Predictive Model of Gait Recovery at One Month after Hip Fracture from a National Cohort of 25,607 Patients: The Hip Fracture Prognosis (HF-Prognosis) Tool
Abstract
1. Introduction
2. Materials and Methods
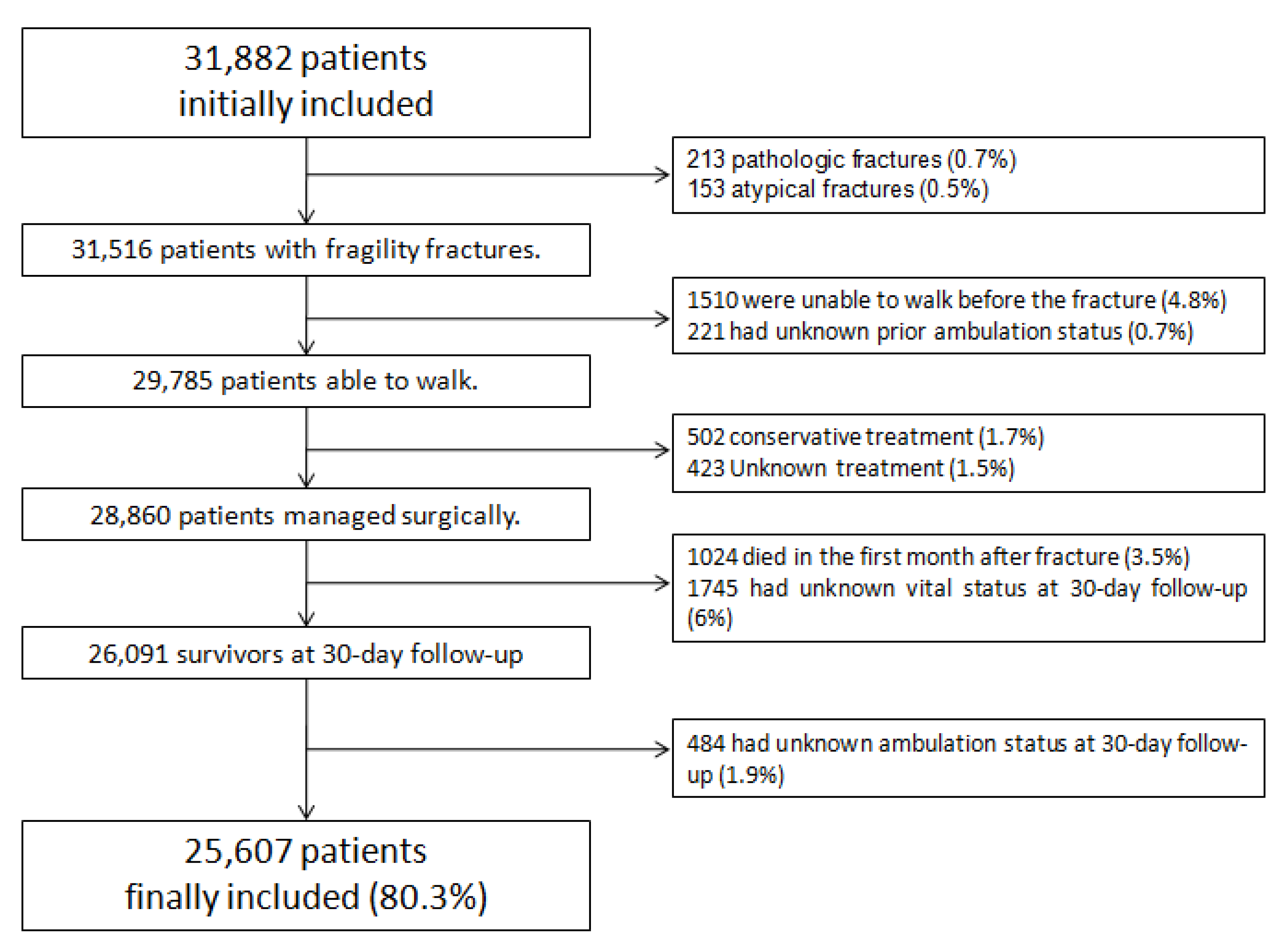
