Environmental Detection of SARS-CoV-2 Virus RNA in Health Facilities in Brazil and a Systematic Review on Contamination Sources
Abstract
1. Introduction
2. Materials and Methods
2.1. Molecular Analysis
2.1.1. Sampling Areas
2.1.2. Detection of Viral RNA on Inert Surfaces
2.2. Statistical Analysis
2.3. Systematic Review: Search Strategy and Selection Criteria
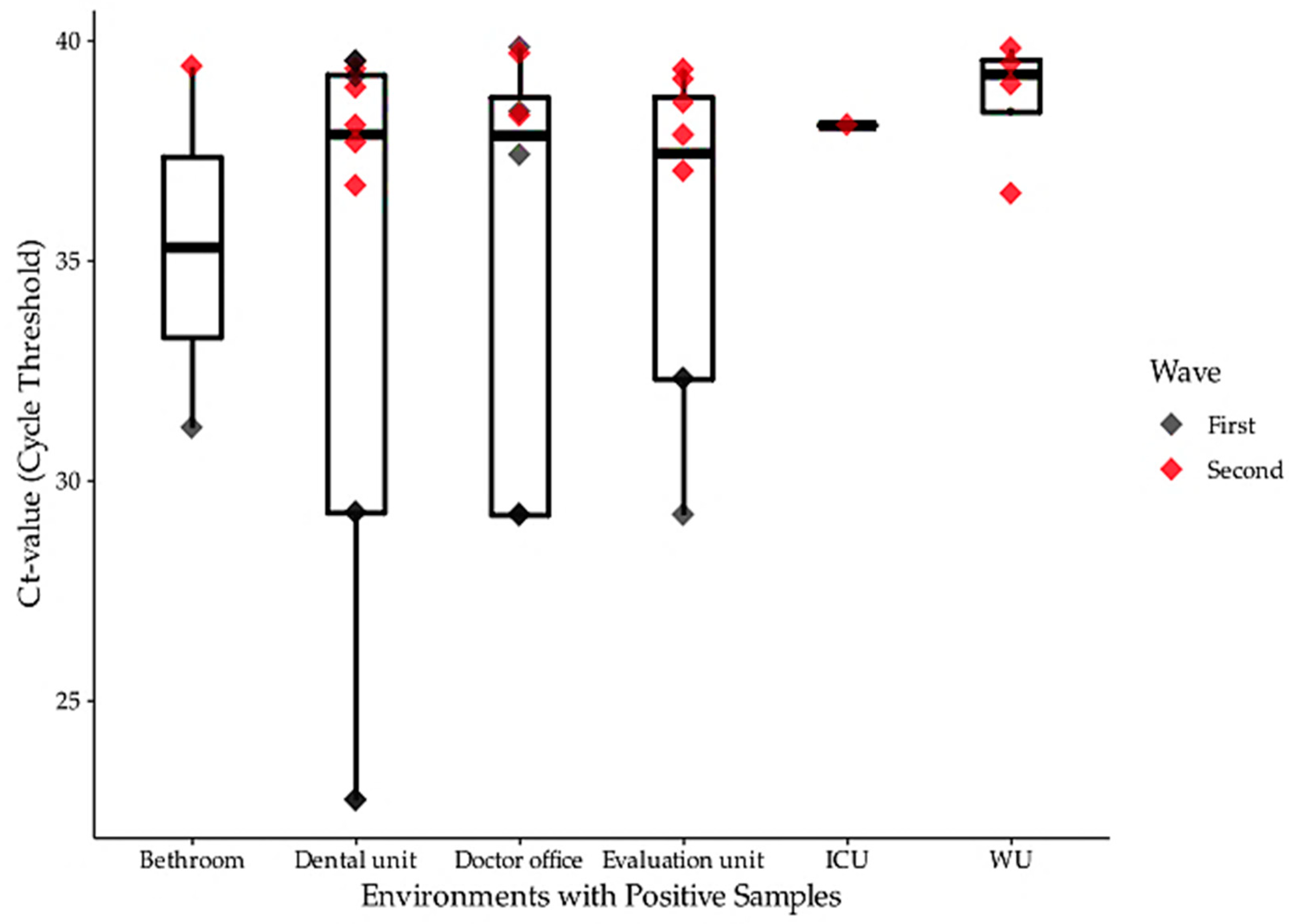
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. Report of the WHO-China Joint Mission on Coronavirus Disease 2019 (COVID-19); World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- Ranzani, O.T.; Bastos, L.S.L.; Gelli, J.G.M.; Marchesi, J.F.; Baião, F.; Hamacher, S.; A Bozza, F. Characterisation of the first 250,000 hospital admissions for COVID-19 in Brazil: A retrospective analysis of nationwide data. Lancet Respir. Med. 2021. [Google Scholar] [CrossRef]
- Kampf, G.; Lemmen, S.; Suchomel, M. Ct values and infectivity of SARS-CoV-2 on surfaces. Lancet Infect. Dis. 2020. [Google Scholar] [CrossRef]
- Lv, J.; Yang, J.; Xue, J.; Zhu, P.; Liu, L.; Li, S. Detection of SARS-CoV-2 RNA residue on object surfaces in nucleic acid testing laboratory using droplet digital PCR. Sci. Total. Environ. 2020, 742, 140370. [Google Scholar] [CrossRef] [PubMed]
- Dehghani, R.; Kassiri, H. A brief review on the possible role of houseflies and cockroaches in the mechanical transmission of coronavirus disease 2019 (COVID-19). Arch. Clin. Infect. Dis. 2020, 15, 102863. [Google Scholar] [CrossRef]
- Ahmed, W.; Angel, N.; Edson, J.; Bibby, K.; Bivins, A.; O’Berien, J.W.; Choi, P.H.; Kitajima, M.; Simpson, S.L.; Li, J.; et al. First confirmed detection of SARS-CoV-2 in untreated wastewater in Australia: A proof of concept for the wastewater surveillance of COVID-19 in the community. Sci. Total. Environ. 2020, 728, 138764. [Google Scholar] [CrossRef] [PubMed]
- WHO; (UNICEF) UNCF. Water, Sanitation, Hygiene and Waste Management for COVID-19: Technical Brief, 03 March 2020; World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- Chin, A.; Chu, J.; Perera, M.; Hui, K.; Yen, H.-L.; Chan, M.; Peiris, M.; Poon, L. Stability of SARS-CoV-2 in different environmental conditions. medRxiv 2020, 1, e146. [Google Scholar] [CrossRef]
