Effect of COVID-19 Pandemic on the Change in Skeletal Muscle Mass in Older Patients with Type 2 Diabetes: A Retrospective Cohort Study
Abstract
1. Introduction
2. Materials and Methods
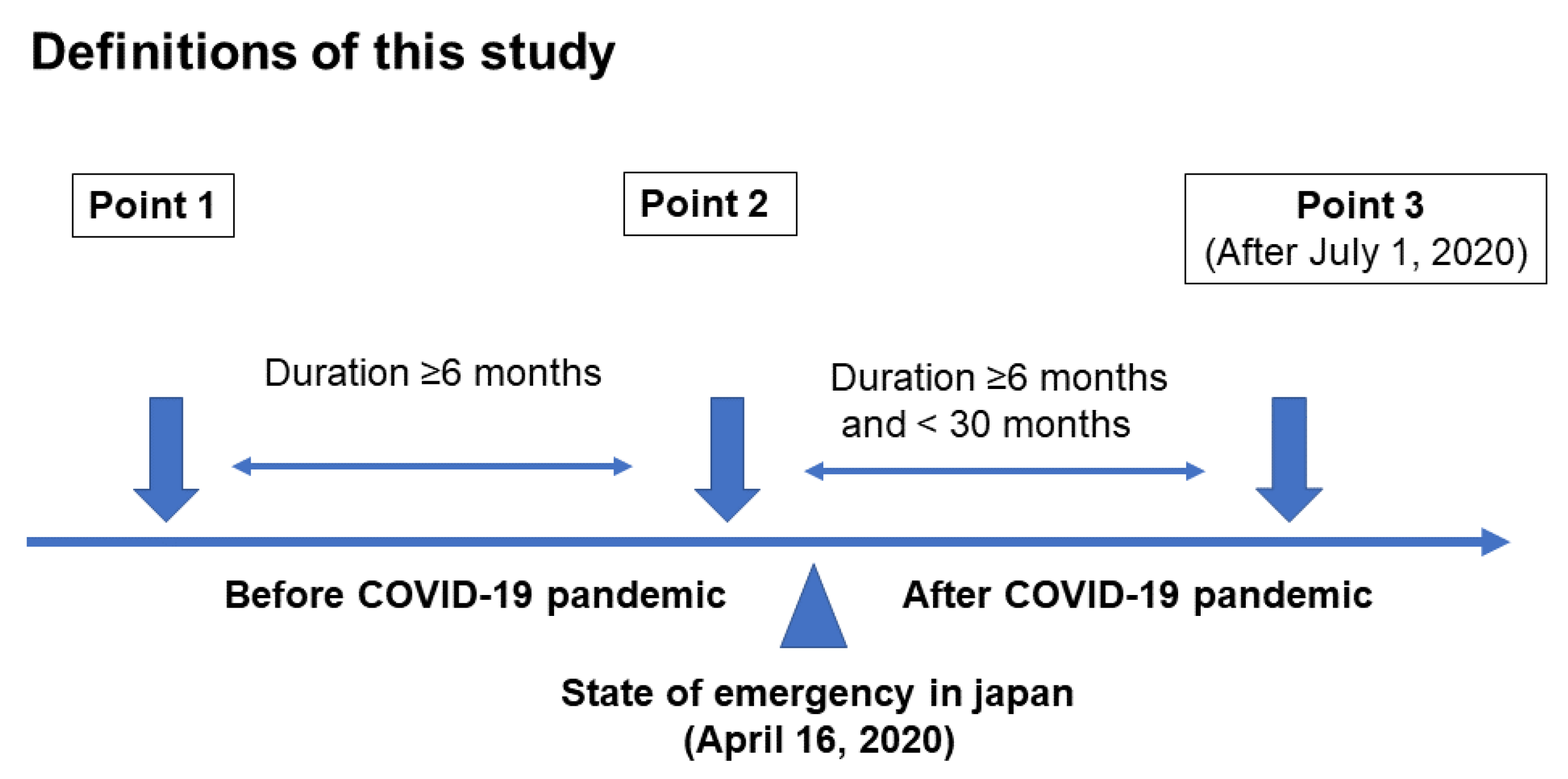
2.1. Participants and Study Design
2.2. Measurement of Body Composition
2.3. Lifestyle, Medications, and Laboratory Data Collection Participants and Study Design
2.4. Questionnaire to Assess Change in Stress Levels and Lifestyle Due to the COVID-19 Pandemic
2.5. Calculation of Sample Size
2.6. Statistical Analyses
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Andersen, K.G.; Rambaut, A.; Lipkin, W.I.; Holmes, E.C.; Garry, R.F. The proximal origin of SARS-CoV-2. Nat. Med. 2020, 26, 450–452. [Google Scholar] [CrossRef]
- World Health Organization World Health Organization. WHO Coronavirus Disease (COVID-19) Dashboard. 2020. Available online: https://covid19.who.int/ (accessed on 8 January 2021).