2.1. Study Sample
2.2. Data Collection
2.3. Outcome Definition
2.4. Statistical Analysis
2.4.1. Training Phase
2.4.2. Test Phase
2.4.3. Validation Phase
3. Results
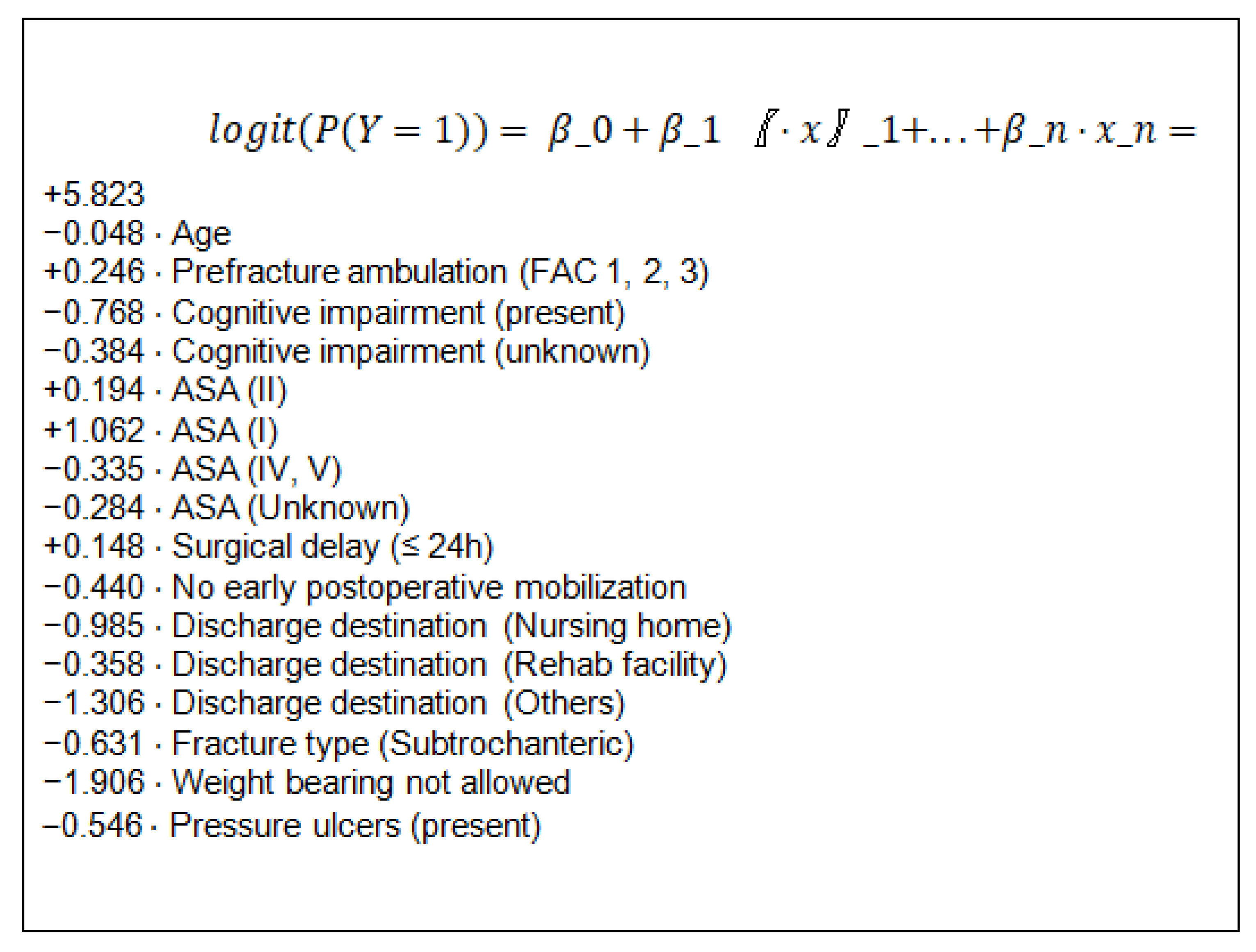
