of the effect of school gathering on the dynamics of dengue epidemics. Glob. Health Action 2016, 9. [Google Scholar] [CrossRef]
- Dhar-Chowdhury, P.; Paul, K.K.; Haque, C.E.; Hossain, S.; Lindsay, L.R.; Dibernardo, A.; Brooks, W.A.; Drebot, M.A. Dengue seroprevalence, seroconversion and risk factors in Dhaka, Bangladesh. PLoS Negl. Trop. Dis. 2017, 11, e0005475. [Google Scholar] [CrossRef]
- Ooi, E.-E.; Gubler, D.J. Global spread of epidemic dengue: The influence of environmental change. Future Virol. 2009, 4, 571–580. [Google Scholar] [CrossRef]
- Khalil, I.; Troeger, C.E.; Blacker, B.F.; Reiner, R.C. Capturing the true burden of Shigella and ETEC: The way forward. Vaccine 2019, 37, 4784–4786. [Google Scholar] [CrossRef]
- Anderson, K.B.; Chunsuttiwat, S.; Nisalak, A.; Mammen, M.P.; Libraty, D.H.; Rothman, A.L.; Green, S.; Vaughn, D.W.; Ennis, F.A.; Endy, T.P. Burden of symptomatic dengue infection in children at primary school in Thailand: A prospective study. Lancet 2007, 369, 1452–1459. [Google Scholar] [CrossRef]
- Bhavsar, A.; Tam, C.C.; Garg, S.; Jammy, G.R.; Taurel, A.-F.; Chong, S.-N.; Nealon, J. Estimated dengue force of infection and burden of primary infections among Indian children. BMC Public Health 2019, 19, 1116. [Google Scholar] [CrossRef]
- Rose, W.; Sindhu, K.N.; Abraham, A.M.; Kang, G.; John, J. Incidence of dengue illness among children in an urban setting in South India: A population based study. Int. J. Infect. Dis. 2019, 84, S15–S18. [Google Scholar] [CrossRef]
- Stein, C. Global Burden of Disease (GBD) Approach and the Use of Disability-Adjusted Life Years (DALY) at the World Health Organization (WHO), 2nd ed.; Nriagu, J.B.T.-E., Ed.; Elsevier: Oxford, UK, 2019; pp. 279–288. ISBN 978-0-444-63952-3. [Google Scholar]
- Thornton, P.E.; Thornton, M.M.; Mayer, B.W.; Wei, Y.; Devarakonda, R.; Vose, R.S.; Cook, R.B. Daymet: Daily Surface Weather Data on a 1-km Grid for North America; Version 3; ORNL DAAC: Oak Ridge, TN, USA, 2016.
- INEGI. National Intercensal Survey. 2015, Recovered 09 September 2020. Available online: https://www.inegi.org.mx/programas/intercensal/2015/ (accessed on 14 April 2021).
- Gorelick, N.; Hancher, M.; Dixon, M.; Ilyushchenko, S.; Thau, D.; Moore, R. Google Earth Engine: Planetary-scale geospatial analysis for everyone. Remote Sens. Environ. 2017, 202, 18–27. [Google Scholar] [CrossRef]
- Rosenberg, D. Trend Analysis and Interpretation. Key Concepts and Methods for Maternal and Child Health Professionals; Division of Science, Education and Analysis Maternal and Child Health Bureau: Rockville, MD, USA, 1997; p. 39.
- Gillis, D.; Edwards, B.P.M. The utility of joinpoint regression for estimating population parameters given changes in population structure. Heliyon 2019, 5. [Google Scholar] [CrossRef]
- Barrio, G.; Pulido, J.; Bravo, M.J.; Lardelli-Claret, P.; Jiménez-Mejías, E.; de la Fuente, L. An example of the usefulness of joinpoint trend analysis for assessing changes in traffic safety policies. Accid. Anal. Prev. 2015, 75, 292–297. [Google Scholar] [CrossRef]
- Butterworth, M.K.; Morin, C.W.; Comrie, A.C. An Analysis of the Potential Impact of Climate Change on Dengue Transmission in the Southeastern United States. Environ. Health Perspect. 2017, 125, 579–585. [Google Scholar] [CrossRef]
- Long, N.P.; Huy, N.T.; Trang, N.T.H.; Luan, N.T.; Anh, N.H.; Nghi, T.D.; Van Hieu, M.; Hirayama, K.; Karbwang, J. Scientific Productivity on Research in Ethical Issues over the Past Half Century: A JoinPoint Regression Analysis. Trop. Med. Health 2014, 42, 121–126. [Google Scholar] [CrossRef]
- López-Campos, J.L.; Ruiz-Ramos, M.; Soriano, J.B. Mortality trends in chronic obstructive pulmonary disease in Europe, 1994-2010: A joinpoint regression analysis. Lancet. Respir. Med. 2014, 2, 54–62. [Google Scholar] [CrossRef]
- Scavuzzo, J.M.; Trucco, F.; Espinosa, M.; Tauro, C.B.; Abril, M.; Scavuzzo, C.M.; Frery, A.C. Modeling Dengue vector population using remotely sensed data and machine learning. Acta Trop. 2018, 185, 167–175. [Google Scholar] [CrossRef]
- Fuller, D.O.; Troyo, A.; Calderón-Arguedas, O.; Beier, J.C. Dengue vector (Aedes aegypti) larval habitats in an urban environment of Costa Rica analysed with ASTER and QuickBird imagery. Int. J. Remote Sens. 2010, 31, 3–11. [Google Scholar] [CrossRef]