- Aung, M.N.; Yuasa, M.; Koyanagi, Y.; Aung, T.N.N.; Moolphate, S.; Matsumoto, H.; Yoshioka, T. Sustainable health promotion for the seniors during COVID-19 outbreak: A lesson from Tokyo. J. Infect. Dev. Ctries. 2020, 14, 328–331. [Google Scholar] [CrossRef] [PubMed]
- Lim, W.; Liang, C.; Assantachai, P.; Auyeung, T.W.; Kang, L.; Lee, W.; Lim, J.; Sugimoto, K.; Akishita, M.; Chia, S.; et al. COVID -19 and older people in Asia: Asian Working Group for Sarcopenia calls to action. Geriatr. Gerontol. Int. 2020, 20, 547–558. [Google Scholar] [CrossRef] [PubMed]
- Cheikh Ismail, L.; Osaili, T.M.; Mohamad, M.N.; Al Marzouqi, A.; Jarrar, A.H.; Abu Jamous, D.O.; Magriplis, E.; Ali, H.I.; Al Sabbah, H.; Hasan, H.; et al. Eating Habits and Lifestyle during COVID-19 Lockdown in the United Arab Emirates: A Cross-Sectional Study. Nutrients 2020, 12, 3314. [Google Scholar] [CrossRef] [PubMed]
- Sidor, A.; Rzymski, P. Dietary Choices and Habits during COVID-19 Lockdown: Experience from Poland. Nutrients 2020, 12, 1657. [Google Scholar] [CrossRef]
- Di Renzo, L.; Gualtieri, P.; Pivari, F.; Soldati, L.; Attinà, A.; Cinelli, G.; Leggeri, C.; Caparello, G.; Barrea, L.; Scerbo, F.; et al. Eating habits and lifestyle changes during COVID-19 lockdown: An Italian survey. J. Transl. Med. 2020, 18, 229. [Google Scholar] [CrossRef]
- Munekawa, C.; Hosomi, Y.; Hashimoto, Y.; Okamura, T.; Takahashi, F.; Kawano, R.; Nakajima, H.; Osaka, T.; Okada, H.; Majima, S.; et al. Effect of coronavirus disease 2019 pandemic on the lifestyle and glycemic control in patients with type 2 diabetes: A cross-section and retrospective cohort study. Endocr. J. 2020, in press. [Google Scholar] [CrossRef]
- DeFronzo, R.A.; Tripathy, D. Skeletal Muscle Insulin Resistance Is the Primary Defect in Type 2 Diabetes. Diabetes Care 2009, 32, S157–S163. [Google Scholar] [CrossRef]
- Umegaki, H. Sarcopenia and frailty in older patients with diabetes mellitus. Geriatr. Gerontol. Int. 2016, 16, 293–299. [Google Scholar] [CrossRef]
- Okamura, T.; Miki, A.; Hashimoto, Y.; Kaji, A.; Sakai, R.; Osaka, T.; Hamaguchi, M.; Yamazaki, M.; Fukui, M. Shortage of energy intake rather than protein intake is associated with sarcopenia in elderly patients with type 2 diabetes: A cross-sectional study of the KAMOGAWA-DM cohort. J. Diabetes 2019, 11, 477–483. [Google Scholar] [CrossRef]
- Ammar, A.; Brach, M.; Trabelsi, K.; Chtourou, H.; Boukhris, O.; Masmoudi, L.; Bouaziz, B.; Bentlage, E.; How, D.; Ahmed, M.; et al. Effects of COVID-19 Home Confinement on Eating Behaviour and Physical Activity: Results of the ECLB-COVID19 International Online Survey. Nutrients 2020, 12, 1583. [Google Scholar] [CrossRef]
- Yamada, K.; Yamaguchi, S.; Sato, K.; Fuji, T.; Ohe, T. The COVID-19 outbreak limits physical activities and increases sedentary behavior: A possible secondary public health crisis for the elderly. J. Orthop. Sci. 2020, 25, 1093–1094. [Google Scholar] [CrossRef]
- López-Sánchez, G.F.; López-Bueno, R.; Gil-Salmerón, A.; Zauder, R.; Skalska, M.; Jastrzębska, J.; Jastrzębski, Z.; Schuch, F.B.; Grabovac, I.; Tully, M.A.; et al. Comparison of physical activity levels in Spanish adults with chronic conditions before and during COVID-19 quarantine. Eur. J. Public Heal. 2021, 31, 161–166. [Google Scholar] [CrossRef]