3.1. Training Set; Logistic Regression Model
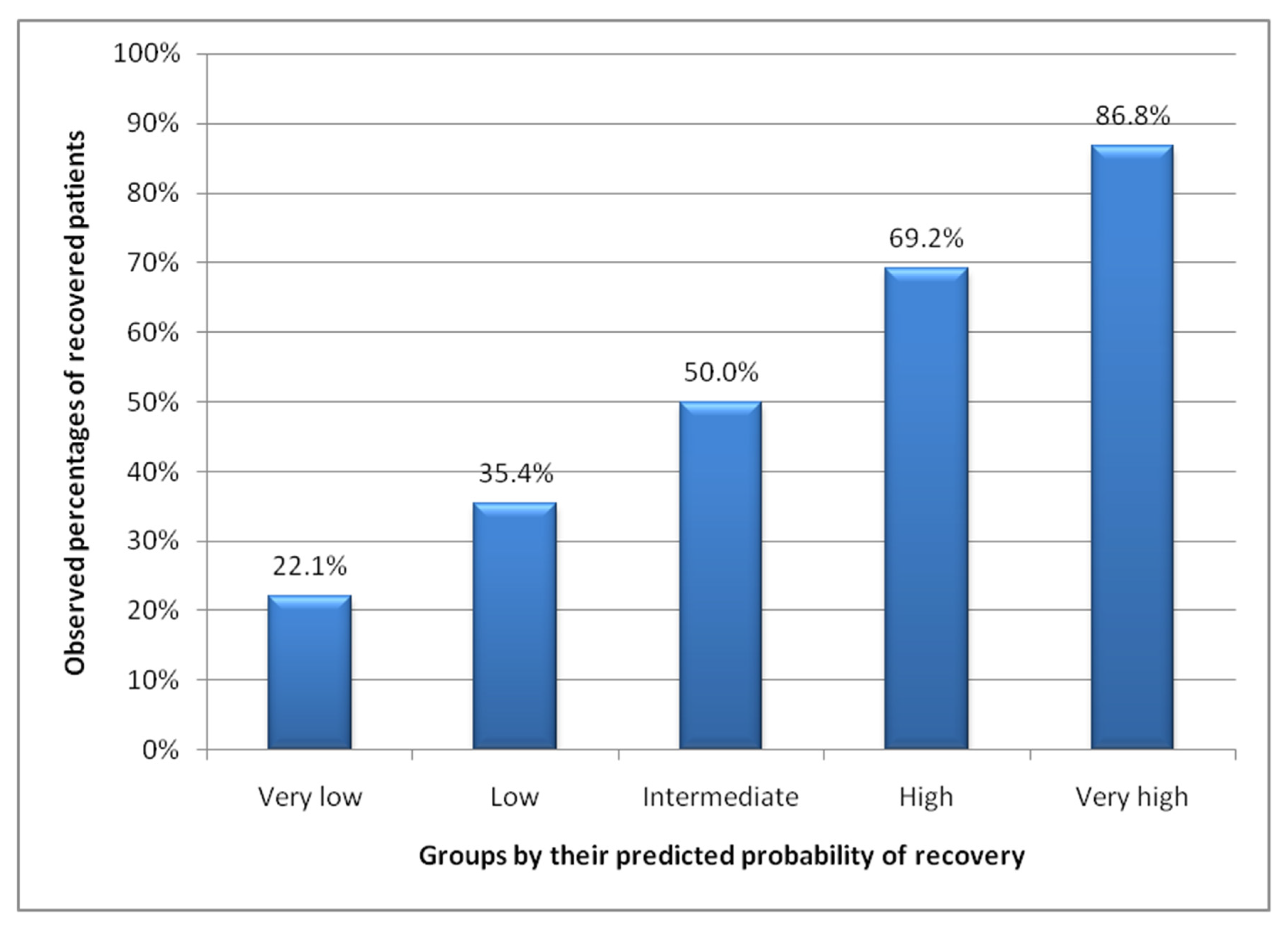
3.2. Performance Measures in Validation
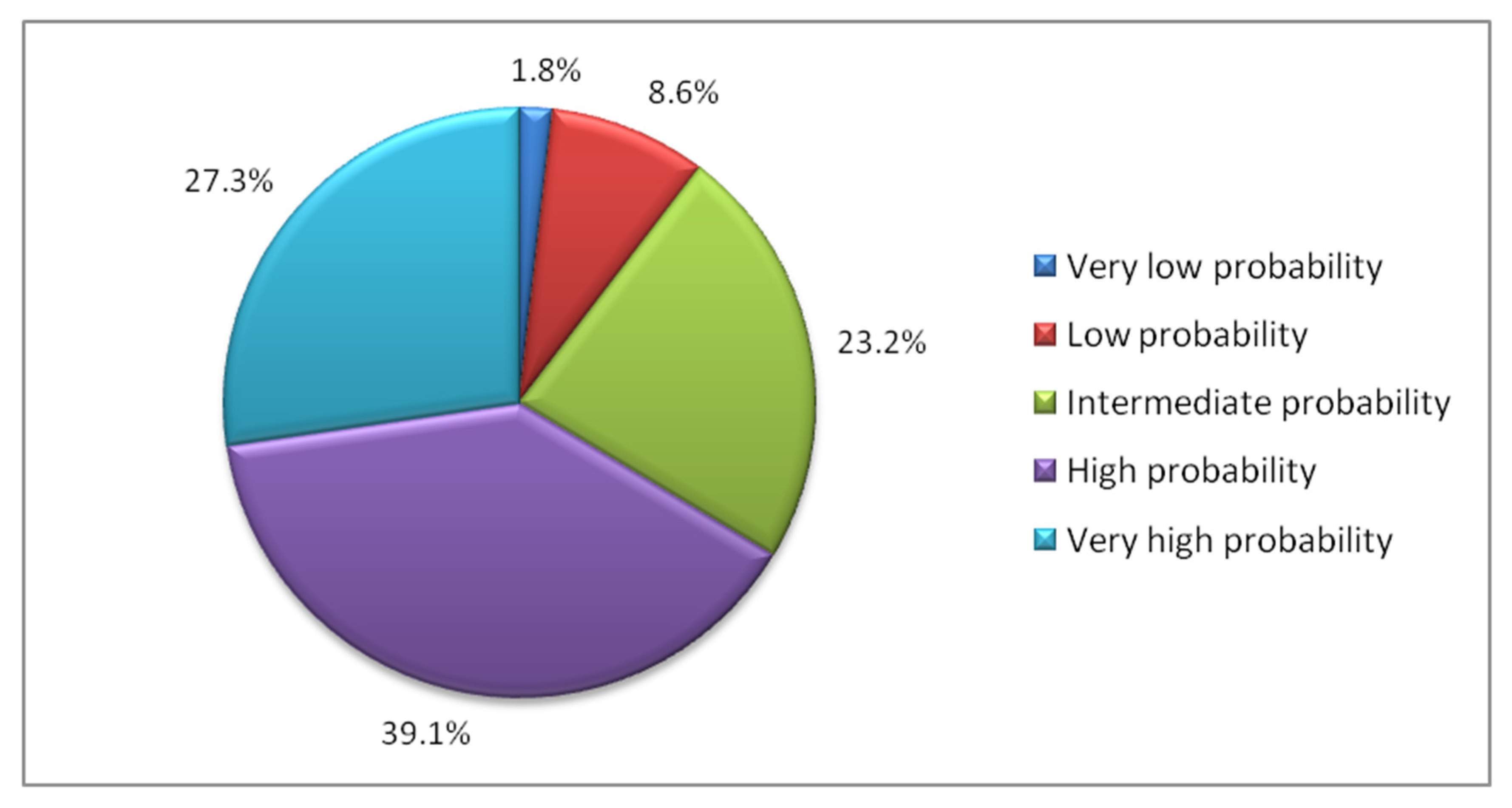
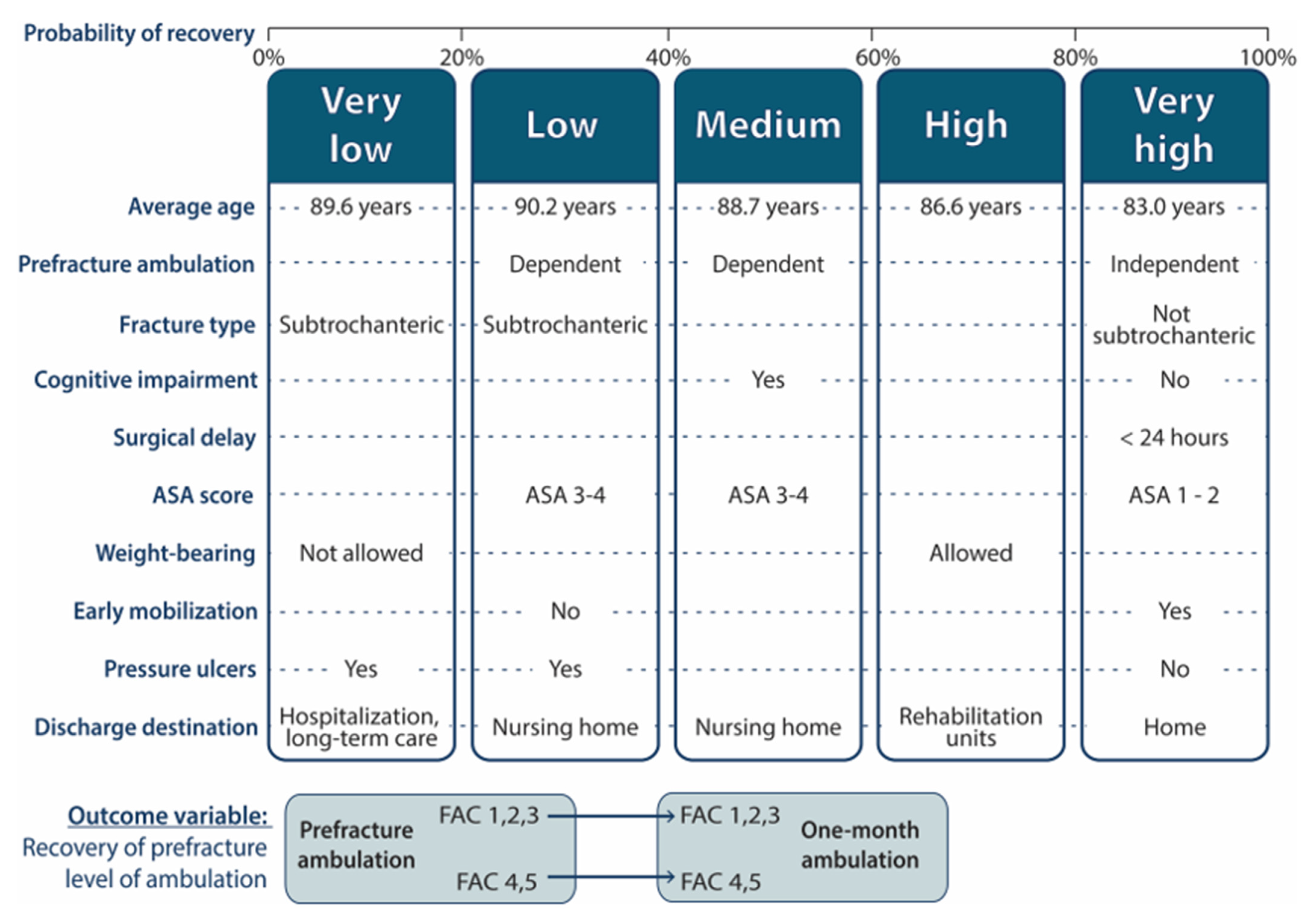
3.3. Groups by Predicted Probability of Recovery
- “Very low” group (less than 0.2 probability). This group represented 1.8% of the sample. With a mean age of 89.6 years, was characterized by patients admitted for subtrochanteric fractures, in whom postoperative weight bearing was not allowed, who develop pressure ulcers and who are discharged to locations other than their home, nursing homes or geriatric rehabilitation units, such as acute hospitalization or long-stay units.