- Huang, Y.; Ding, Z.; Chen, Q.; Wu, L.; Guo, L.; Zhao, C.; Sha, L.; Sun, H. Environmental virus detection associated with asymptomatic SARS-CoV-2-infected individuals with positive anal swabs. Sci. Total. Environ. 2020, 753, 142289. [Google Scholar] [CrossRef] [PubMed]
- WHO. Modes of Transmission of Virus Causing COVID-19: Implications for IPC Precaution Recommendations: Scientific Brief, 27 March 2020; World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- Barratt, R.; Shaban, R.Z.; Gilbert, G.L. Clinician perceptions of respiratory infection risk; a rationale for research into mask use in routine practice. Infect. Dis. Heal. 2019, 24, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Lai, T.; Xiang, F.; Zeng, J.; Huang, Y.; Jia, L.; Chen, H.; Wu, J.; Xie, J.; Liu, S.; Deng, W.; et al. Reliability of induced sputum test is greater than that of throat swab test for detecting SARS-CoV-2 in patients with COVID-19: A multi-center cross-sectional study. Virulence 2020, 11, 1394–1401. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, C.; Lin, M.; Deng, Q.; Ye, Y.; Li, Z.; Qiu, L.; Wang, Z. Analysis of the virus contamination and disinfection effect in isolation ward of patients with COVID-19. Front. Public Health 2020, 8, 8. [Google Scholar] [CrossRef]
- Kampf, G.; Todt, D.; Pfaender, S.; Steinmann, E. Persistence of coronaviruses on inanimate surfaces and their inactivation with biocidal agents. J. Hosp. Infect. 2020, 104, 246–251. [Google Scholar] [CrossRef] [PubMed]
- Eslami, H.; Jalili, M. The role of environmental factors to transmission of SARS-CoV-2 (COVID-19). AMB Express 2020, 10, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Ye, G.; Lin, H.; Chen, S.; Wang, S.; Zeng, Z.; Wang, W.; Zhang, S.; Rebmann, T.; Li, Y.; Pan, Z.; et al. Environmental contamination of SARS-CoV-2 in healthcare premises. J. Infect. 2020, 81, e1–e5. [Google Scholar] [CrossRef] [PubMed]
- Lewis, D. COVID-19 rarely spreads through surfaces. So why are we still deep cleaning? Nat. Cell Biol. 2021, 590, 26–28. [Google Scholar] [CrossRef]
- Wang, D.; Hu, B.; Hu, C.; Zhu, F.; Liu, X.; Zhang, J.; Wang, B.; Xiang, H.; Cheng, Z.; Xiong, Y.; et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA 2020, 323, 1061–1069. [Google Scholar] [CrossRef] [PubMed]