- Wang, P.-Y.; Li, Y.; Wang, Q. Sarcopenia: An underlying treatment target during the COVID-19 pandemic. Nutrition 2021, 84, 111104. [Google Scholar] [CrossRef]
- Welch, C.; Greig, C.; Masud, T.; Wilson, D.; A Jackson, T. COVID-19 and Acute Sarcopenia. Aging Dis. 2020, 11, 1345–1351. [Google Scholar] [CrossRef] [PubMed]
- Kirwan, R.; McCullough, D.; Butler, T.; De Heredia, F.P.; Davies, I.G.; Stewart, C. Sarcopenia during COVID-19 lockdown restrictions: Long-term health effects of short-term muscle loss. GeroScience 2020, 42, 1547–1578. [Google Scholar] [CrossRef] [PubMed]
- Morley, J.E.; Kalantar-Zadeh, K.; Anker, S.D. COVID-19: A major cause of cachexia and sarcopenia? J. Cachex Sarcopenia Muscle 2020, 11, 863–865. [Google Scholar] [CrossRef] [PubMed]
- Sakai, R.; Hashimoto, Y.; Ushigome, E.; Miki, A.; Okamura, T.; Matsugasumi, M.; Fukuda, T.; Majima, S.; Matsumoto, S.; Senmaru, T.; et al. Late-night-dinner is associated with poor glycemic control in people with type 2 diabetes: The KAMOGAWA-DM cohort study. Endocr. J. 2018, 65, 395–402. [Google Scholar] [CrossRef]
- Shafiee, G.; Keshtkar, A.; Soltani, A.; Ahadi, Z.; Larijani, B.; Heshmat, R. Prevalence of sarcopenia in the world: A systematic review and meta- analysis of general population studies. J. Diabetes Metab. Disord. 2017, 16, 1–10. [Google Scholar] [CrossRef]
- Haneda, M.; Utsunomiya, K.; Koya, D.; Babazono, T.; Moriya, T.; Makino, H.; Kimura, K.; Suzuki, Y.; Wada, T.; Ogawa, S.; et al. A new Classification of Diabetic Nephropathy 2014: A report from Joint Committee on Diabetic Nephropathy. J. Diabetes Investig. 2015, 6, 242–246. [Google Scholar] [CrossRef]
- Dickstein, K.; Cohen-Solal, A.; Filippatos, G.; McMurray, J.J.; Ponikowski, P.; Poole-Wilson, P.A.; Strömberg, A.; Van Veldhuisen, D.J.; Atar, D.; Hoes, A.W.; et al. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2008: The task force for the diagnosis and treatment of Acute and Chronic Heart Failure 2008 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association of the ESC (HFA) and endorsed by the European Society of Intensive Care Medicine (ESICM). Eur. Hear. J. 2008, 29, 2388–2442. [Google Scholar] [CrossRef]
- Kim, M.; Shinkai, S.; Murayama, H.; Mori, S. Comparison of segmental multifrequency bioelectrical impedance analysis with dual-energy X-ray absorptiometry for the assessment of body composition in a community-dwelling older population. Geriatr. Gerontol. Int. 2015, 15, 1013–1022. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.-K.; Woo, J.; Assantachai, P.; Auyeung, T.-W.; Chou, M.-Y.; Iijima, K.; Jang, H.C.; Kang, L.; Kim, M.; Kim, S.; et al. Asian Working Group for Sarcopenia: 2019 Consensus Update on Sarcopenia Diagnosis and Treatment. J. Am. Med. Dir. Assoc. 2020, 21, 300–307.e2. [Google Scholar] [CrossRef]