- Moreno-Madriñán, M.; Crosson, W.; Eisen, L.; Estes, S.; Estes, M., Jr.; Hayden, M.; Hemmings, S.; Irwin, D.; Lozano-Fuentes, S.; Monaghan, A.; et al. Correlating Remote Sensing Data with the Abundance of Pupae of the Dengue Virus Mosquito Vector, Aedes aegypti, in Central Mexico. ISPRS Int. J. Geo-Inf. 2014, 3, 732–749. [Google Scholar] [CrossRef]
- Parra-henao, G. Sistemas de información geográfica y sensores remotos: Aplicaciones enenfermedades transmitidas por vectores. CES Med. 2010, 24, 75–89. [Google Scholar]
- Goindin, D.; Delannay, C.; Ramdini, C.; Gustave, J.; Fouque, F. Parity and longevity of Aedes aegypti according to temperatures in controlled conditions and consequences on dengue transmission risks. PLoS ONE 2015, 10, e0135489. [Google Scholar] [CrossRef]
- Gubler, D. Dengue, urbanization and globalization: The unholy trinity of the 21st century. Int. J. Infect. Dis. 2012, 16, e2. [Google Scholar] [CrossRef]
- Wu, P.-C.; Lay, J.-G.; Guo, H.-R.; Lin, C.-Y.; Lung, S.-C.; Su, H.-J. Higher temperature and urbanization affect the spatial patterns of dengue fever transmission in subtropical Taiwan. Sci. Total Environ. 2009, 407, 2224–2233. [Google Scholar] [CrossRef]
- Lindgren, E.; Andersson, Y.; Suk, J.E.; Sudre, B.; Semenza, J.C. Monitoring EU Emerging Infectious Disease Risk Due to Climate Change. Science 2012, 336, 418–419. [Google Scholar] [CrossRef]
- Dictionary, A.H. The American Heritage medical dictionary. Med. Dict. 2007, xxxii, 909. [Google Scholar]
- Tjaden, N.B.; Thomas, S.M.; Fischer, D.; Beierkuhnlein, C. Extrinsic Incubation Period of Dengue: Knowledge, Backlog, and Applications of Temperature Dependence. PLoS Negl. Trop. Dis. 2013, 7, e2207. [Google Scholar] [CrossRef]
- Barbazan, P.; Guiserix, M.; Boonyuan, W.; Tuntaprasart, W.; Pontier, D.; Gonzalez, J.-P. Modelling the effect of temperature on transmission of dengue. Med. Vet. Entomol. 2010, 24, 66–73. [Google Scholar] [CrossRef]
- Siraj, A.S.; Oidtman, R.J.; Huber, J.H.; Kraemer, M.U.G.; Brady, O.J.; Johansson, M.A.; Perkins, T.A. Temperature modulates dengue virus epidemic growth rates through its effects on reproduction numbers and generation intervals. PLoS Negl. Trop. Dis. 2017, 11, e0005797. [Google Scholar] [CrossRef]
- Lafferty, K.D. The ecology of climate change and infectious diseases. Ecology 2009, 90, 888–900. [Google Scholar] [CrossRef]
- Beck-Johnson, L.M.; Nelson, W.A.; Paaijmans, K.P.; Read, A.F.; Thomas, M.B.; Bjørnstad, O.N. The Effect of Temperature on Anopheles Mosquito Population Dynamics and the Potential for Malaria Transmission. PLoS ONE 2013, 8, e79276. [Google Scholar] [CrossRef]
| Period | AAPC | (95% CI) | p |
|---|---|---|---|
| Overall (1990–2017) | 64 | (44, 87) | <0.001 |
| First (1990–1999) | 2 | (−68, 227) | 0.976 |
| Second (2000–2009) | 185 | (18, 588) | 0.020 |
| Third (2010–2017) | −5 | (−18, 10) | 0.503 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mendoza-Cano, O.; Rincón-Avalos, P.; Watson, V.; Khouakhi, A.; Cruz, J.L.-d.l.; Ruiz-Montero, A.P.; Nava-Garibaldi, C.M.; Lopez-Rojas, M.; Murillo-Zamora, E. The Burden of Dengue in Children by Calculating Spatial Temperature: A Methodological Approach Using Remote Sensing Techniques. Int. J. Environ. Res. Public Health 2021, 18, 4230. https://doi.org/10.3390/ijerph18084230
Mendoza-Cano O, Rincón-Avalos P, Watson V, Khouakhi A, Cruz JL-dl, Ruiz-Montero AP, Nava-Garibaldi CM, Lopez-Rojas M, Murillo-Zamora E. The Burden of Dengue in Children by Calculating Spatial Temperature: A Methodological Approach Using Remote Sensing Techniques. International Journal of Environmental Research and Public Health. 2021; 18(8):4230. https://doi.org/10.3390/ijerph18084230
Chicago/Turabian StyleMendoza-Cano, Oliver, Pedro Rincón-Avalos, Verity Watson, Abdou Khouakhi, Jesús López-de la Cruz, Angelica Patricia Ruiz-Montero, Cynthia Monique Nava-Garibaldi, Mario Lopez-Rojas, and Efrén Murillo-Zamora. 2021. "The Burden of Dengue in Children by Calculating Spatial Temperature: A Methodological Approach Using Remote Sensing Techniques" International Journal of Environmental Research and Public Health 18, no. 8: 4230. https://doi.org/10.3390/ijerph18084230
APA StyleMendoza-Cano, O., Rincón-Avalos, P., Watson, V., Khouakhi, A., Cruz, J. L.-d. l., Ruiz-Montero, A. P., Nava-Garibaldi, C. M., Lopez-Rojas, M., & Murillo-Zamora, E. (2021). The Burden of Dengue in Children by Calculating Spatial Temperature: A Methodological Approach Using Remote Sensing Techniques. International Journal of Environmental Research and Public Health, 18(8), 4230. https://doi.org/10.3390/ijerph18084230