- Kimball, A.; Hatfield, K.M.; Arons, M.; James, A.; Taylor, J.; Spicer, K.; Bardossy, A.C.; Oakley, L.P.; Tanwar, S.; Chisty, Z.; et al. Asymptomatic and presymptomatic SARS-CoV-2 infections in residents of a long-term care skilled nursing facility—King County, Washington, March 2020. MMWR Morb. Mortal. Wkly. Rep. 2020, 69, 377–381. [Google Scholar] [CrossRef] [PubMed]
- Santarpia, J.L.; Rivera, D.N.; Herrera, V.L.; Morwitzer, M.J.; Creager, H.M.; Santarpia, G.W.; Crown, K.K.; Brett-Major, D.M.; Schnaubelt, E.R.; Broadhurst, M.J.; et al. Aerosol and surface contamination of SARS-CoV-2 observed in quarantine and isolation care. Sci. Rep. 2020, 10, 12732. [Google Scholar] [CrossRef]
- Medema, G.; Heijnen, L.; Elsinga, G.; Italiaander, R.; Brouwer, A. Presence of SARS-Coronavirus-2 RNA in sewage and correlation with reported COVID-19 prevalence in the early stage of the epidemic in The Netherlands. Environ. Sci. Technol. Lett. 2020, 7, 511–516. [Google Scholar] [CrossRef]
- Pasquarella, C.; Colucci, M.E.; Bizzarro, A.; Veronesi, L.; Affanni, P.; Meschi, T.; Brianti, E.; Vitali, P.; Albertini, R. Detection of SARS-CoV-2 on hospital surfaces. Acta Biomed. 2020, 91, 76–78. [Google Scholar] [CrossRef]
- Wu, F.; Xiao, A.; Zhang, J.; Moniz, K.; Endo, N.; Armas, F.; Bonneau, R.; Brown, M.A.; Bushman, M.; Chai, P.R.; et al. SARS-CoV-2 titers in wastewater foreshadow dynamics and clinical presentation of new COVID-19 cases. medRxiv Prepr. Serv. Health Sci. 2020. published online June. [Google Scholar] [CrossRef]
- Ahmed, W.; Payyappat, S.; Cassidy, M.; Harrison, N.; Besley, C. Sewage-associated marker genes illustrate the impact of wet weather overflows and dry weather leakage in urban estuarine waters of Sydney, Australia. Sci. Total. Environ. 2020, 705, 135390. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.; Vieira, G.; Vanny, P. Procedimento de Higienização de Superfícies das Salas de Espera em ambientes de atendimento de pacientes com suspeita ou confirmação de Covid-19. Available online: http://www2.ebserh.gov.br/documents/2016343/5135576/POP+Higienização+Superf%C3%ADcies+Sala+de+Espera.pdf/c0dede3f-9fe0-4ba1-a8d0-fb75ce0cf67a (accessed on 20 August 2020).
- Parana MBI of KIT BIOMOL OneStep/COVID-19. 2020. Available online: http://www.ibmp.org.br/pt-br/wp-content/uploads/2020/05/Instrução-de-Uso-Kit-BIOMOL-OneStep_COVID-19-rev-02.pdf. (accessed on 28 July 2020).