- Hashimoto, Y.; Tanaka, M.; Okada, H.; Senmaru, T.; Hamaguchi, M.; Asano, M.; Yamazaki, M.; Oda, Y.; Hasegawa, G.; Toda, H.; et al. Metabolically Healthy Obesity and Risk of Incident CKD. Clin. J. Am. Soc. Nephrol. 2015, 10, 578–583. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, Y.; Tanaka, M.; Kimura, T.; Kitagawa, N.; Hamaguchi, M.; Asano, M.; Yamazaki, M.; Oda, Y.; Toda, H.; Nakamura, N.; et al. Hemoglobin concentration and incident metabolic syndrome: A population-based large-scale cohort study. Endocr. 2015, 50, 390–396. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, S.; Imai, E.; Horio, M.; Yasuda, Y.; Tomita, K.; Nitta, K.; Yamagata, K.; Tomino, Y.; Yokoyama, H.; Hishida, A. Revised Equations for Estimated GFR From Serum Creatinine in Japan. Am. J. Kidney Dis. 2009, 53, 982–992. [Google Scholar] [CrossRef] [PubMed]
- Tsekoura, M.; Kastrinis, A.; Katsoulaki, M.; Billis, E.; Gliatis, J.; Vlamos, P. Sarcopenia and Its Impact on Quality of Life. Adv. Exp. Med. Biol. 2017, 987, 213–218. [Google Scholar] [CrossRef] [PubMed]
- Lai, S.; Muscaritoli, M.; Andreozzi, P.; Sgreccia, A.; De Leo, S.; Mazzaferro, S.; Mitterhofer, A.P.; Pasquali, M.; Protopapa, P.; Spagnoli, A.; et al. Sarcopenia and cardiovascular risk indices in patients with chronic kidney disease on conservative and replacement therapy. Nutrition 2019, 62, 108–114. [Google Scholar] [CrossRef]
- Hashimoto, Y.; Kaji, A.; Sakai, R.; Hamaguchi, M.; Okada, H.; Ushigome, E.; Asano, M.; Yamazaki, M.; Fukui, M. Sarcopenia is associated with blood pressure variability in older patients with type 2 diabetes: A cross-sectional study of the KAMOGAWA-DM cohort study. Geriatr. Gerontol. Int. 2018, 18, 1345–1349. [Google Scholar] [CrossRef]
- Miyake, H.; Kanazawa, I.; Tanaka, K.-I.; Sugimoto, T. Low skeletal muscle mass is associated with the risk of all-cause mortality in patients with type 2 diabetes mellitus. Ther. Adv. Endocrinol. Metab. 2019, 10, 2042018819842971. [Google Scholar] [CrossRef]
- Lee, C.G.; Boyko, E.J.; Barrett-Connor, E.; Miljkovic, I.; Hoffman, A.R.; Everson-Rose, S.A.; Lewis, C.E.; Cawthon, P.M.; Strotmeyer, E.S.; Orwoll, E.S.; et al. Insulin Sensitizers May Attenuate Lean Mass Loss in Older Men with Diabetes. Diabetes Care 2011, 34, 2381–2386. [Google Scholar] [CrossRef]
- Park, S.W.; Goodpaster, B.H.; Lee, J.S.; Kuller, L.H.; Boudreau, R.; De Rekeneire, N.; Harris, T.B.; Kritchevsky, S.; Tylavsky, F.A.; Nevitt, M.; et al. Excessive Loss of Skeletal Muscle Mass in Older Adults with Type 2 Diabetes. Diabetes Care 2009, 32, 1993–1997. [Google Scholar] [CrossRef]
- Sugimoto, K.; Ikegami, H.; Takata, Y.; Katsuya, T.; Fukuda, M.; Akasaka, H.; Tabara, Y.; Osawa, H.; Hiromine, Y.; Rakugi, H. Glycemic Control and Insulin Improve Muscle Mass and Gait Speed in Type 2 Diabetes: The MUSCLES-DM Study. J. Am. Med Dir. Assoc. 2020, in press. [Google Scholar]
- Park, S.W.; Goodpaster, B.H.; Strotmeyer, E.S.; De Rekeneire, N.; Harris, T.B.; Schwartz, A.V.; Tylavsky, F.A.; Newman, A.B. Decreased Muscle Strength and Quality in Older Adults With Type 2 Diabetes: The Health, Aging, and Body Composition Study. Diabetes 2006, 55, 1813–1818. [Google Scholar] [CrossRef] [PubMed]