- “Low” group (probability 0.2–0.4). This group represented 8.6% of the sample. Patients discharged to nursing homes, suffering subtrochanteric fractures, developing pressure ulcers, and not mobilized on the first postoperative day, characterized this group, that had an average age of 90.2 years. The scarcity of mild systemic disease (ASA = I–II) also stand out.
- “Medium” group (probability 0.4–0.6). This group represented 23.2% of the sample. With an average age of 88.7 years, was characterized by patients who walked with assistance (FAC 1–3) before the fracture, discharged to nursing homes, and who had cognitive impairment. The scarcity of mild systemic disease (ASA = I–II) was also noteworthy.
- “High” group (probability 0.6–0.8): This group represented 39.1% of the sample. With an average age of 86.6 years, was characterized by the scarcity of patients discharged to nursing homes and by the predominance of patients in whom weight bearing was allowed and who were sent to geriatric rehabilitation units at discharge.
- “Very high” group (probability greater than 0.8): This group represented 27.3% of the sample. The mean age of this group was 83 years. It was characterized by the scarcity of patients dependent for walking at baseline, as well as the scarcity of cognitive impairment, subtrochanteric fractures and pressure ulcers. ASA levels = I and II stand out as well as mobilization on the first postoperative day. There is also a predominance of short operative delay (less than 24 h) and discharge back home.
3.4. Examples of Hypothetical Patients with Different Probabilities of Recovery
- -
- If weight bearing had not been authorized, probability of recovery would drop to 0.343.
- -
- If weight bearing had not been authorized in addition to discharge to a nursing home, the probability of recovery would fall to 0.163.
- -
- Had undergone surgery within 24 h after admission, the probability of recovery would be 0.543.
- -
- Had been mobilized the first day after surgery, the probability of recovery would be 0.614.
- -
- If both situations had occurred, the probability would reach 0.649.
- -
- If weight bearing had not been authorized then the probability of recovery would fall to 0.132.
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kanis, J.A.; on behalf of the IOF Working Group on Epidemiology and Quality of Life; Odén, A.; McCloskey, E.V.; Johansson, H.; Wahl, D.A.; Cooper, C. A systematic review of hip fracture incidence and probability of fracture worldwide. Osteoporos. Int. 2012, 23, 2239–2256. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Nebreda, M.L.; Jiménez, A.B.; Rodríguez, P.; Serra, J.A. Epidemiology of hip fracture in the elderly in Spain. Bone 2008, 42, 278–285. [Google Scholar] [CrossRef] [PubMed]
- Azagra, R.; López-Expósito, F.; Martin-Sánchez, J.C.; Aguyé, A.; Moreno, N.; Cooper, C.; Díez-Pérez, A.; Dennison, E.M. Changing trends in the epidemiology of hip fracture in Spain. Osteoporos. Int. 2014, 25, 1267–1274. [Google Scholar] [CrossRef]
- Haleem, S.; Lutchman, L.; Mayahi, R.; Grice, J.; Parker, M. Mortality following hip fracture: Trends and geographical variations over the last 40 years. Injury 2008, 39, 1157–1163. [Google Scholar] [CrossRef]
- Giversen, I.M. Time trends of mortality after first hip fractures. Osteoporos. Int. 2007, 18, 721–732. [Google Scholar] [CrossRef] [PubMed]
- Pollock, F.H.; Bethea, A.; Samanta, D.; Modak, A.; Maurer, J.P.; Chumbe, J.T. Readmission within 30 days of discharge after hip fracture care. Orthopedics 2015, 38, e7–e13. [Google Scholar] [CrossRef]
- Alarcón, T.A.; González-Montalvo, J.I. Fractura osteoporótica de cadera: Factores predictivos de recuperación funcional a corto y largo plazo. An. Med. Interna 2004, 21, 49–58. [Google Scholar] [CrossRef]