- Shamseer, L.; Moher, D.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A.; PRISMA-P Group. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: Elaboration and explanation. BMJ 2015, 349, g7647. [Google Scholar] [CrossRef] [PubMed]
- Viechtbauer, W. Conducting meta-analyses in R with the metafor package. J. Stat. Softw. 2010, 36, 1–48. [Google Scholar] [CrossRef]
- Sultan, A.; Singh, N.; Juneja, A. Dentistry and risk management–A challenging balance in an era of COVID-19. JICDRO 2020, 12, 94. [Google Scholar]
- Peng, X.; Xu, X.; Li, Y.; Cheng, L.; Zhou, X.; Ren, B. Transmission routes of 2019-nCoV and controls in dental practice. Int. J. Oral Sci. 2020, 12, 1–6. [Google Scholar] [CrossRef]
- Razzini, K.; Castrica, M.; Menchetti, L.; Maggi, L.; Negroni, L.; Orfeo, N.V.; Pizzoccheri, A.; Stocco, M.; Stocco, S.K.; Castrica, M.; et al. SARS-CoV-2 RNA detection in the air and on surfaces in the COVID-19 ward of a hospital in Milan, Italy. Sci. Total. Environ. 2020, 742, 140540. [Google Scholar] [CrossRef]
- Otter, J.A.; Donskey, C.; Yezli, S.; Douthwaite, S.; Goldenberg, S.; Weber, D.J. Transmission of SARS and MERS coronaviruses and influenza virus in healthcare settings: The possible role of dry surface contamination. J. Hosp. Infect. 2016, 92, 235–250. [Google Scholar] [CrossRef]
- Ryu, B.-H.; Cho, Y.; Cho, O.-H.; Hong, S.I.; Kim, S.; Lee, S. Environmental contamination of SARS-CoV-2 during the COVID-19 outbreak in South Korea. Am. J. Infect. Control. 2020, 48, 875–879. [Google Scholar] [CrossRef]
- Hu, X.; Xing, Y.; Ni, W.; Zhang, F.; Lu, S.; Wang, Z.; Gao, R.; Jiang, F. Environmental contamination by SARS-CoV-2 of an imported case during incubation period. Sci. Total. Environ. 2020, 742, 140620. [Google Scholar] [CrossRef]
- Li, Y.H.; Fan, Y.Z.; Jiang, L.; Wang, H.B. Aerosol and environmental surface monitoring for SARS-CoV-2 RNA in a designated hospital for severe COVID-19 patients. Epidemiol. Infect. 2020, 148, e154. [Google Scholar] [CrossRef]
- Ian, W.L.E.; Sim, X.Y.J.; Conceicao, E.P.; Aung, M.K.; Tan, K.Y.; Ko, K.K.K.; Wong, H.M.; Wijaya, L.; Tan, B.H.; Venkatachalam, I.; et al. Containing COVID-19 outside the isolation ward: The impact of an infection control bundle on environmental contamination and transmission in a cohorted general ward. Am. J. Infect. Control. 2020, 48, 1056–1061. [Google Scholar]
- Colaneri, M.; Seminari, E.; Novati, S.; Asperges, E.; Biscarini, S.; Piralla, A.; Percivalle, E.; Cassaniti, I.; Baldanti, F.; Bruno, R.; et al. Severe acute respiratory syndrome coronavirus 2 RNA contamination of inanimate surfaces and virus viability in a health care emergency unit. Clin. Microbiol. Infect. 2020, 26, 1094.e1–1094.e5. [Google Scholar] [CrossRef] [PubMed]