- Almurdhi, M.M.; Reeves, N.D.; Bowling, F.L.; Boulton, A.J.; Jeziorska, M.; Malik, R.A. Reduced Lower-Limb Muscle Strength and Volume in Patients with Type 2 Diabetes in Relation to Neuropathy, Intramuscular Fat, and Vitamin D Levels. Diabetes Care. 2016, 39, 441–447. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Roso, M.B.; Knott-Torcal, C.; Matilla-Escalante, D.C.; Garcimartín, A.; Sampedro-Nuñez, M.A.; Dávalos, A.; Marazuela, M. COVID-19 Lockdown and Changes of the Dietary Pattern and Physical Activity Habits in a Cohort of Patients with Type 2 Diabetes Mellitus. Nutrients 2020, 12, 2327. [Google Scholar] [CrossRef]
- Sun, S.; Folarin, A.A.; Ranjan, Y.; Rashid, Z.; Conde, P.; Stewart, C.; Cummins, N.; Matcham, F.; Costa, G.D.; Simblett, S.; et al. Using Smartphones and Wearable Devices to Monitor Behavioral Changes During COVID-19. J. Med. Internet Res. 2020, 22, e19992. [Google Scholar] [CrossRef]
- Cunningham, C.; Sullivan, R.O.; Caserotti, P.; Tully, M.A. Consequences of physical inactivity in older adults: A systematic review of reviews and meta-analyses. Scand J. Med. Sci. Sports 2020, 30, 816–827. [Google Scholar] [CrossRef]
- Patterson, R.; McNamara, E.; Tainio, M.; De Sá, T.H.; Smith, A.D.; Sharp, S.J.; Edwards, P.; Woodcock, J.; Brage, S.; Wijndaele, K. Sedentary behaviour and risk of all-cause, cardiovascular and cancer mortality, and incident type 2 diabetes: A systematic review and dose response meta-analysis. Eur. J. Epidemiol. 2018, 33, 811–829. [Google Scholar] [CrossRef]
- Zhao, R.; Bu, W.; Chen, Y.; Chen, X. The Dose-Response Associations of Sedentary Time with Chronic Diseases and the Risk for All-Cause Mortality Affected by Different Health Status: A Systematic Review and Meta-Analysis. J. Nutr. Heal. Aging 2020, 24, 63–70. [Google Scholar] [CrossRef]
- Marçal, I.R.; Fernandes, B.; Viana, A.A.; Ciolac, E.G. The Urgent Need for Recommending Physical Activity for the Management of Diabetes During and Beyond COVID-19 Outbreak. Front. Endocrinol. 2020, 11, 584642. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Ferran, M.; De La Guía-Galipienso, F.; Sanchis-Gomar, F.; Pareja-Galeano, H. Metabolic Impacts of Confinement during the COVID-19 Pandemic Due to Modified Diet and Physical Activity Habits. Nutrients 2020, 12, 1549. [Google Scholar] [CrossRef]
- Zhu, L.; She, Z.-G.; Cheng, X.; Qin, J.-J.; Zhang, X.-J.; Cai, J.; Lei, F.; Wang, H.; Xie, J.; Wang, W.; et al. Association of Blood Glucose Control and Outcomes in Patients with COVID-19 and Pre-existing Type 2 Diabetes. Cell Metab. 2020, 31, 1068–1077.e3. [Google Scholar] [CrossRef] [PubMed]
- Machado, C.L.F.; Pinto, R.S.; Brusco, C.M.; Cadore, E.L.; Radaelli, R. COVID-19 pandemic is an urgent time for older people to practice resistance exercise at home. Exp. Gerontol. 2020, 141, 111101. [Google Scholar] [CrossRef] [PubMed]
- Guadalupe-Grau, A.; López-Torres, O.; Martos-Bermúdez, Á.; González-Gross, M. Home-based training strategy to maintain muscle function in older adults with diabetes during COVID -19 confinement. J. Diabetes 2020, 12, 701–702. [Google Scholar] [CrossRef] [PubMed]
- Ghram, A.; Briki, W.; Mansoor, H.; Al-Mohannadi, A.S.; Lavie, C.J.; Chamari, K. Home-based exercise can be beneficial for counteracting sedentary behavior and physical inactivity during the COVID-19 pandemic in older adults. Postgrad. Med. 2020, 2020, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Pavón, D.; Carbonell-Baeza, A.; Lavie, C.J. Physical exercise as therapy to fight against the mental and physical consequences of COVID-19 quarantine: Special focus in older people. Prog. Cardiovasc. Dis. 2020, 63, 386–388. [Google Scholar] [CrossRef]