- Liem, I.S.L.; Kammerlander, C.; Suhm, N.; Kates, S.L.; Blauth, M. Literature review of outcome parameters used in studies of geriatric fracture centers. Arch. Orthop. Trauma Surg. 2012, 134, 181–187. [Google Scholar] [CrossRef]
- Liem, I.; Kammerlander, C.; Suhm, N.; Blauth, M.; Roth, T.; Gosch, M.; Hoang-Kim, A.; Mendelson, D.; Zuckerman, J.; Leung, F.; et al. Identifying a standard set of outcome parameters for the evaluation of orthogeriatric co-management for hip fractures. Injury 2013, 44, 1403–1412. [Google Scholar] [CrossRef]
- Eastwood, E.A.; Magaziner, J.; Wang, J.; Silberzweig, S.B.; Hannan, E.L.; Strauss, E.; Siu, A.L. Patients with hip fracture: Subgroups and their outcomes. J. Am. Geriatr. Soc. 2002, 50, 1240–1249. [Google Scholar] [CrossRef]
- Colón-Emeric, C.; Whitson, H.E.; Pieper, C.F.; Sloane, R.; Orwig, D.; Huffman, K.M.; Bettger, J.P.; Parker, D.; Crabtree, D.M.; Gruber-Baldini, A.; et al. Resiliency Groups Following Hip Fracture in Older Adults. J. Am. Geriatr. Soc. 2019, 67, 2519–2527. [Google Scholar] [CrossRef]
- Michel, J.-P.; Hoffmeyer, P.; Klopfenstein, C.; Bruchez, M.; Grab, B.; D’Epinay, C.L. Prognosis of Functional Recovery 1 Year after Hip Fracture: Typical Patient Profiles Through Cluster Analysis. J. Gerontol. Ser. A Boil. Sci. Med. Sci. 2000, 55, M508–M515. [Google Scholar] [CrossRef]
- Ojeda-Thies, C.; Rnfc, O.B.O.T.P.I.T.; Sáez-López, P.; Currie, C.; Tarazona-Santalbina, F.; Alarcón, T.; Muñoz-Pascual, A.; Pareja, T.; Gómez-Campelo, P.; Montero-Fernández, N.; et al. Spanish National Hip Fracture Registry (RNFC): Analysis of its first annual report and international comparison with other established registries. Osteoporos. Int. 2019, 30, 1243–1254. [Google Scholar] [CrossRef] [PubMed]
- Condorhuamán-Alvarado, P.Y.; Pareja-Sierra, T.; Muñoz-Pascual, A.; Sáez-López, P.; Ojeda-Thies, C.; Alarcón-Alarcón, T.; Cassinello-Ogea, M.C.; Pérez-Castrillón, J.L.; Gómez-Campelo, P.; Navarro-Castellanos, L.; et al. First proposal of quality indicators and standards and recommendations to improve the healthcare in the Spanish National Registry of Hip Fracture. Rev. Española Geriatría Gerontol. 2019, 54, 257–264. [Google Scholar] [CrossRef]
- Holden, M.K.; Gill, K.M.; Magliozzi, M.R.; Nathan, J.; Piehl-Baker, L. Clinical Gait Assessment in the Neurologically Impaired. Phys. Ther. 1984, 64, 35–40. [Google Scholar] [CrossRef]
- Pfeiffer, E. A Short Portable Mental Status Questionnaire for the Assessment of Organic Brain Deficit in Elderly Patients. J. Am. Geriatr. Soc. 1975, 23, 433–441. [Google Scholar] [CrossRef]
- De La Iglesiaa, J.M.; Dueñasherrerob, R.; Vilchesa, M.C.O.; Tabernéa, C.A.; Colomerc, C.A.; Luquec, R.L. Adaptación y validación al castellano del cuestionario de Pfeiffer (SPMSQ) para detectar la existencia de deterioro cognitivo en personas mayores e 65 años. Med. Clínica 2001, 117, 129–134. [Google Scholar] [CrossRef]
- Owens, W.D.; Felts, J.A.; Spitznagel, E.L. ASA physical status classifications: A study of consistency of ratings. Anesthe-Siology 1978, 49, 239–243. [Google Scholar] [CrossRef]
- Viosca, E.; Martínez, J.L.; Almagro, P.L.; Gracia, A.; González, C. Proposal and Validation of a New Functional Ambulation Classification Scale for Clinical Use. Arch. Phys. Med. Rehabil. 2005, 86, 1234–1238. [Google Scholar] [CrossRef]
- Efron, B. The Jackknife, the Bootstrap and Other Resampling Plans. Soc. Ind. Appl. Math. 1982. [Google Scholar] [CrossRef]
- James, G.; Witten, D.; Hastie, T.; Tibshirani, R. An Introduction to Statistical Learning: With Applications in R; Springer: New York, NY, USA, 2013; Volume 112, p. 18. [Google Scholar]
- Applied Logistic Regression, 3rd ed.; Wiley: Hoboken, NJ, USA; Available online: https://www.wiley.com/en-us/Applied+Logistic+Regression%2C+3rd+Edition-p-9780470582473 (accessed on 26 December 2020).