- Abrahão, J.S.; Sacchetto, L.; Rezende, I.M.; Rodrigues, R.A.L.; Crispim, A.P.C.; Moura, C.; Mendonça, D.C.; Reis, E.; Souza, F.; Oliveira, G.F.G.; et al. Detection of SARS-CoV-2 RNA on public surfaces in a densely populated urban area of Brazil: A potential tool for monitoring the circulation of infected patients. Sci. Total. Environ. 2020, 766, 142645. [Google Scholar] [CrossRef] [PubMed]
- Biryukov, J.; Boydston, J.A.; Dunning, R.A.; Yeager, J.J.; Wood, S.; Reese, A.L.; Ferris, A.; Miller, D.; Weaver, W.; Zeitouni, N.E.; et al. Increasing temperature and relative humidity accelerates inactivation of SARS-CoV-2 on surfaces. mSphere 2020, 5, 00441-20. [Google Scholar] [CrossRef] [PubMed]
- Carraturo, F.; Del Giudice, C.; Morelli, M.; Cerullo, V.; Libralato, G.; Galdiero, E.; Guida, M. Persistence of SARS-CoV-2 in the environment and COVID-19 transmission risk from environmental matrices and surfaces. Environ. Pollut. 2020, 265, 115010. [Google Scholar] [CrossRef]
- Morris, D.H.; Yinda, K.C.; Gamble, A.; Rossine, F.W.; Huang, Q.; Bushmaker, T.; Fischer, R.J.; Matson, M.J.; van Doremalen, N.; Vikesland, P.J.; et al. The effect of temperature and humidity on the stability of SARS-CoV-2 and other enveloped viruses. bioRxiv 2020. [Google Scholar] [CrossRef]
- Van Doremalen, N.; Bushmaker, T.; Lloyd-Smith, J.O.; De Wit, E.; Munster, V.J.; Morris, D.H.; Holbrook, M.G.; Gamble, A.; Williamson, B.N.; Tamin, A.; et al. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N. Engl. J. Med. 2020, 382, 1564–1567. [Google Scholar] [CrossRef]
- Marquès, M.; Domingo, J.L. Contamination of inert surfaces by SARS-CoV-2: Persistence, stability and infectivity. A review. Environ. Res. 2021, 193, 110559. [Google Scholar] [CrossRef]
- Rimoldi, S.G.; Stefani, F.; Gigantiello, A.; Polesello, S.; Comandatore, F.; Mileto, D.; Marecas, M.; Longobardi, C.; Manco, A.; Romeir, F.; et al. Presence and infectivity of SARS-CoV-2 virus in wastewaters and rivers. Sci. Total. Environ. 2020, 744, 140911. [Google Scholar] [CrossRef]
- Wang, J.; Feng, H.; Zhang, S.; Ni, Z.; Ni, L.; Chen, Y.; Zhuo, L.; Zhong, Z.; Qu, T. SARS-CoV-2 RNA detection of hospital isolation wards hygiene monitoring during the Coronavirus Disease 2019 outbreak in a Chinese hospital. Int. J. Infect. Dis. 2020, 94, 103–106. [Google Scholar] [CrossRef]
- Chia, P.Y.; Coleman, K.K.; Tan, Y.K.; Ong, S.W.X.; Gum, M.; Lau, S.K.; Lim, X.F.; Lim, A.S.; Sutjipto, S.; Lee, P.H.; et al. Singapore novel coronavirus outbreak research T (2020) detection of air and surface contamination by SARS-CoV-2 in hospital rooms of infected patients. Nat. Commun. 2020, 11, 2800. [Google Scholar] [CrossRef] [PubMed]