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef]
- Petrilli, C.M.; Jones, S.A.; Yang, J.; Rajagopalan, H.; O’Donnell, L.; Chernyak, Y.; Tobin, K.A.; Cerfolio, R.J.; Francois, F.; Horwitz, L.I. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: Prospective cohort study. BMJ 2020, 369, m1966. [Google Scholar] [CrossRef]
- Yan, Y.; Yang, Y.; Wang, F.; Ren, H.; Zhang, S.; Shi, X.; Yu, X.; Dong, K. Clinical characteristics and outcomes of patients with severe covid-19 with diabetes. BMJ Open Diabetes Res. Care 2020, 8, e001343. [Google Scholar] [CrossRef]
- Patanavanich, R.; Glantz, S.A. Smoking Is Associated with COVID-19 Progression: A Meta-analysis. Nicotine Tob. Res. 2020, 22, 1653–1656. [Google Scholar] [CrossRef]
- Cheng, Q.; Zhu, X.; Zhang, X.; Li, H.; Du, Y.; Hong, W.; Xue, S.; Zhu, H. A cross-sectional study of loss of muscle mass corresponding to sarcopenia in healthy Chinese men and women: Reference values, prevalence, and association with bone mass. J. Bone Miner. Metab. 2013, 32, 78–88. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Hirayama, K.; Han, T.-F.; Izutsu, M.; Yuki, M. Sarcopenia Prevalence and Risk Factors among Japanese Community Dwelling Older Adults Living in a Snow-Covered City According to EWGSOP2. J. Clin. Med. 2019, 8, 291. [Google Scholar] [CrossRef]
- Hashimoto, Y.; Kaji, A.; Sakai, R.; Takahashi, F.; Kawano, R.; Hamaguchi, M.; Fukui, M. Effect of Exercise Habit on Skeletal Muscle Mass Varies with Protein Intake in Elderly Patients with Type 2 Diabetes: A Retrospective Cohort Study. Nutrients 2020, 12, 3220. [Google Scholar] [CrossRef] [PubMed]
- Noh, H.-M.; Park, Y.S. Handgrip strength, dynapenia, and mental health in older Koreans. Sci. Rep. 2020, 10, 1–9. [Google Scholar] [CrossRef]
- Ida, S.; Kaneko, R.; Nagata, H.; Noguchi, Y.; Araki, Y.; Nakai, M.; Ito, S.; Ishihara, Y.; Imataka, K.; Murata, K. Association between sarcopenia and sleep disorder in older patients with diabetes. Geriatr. Gerontol. Int. 2019, 19, 399–403. [Google Scholar] [CrossRef]

| N = 56 | Point 1 | Point 2 | Point 3 |
|---|---|---|---|
| Age, years | - | 75.2 (7.1) | - |
| Men | - | 35 (62.5) | - |
| Duration of diabetes, years | - | 19.7 (8.2) | - |
| Smokers | - | 25 (44.6) | - |
| Exercisers | - | 34 (60.7) | - |
| Alcohol consumers | - | 26 (46.4) | - |
| Height, cm | - | 160.5 (8.2) | - |
| HbA1c, % | 7.2 (0.8) | 7.1 (0.7) | 7.1 (0.7) |
| HbA1c, mmol/mol | 55.1 (9.1) | 54.4 (7.9) | 54.4 (7.9) |
| Insulin secretagogues | 49 (86.0) | 49 (86.0) | 51 (89.5) |
| Insulin sensitizers | 23 (40.4) | 24 (42.1) | 28 (49.1) |
| α-glucosidase inhibitors | 11 (19.3) | 11 (19.3) | 13 (22.8) |
| Sodium glucose cotransporter 2 inhibitors | 10 (17.5) | 16 (28.1) | 19 (33.3) |
| GLP-1 receptor agonists | 3 (5.2) | 4 (7.0) | 5 (8.8) |
| Insulin | 14 (24.6) | 14 (24.6) | 15 (26.3) |
| Renin-angiotensin system inhibitors | - | 34 (59.6) | - |
| Calcium channel blockers | - | 19 (33.3) | - |
| Other antihypertension drug | - | 15 (26.3) | - |