- Ding, Z.; Qian, H.; Xu, B.; Huang, Y.; Miao, T.; Yen, H.-L.; Xiao, S.; Cui, L.; Wu, X.; Shao, W.; et al. Toilets dominate environmental detection of SARS-CoV-2 virus in a hospital. medRxiv 2020. [Google Scholar] [CrossRef]
- Faridi, S.; Niazi, S.; Sadeghi, K.; Naddafi, K.; Yavarian, J.; Shamsipour, M.; Jandaghi, N.Z.S.; Sadeghniiat, K.; Nabizadeh, R.; Yunesian, M.; et al. A field indoor air measurement of SARS-CoV-2 in the patient rooms of the largest hospital in Iran. Sci. Total. Environ. 2020, 725, 138401. [Google Scholar] [CrossRef] [PubMed]
- Ge, X.-Y.; Pu, Y.; Liao, C.-H.; Huang, W.-F.; Zeng, Q.; Zhou, H.; Yi, B.; Wang, A.-M.; Dou, Q.-Y.; Zhou, P.-C.; et al. Evaluation of the exposure risk of SARS-CoV-2 in different hospital environment. Sustain. Cities Soc. 2020, 61, 102413. [Google Scholar] [CrossRef]
- Kim, U.J.; Lee, S.Y.; Lee, J.Y.; Lee, A.; Kim, S.E.; Choi, O.-J.; Kee, S.-J.; Jang, H.-C. Air and environmental contamination caused by COVID-19 patients: A multi-center study. J. Korean Med Sci. 2020, 35, 332. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Lin, J.; Duan, X.; Huang, W.; Lu, X.; Zhou, J.; Zong, Z. Asymptomatic COVID-19 patients can contaminate their surroundings: An environment sampling study. mSphere 2020, 5, 5. [Google Scholar] [CrossRef]
- Wu, S.; Wang, Y.; Jin, X.; Tian, J.; Liu, J.; Mao, Y. Environmental contamination by SARS-CoV-2 in a designated hospital for coronavirus disease 2019. Am. J. Infect. Control. 2020, 48, 910. [Google Scholar] [CrossRef] [PubMed]
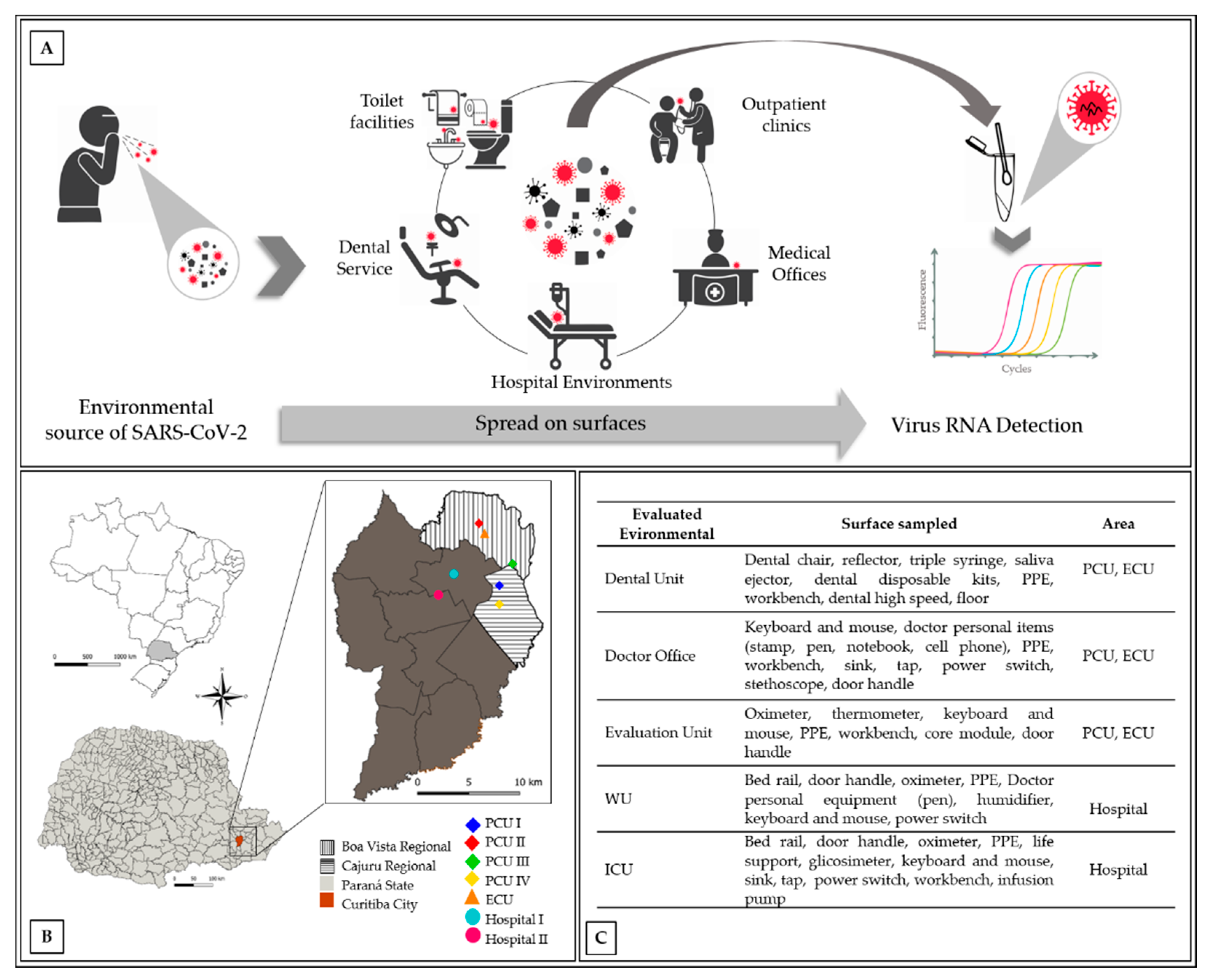

| Location | Month | Sample Description | Pd | CT—Orf1 | CT—N |
|---|---|---|---|---|---|
| PCU | July | Dental triple syringe | No | - | 39.18 |
| PCU | July | Sink and tap from the evaluation unit | No | 30.51 | 31.88 |
| PCU | July | Dental chair | Yes | 22.74 | - |
| PCU | July | Dental triple syringe | Yes | 22.74 | - |
| PCU | August | Oximeter from the doctor room | No | 38.38 | - |
| PCU | August | Patient armchair | No | - | 39.84 |
| ECU | August | Oximeter from the evaluation unit | No | 32.31 | - |
| ECU | August | Thermometer from the evaluation unit | No | 32.31 | - |
| ECU | September | Dental reflector | No | - | 39.53 |
| ECU | September | Dental saliva ejector | No | - | 39.53 |
| PCU | September | Dental disposable kit | No | 29.27 | - |
| PCU | September | Keyboard and mouse from the doctor room | No | 29.22 | - |
| PCU | September | Doctor personal item—notebook | No | 29.22 | - |
| PCU | September | Workbench disposable gloves workbench | No | 29.27 | - |
| PCU | September | Doctor personal item—pen and stamp | No | 29.22 | - |
| PCU | September | Patient armchair from the doctor room | No | 37.40 | - |
| PCU | September | Keyboard and mouse from the evaluation unit | No | 29.22 | - |
| ECU | December | Doctor personal item—stamp | No | - | 39.70 |
| ECU | December | Door handle from inside of the doctor room | No | - | 38.29 |
| ECU | December | Toilet discharge from the evaluation unit | No | - | 39.41 |
| PCU | December | Patient armchair from the covid-19 evaluation unit | No | - | 39.12 |
| PCU | December | Keyboard and mouse from the covid-19 evaluation unit | No | 37.03 | - |
| PCU | December | Doctor personal item—pen from the covid-19 evaluation unit | No | - | 38.59 |
| PCU | December | Dental chair | Yes | 36.87 | 38.48 |
| PCU | December | Dental reflector | Yes | - | 39.35 |
| PCU | December | Dental saliva ejector | No | - | 38.93 |
| PCU | December | Dental saliva ejector | No | 38.07 | - |
| PCU | December | Dental disposable kit | No | 36.70 | - |
| WU | December | Bed rail from the hospital covid-19 ward | No | 38.08 | 39.92 |
| WU | December | Sink and Tap of the hospital covid-19 ward | No | 36.13 | 36.91 |
| WU | December | Door handle from the hospital covid-19 ward | No | - | 39.48 |
| ICU | December | Bed rail from the hospital intense care unit | No | 38.08 | - |
| WU | December | Life support—humidifier from the hospital covid-19 ward | No | - | 39.82 |
| ECU | December | X-ray bucky wall | No | - | 37.85 |
| ECU | December | Door handle from the X-ray room | No | 39.43 | 39.25 |
| Sample N° | N Gene | % | IC (95%) | p | Orf1 Gene | % | IC (95%) | p | |
|---|---|---|---|---|---|---|---|---|---|
| Health Unit | |||||||||
| PCU | 365 | 8 | 2.19 | 2.18–2.19 | 0.668 | 16 | 4.38 | 4.38–4.39 | 0.092 |
| ECU | 235 | 7 | 2.98 | 2.98–2.98 | 3 | 1.28 | 1.27–1.28 | ||
| Hospital | 111 | 4 | 3.60 | 3.60–3.61 | 3 | 2.70 | 2.70–2.71 | ||
| Health place | |||||||||
| PCU I | 78 | 4 | 5.13 | 5.15–5.11 | 0.063 | 2 | 2.56 | 2.53–2.60 | <0.001 |
| PCU II | 57 | 0 | 0.00 | −0.02–0.02 | 8 | 14.04 | 14.00–14.07 | ||
| PCU III | 22 | 1 | 4.55 | 4.53–4.56 | 2 | 9.09 | 9.06–9.13 | ||
| PCU IV | 208 | 3 | 1.44 | 1.42–1.46 | 4 | 1.92 | 1.89–1.96 | ||
| ECU | 235 | 7 | 2.98 | 2.96–3.00 | 3 | 1.28 | 1.24–1.31 | ||
| Hospital 1 | 73 | 1 | 1.37 | 1.35–1.39 | 1 | 1.37 | 1.33–1.41 | ||
| Hospital II | 38 | 3 | 7.89 | 7.88–7.91 | 2 | 5.26 | 5.23–5.30 | ||
| Evaluated Environment | |||||||||
| Dental Unit | 234 | 6 | 2.56 | 2.54–2.58 | 0.051 | 7 | 2.99 | 2.99–3.00 | 0.763 |
| Doctor office | 177 | 3 | 1.69 | 1.67–1.71 | 6 | 3.39 | 3.38–3.40 | ||
| Evaluation Unit | 160 | 4 | 2.50 | 2.48–2.52 | 5 | 3.13 | 3.12–3.13 | ||
| WU | 45 | 4 | 8.89 | 8.87–8.91 | 2 | 4.44 | 4.44–4.45 | ||
| ICU | 55 | 0 | 0.00 | −0.02–0.02 | 1 | 1.82 | 1.81–1.82 | ||
| Bathroom | 40 | 2 | 5.00 | 4.98–5.02 | 1 | 2.50 | 2.49–2.51 | ||