| Medication for dyslipidemia | - | 33 (57.8) | - |
| N = 22 | |||
| Age, years | - | 75.3 (6.3) | - |
| Men | - | 15 (68.1) | - |
| Duration of diabetes, years | - | 21.2 (9.8) | - |
| Smokers | - | 14 (63.6) | - |
| Exercisers | - | 14 (63.6) | - |
| Alcohol consumers | - | 10 (45.4) | - |
| Height, cm | - | 155.1 (24.7) | - |
| HbA1c, % | - | 7.4 (0.7) | - |
| HbA1c, mmol/mol | - | 57.4 (7.6) | - |
| Insulin secretagogues | - | 22 (100.0) | - |
| Insulin sensitizers | - | 14 (63.6) | - |
| α-Glucosidase inhibitors | - | 9 (40.9) | - |
| Sodium glucose cotransporter 2 inhibitors | - | 7 (31.8) | - |
| GLP-1 receptor agonists | - | 1 (4.5) | - |
| Insulin | - | 6 (27.2) | - |
| Renin-angiotensin system inhibitors | - | 15 (68.1) | - |
| Calcium channel blockers | - | 3 (13.6) | - |
| Other antihypertension drug | - | 5 (22.7) | - |
| Medication for dyslipidemia | - | 13 (59.0) | - |
| Stress increased | - | 5 (22.7) | - |
| Sleep duration decreased | - | 4 (18.1) | - |
| Exercise decreased | - | 11 (50.0) | - |
| Point 1 | Point 2 | Point 3 | * p Value | Change between Point 1 and Point 2 | Change between Point 2 and Point 3 | ** p Value | |
|---|---|---|---|---|---|---|---|
| Body weight, kg | 59.3 (10.3) | 59.1 (10.2) | 58.1 (10.5) †‡ | <0.001 | −0.223 (1.621) | −0.524 (1.436) | 0.337 |
| Appendicular muscle mass, kg | 17.5 (3.6) | 17.6 (3.7) | 17.1 (3.7) †‡ | <0.001 | 0.018 (0.740) | −0.302 (0.611) | 0.047 |
| SMI, kg/m2 | 6.7 (0.9) | 6.8 (0.9) | 6.6 (0.9) †‡ | <0.001 | 0.005 (0.289) | −0.117 (0.240) | 0.049 |
| Body fat, kg | 16.7 (6.4) | 16.5 (6.4) | 16.1 (6.5) | 0.274 | −0.177 (1.324) | −0.142 (1.762) | 0.904 |
| Percent body fat, % | 27.6 (7.8) | 27.4 (7.8) | 27.7 (7.4) | 0.692 | −0.160 (1.799) | 0.269 (1.856) | 0.276 |
| Point 1 | Point 2 | Point 3 | * p Value | Change between Point 1 and Point 2 | Change between Point 2 and Point 3 | ** p Value | ||
|---|---|---|---|---|---|---|---|---|
| Sex | Men, n = 35 | 7.1 (0.7) | 7.2 (0.8) | 7.0 (0.8) †‡ | <0.001 | 0.031 (0.325) | −0.159 (0.257) | 0.038 |
| Women, n = 21 | 6.0 (0.6) | 6.0 (0.6) | 5.9 (0.7) | 0.301 | −0.038 (0.217) | −0.048 (0.198) | 0.885 | |
| Exercise habit | (−), n = 22 | 6.9 (0.7) | 6.9 (0.7) | 6.7 (0.7) †‡ | 0.018 | 0.001 (0.386) | −0.162 (0.289) | 0.229 |
| (+), n = 34 | 6.6 (1.0) | 6.6 (1.0) | 6.5 (1.0) ‡ | 0.028 | 0.008 (0.212) | −0.088 (0.203) | 0.088 | |
| Smoking | (−), n = 31 | 6.5 (0.9) | 6.5 (0.9) | 6.4 (0.9) | 0.143 | −0.000 (0.298) | −0.081 (0.256) | 0.349 |
| (+), n = 25 | 7.0 (0.8) | 7.1 (0.9) | 6.8 (0.8) †‡ | <0.001 | 0.012 (0.285) | −0.161 (0.219) | 0.057 | |
| Alcohol | (−), n = 30 | 6.7 (0.9) | 6.7 (1.0) | 6.5 (1.0) | 0.060 | −0.025 (0.278) | −0.091 (0.256) | 0.402 |
| (+), n = 26 | 6.8(0.8) | 6.8 (0.9) | 6.6 (0.8) †‡ | 0.003 | 0.040 (0.303) | −0.147 (0.224) | 0.062 | |
| HbA1c | <7.0%, n = 28 | 6.7 (0.9) | 6.7 (0.8) | 6.7 (0.9) | 0.405 | −0.021 (0.297) | −0.064 (0.207) | 0.570 |
| ≥7.0%, n = 28 | 6.7 (0.8) | 6.8 (1.0) | 6.5 (0.9) †‡ | <0.001 | 0.031 (0.285) | −0.170 (0.264) | 0.042 | |
| Obesity | (−), n = 41 | 6.5 (0.7) | 6.5 (0.8) | 6.3 (0.8) †‡ | <0.001 | 0.010 (0.297) | −0.133 (0.239) | 0.067 |
| (+), n = 15 | 7.4 (0.9) | 7.4 (0.8) | 7.3 (0.7) | 0.414 | −0.009 (0.276) | −0.070 (0.249) | 0.494 | |
| Duration | <20 years, n = 36 | 6.8 (1.0) | 6.8 (1.0) | 6.6 (1.0) | 0.026 | −0.037 (0.283) | −0.097 (0.248) | 0.402 |
| ≥20 years, n = 20 | 6.6 (0.6) | 6.7 (0.8) | 6.4 (0.6) †‡ | 0.007 | 0.082 (0.291) | −0.153 (0.229) | 0.049 |
| P | η2 | |
|---|---|---|
| Time | 0.029 | 0.108 |
| Time * sex | 0.511 | 0.009 |
| Time * age | 0.110 | 0.057 |
| Time * BMI | 0.535 | 0.008 |
| Time * exercise | 0.669 | 0.004 |
| Time * smoking | 0.839 | 0.001 |
| Time * alcohol | 0.517 | 0.009 |
| Time * duration | 0.138 | 0.048 |
| Time * HbA1c | 0.389 | 0.016 |
| Point 1 | Point 2 | Point 3 | *p Value | Change between Point 1 and Point 2 | Change between Point 2 and Point 3 | ** p Value | |
|---|---|---|---|---|---|---|---|
| Stress (−), n = 17 | 6.6 (0.8) | 6.6 (0.9) | 6.5 (0.9) | 0.042 | −0.013 (0.152) | −0.071 (0.155) | 0.399 |
| Stress (+), n = 5 | 6.7 (1.3) | 6.9 (1.2) | 6.8 (1.3) | 0.402 | 0.202 (0.153) | −0.060 (0.296) | 0.108 |
| Sleep duration decrease (−), n = 18 | 6.5 (0.8) | 6.6 (0.9) | 6.5 (0.9) | 0.302 | 0.032 (0.199) | −0.065 (0.217) | 0.242 |
| Sleep duration decrease (+), n = 4 | 7.0 (1.2) | 7.1 (1.3) † | 6.9 (1.3) | 0.072 | 0.084 (0.032) | −0.082 (0.073) | 0.005 |
| Exercise decrease (−), n = 11 | 6.5 (0.5) | 6.5 (0.5) | 6.4 (0.7) | 0.874 | 0.024 (0.205) | −0.027 (0.255) | 0.643 |
| Exercise decrease (+), n = 11 | 6.8 (1.2) | 6.9 (1.2) | 6.7(1.2) ‡ | 0.006 | 0.061 (0.154) | −0.107 (0.111) | 0.036 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hasegawa, Y.; Takahashi, F.; Hashimoto, Y.; Munekawa, C.; Hosomi, Y.; Okamura, T.; Okada, H.; Senmaru, T.; Nakanishi, N.; Majima, S.; et al. Effect of COVID-19 Pandemic on the Change in Skeletal Muscle Mass in Older Patients with Type 2 Diabetes: A Retrospective Cohort Study. Int. J. Environ. Res. Public Health 2021, 18, 4188. https://doi.org/10.3390/ijerph18084188
Hasegawa Y, Takahashi F, Hashimoto Y, Munekawa C, Hosomi Y, Okamura T, Okada H, Senmaru T, Nakanishi N, Majima S, et al. Effect of COVID-19 Pandemic on the Change in Skeletal Muscle Mass in Older Patients with Type 2 Diabetes: A Retrospective Cohort Study. International Journal of Environmental Research and Public Health. 2021; 18(8):4188. https://doi.org/10.3390/ijerph18084188
Chicago/Turabian StyleHasegawa, Yuka, Fuyuko Takahashi, Yoshitaka Hashimoto, Chihiro Munekawa, Yukako Hosomi, Takuro Okamura, Hiroshi Okada, Takafumi Senmaru, Naoko Nakanishi, Saori Majima, and et al. 2021. "Effect of COVID-19 Pandemic on the Change in Skeletal Muscle Mass in Older Patients with Type 2 Diabetes: A Retrospective Cohort Study" International Journal of Environmental Research and Public Health 18, no. 8: 4188. https://doi.org/10.3390/ijerph18084188
APA StyleHasegawa, Y., Takahashi, F., Hashimoto, Y., Munekawa, C., Hosomi, Y., Okamura, T., Okada, H., Senmaru, T., Nakanishi, N., Majima, S., Ushigome, E., Hamaguchi, M., Yamazaki, M., & Fukui, M. (2021). Effect of COVID-19 Pandemic on the Change in Skeletal Muscle Mass in Older Patients with Type 2 Diabetes: A Retrospective Cohort Study. International Journal of Environmental Research and Public Health, 18(8), 4188. https://doi.org/10.3390/ijerph18084188