| Month of sampled | |||||||||
| July | 83 | 2 | 2.41 | 2.40–2.42 | 0.412 | 3 | 3.61 | 3.60–3.63 | 0.525 |
| August | 176 | 1 | 0.57 | 0.56–0.58 | 3 | 1.70 | 1.69–1.72 | ||
| September | 104 | 2 | 1.92 | 1.92–1.93 | 8 | 7.69 | 7.68–7.71 | ||
| December | 348 | 14 | 4.02 | 4.02–4.03 | 8 | 2.30 | 2.29–2.31 | ||
| Presence of COVID-19 patient | |||||||||
| Positive | 411 | 10 | 2.43 | 2.42–2.44 | <0.001 | 14 | 3.41 | 3.40–3.42 | 0.938 |
| Negative | 24 | 0 | 0.00 | −0.01–0.01 | 0 | 0.00 | −0.01–0.01 | ||
| Indifferent | 276 | 9 | 3.26 | 3.25–3.27 | 8 | 2.90 | 2.89–2.91 | ||
| Disinfected surface | |||||||||
| Yes | 206 | 2 | 0.97 | 0.96–0.98 | 0.224 | 4 | 1.94 | 1.93–1.95 | 0.149 |
| No | 505 | 17 | 3.37 | 3.36–3.38 | 18 | 3.56 | 3.56–3.57 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vicente, V.A.; Lustosa, B.P.R.; Grisolia, M.E.; Pavini Beato, C.; Balsanelli, E.; de Souza Gubert Fruet, V.; Bordignon Nogueira, M.; Raboni, S.M.; Carvalho, K.A.T.; Flôr, I.C.; et al. Environmental Detection of SARS-CoV-2 Virus RNA in Health Facilities in Brazil and a Systematic Review on Contamination Sources. Int. J. Environ. Res. Public Health 2021, 18, 3824. https://doi.org/10.3390/ijerph18073824
Vicente VA, Lustosa BPR, Grisolia ME, Pavini Beato C, Balsanelli E, de Souza Gubert Fruet V, Bordignon Nogueira M, Raboni SM, Carvalho KAT, Flôr IC, et al. Environmental Detection of SARS-CoV-2 Virus RNA in Health Facilities in Brazil and a Systematic Review on Contamination Sources. International Journal of Environmental Research and Public Health. 2021; 18(7):3824. https://doi.org/10.3390/ijerph18073824
Chicago/Turabian StyleVicente, Vania Aparecida, Bruno Paulo Rodrigues Lustosa, Maria Eduarda Grisolia, Caroline Pavini Beato, Eduardo Balsanelli, Viviane de Souza Gubert Fruet, Meri Bordignon Nogueira, Sonia Maria Raboni, Katherine Athayde Teixeira Carvalho, Izadora Cervelin Flôr, and et al. 2021. "Environmental Detection of SARS-CoV-2 Virus RNA in Health Facilities in Brazil and a Systematic Review on Contamination Sources" International Journal of Environmental Research and Public Health 18, no. 7: 3824. https://doi.org/10.3390/ijerph18073824
APA StyleVicente, V. A., Lustosa, B. P. R., Grisolia, M. E., Pavini Beato, C., Balsanelli, E., de Souza Gubert Fruet, V., Bordignon Nogueira, M., Raboni, S. M., Carvalho, K. A. T., Flôr, I. C., Ferreira Voidaleski, M., Etchepare, R. G., Meis, J. F., Soccol, V. T., & Souza, E. M. (2021). Environmental Detection of SARS-CoV-2 Virus RNA in Health Facilities in Brazil and a Systematic Review on Contamination Sources. International Journal of Environmental Research and Public Health, 18(7), 3824. https://doi.org/10.3390/ijerph18073824