- Hannan, E.L.; Magaziner, J.; Wang, J.J.; Eastwood, E.A.; Silberzweig, S.B.; Gilbert, M.; Morrison, R.S.; McLaughlin, M.A.; Orosz, G.M.; Siu, A.L. Mortality and Locomotion 6 Months After Hospitalization for Hip Fracture: Risk factors and risk-adjusted hospital outcomes. JAMA 2001, 285, 2736–2742. [Google Scholar] [CrossRef]
- Kim, S.-M.; Moon, Y.-W.; Lim, S.-J.; Yoon, B.-K.; Min, Y.-K.; Lee, D.-Y.; Park, Y.-S. Prediction of survival, second fracture, and functional recovery following the first hip fracture surgery in elderly patients. Bone 2012, 50, 1343–1350. [Google Scholar] [CrossRef]
- Tang, V.L.; Sudore, R.; Cenzer, I.S.; Boscardin, W.J.; Smith, A.; Ritchie, C.; Wallhagen, M.; Finlayson, E.; Petrillo, L.; Covinsky, K. Rates of Recovery to Pre-Fracture Function in Older Persons with Hip Fracture: An Observational Study. J. Gen. Intern. Med. 2017, 32, 153–158. [Google Scholar] [CrossRef]
- Pajulammi, H.M.; Pihlajamäki, H.K.; Luukkaala, T.H.; Nuotio, M.S. Pre- and perioperative predictors of changes in mobility and living arrangements after hip fracture—A population-based study. Arch. Gerontol. Geriatr. 2015, 61, 182–189. [Google Scholar] [CrossRef]
- Pioli, G.; Lauretani, F.; Pellicciotti, F.; Pignedoli, P.; Bendini, C.; Davoli, M.L.; Martini, E.; Zagatti, A.; Giordano, A.; Nardelli, A.; et al. Modifiable and non-modifiable risk factors affecting walking recovery after hip fracture. Osteoporos. Int. 2016, 27, 2009–2016. [Google Scholar] [CrossRef]
- Ouellet, J.A.; Ouellet, G.M.; Romegialli, A.M.; Hirsch, M.; Berardi, L.; Ramsey, C.M., Jr.; Walke, L.M. Functional Outcomes after Hip Fracture in Independent Community-Dwelling Patients. J. Am. Geriatr. Soc. 2019, 67, 1386–1392. [Google Scholar] [CrossRef]
- Sterling, R.S. Gender and Race/Ethnicity Differences in Hip Fracture Incidence, Morbidity, Mortality, and Function. Clin. Orthop. Relat. Res. 2011, 469, 1913–1918. [Google Scholar] [CrossRef]
- Endo, Y.; Aharonoff, G.B.; Zuckerman, J.D.; Egol, K.A.; Koval, K.J. Gender Differences in Patients With Hip Fracture: A Greater Risk of Morbidity and Mortality in Men. J. Orthop. Trauma 2005, 19, 29–35. [Google Scholar] [CrossRef]
- Woodward, L.M.; Clemson, L.; Moseley, A.M.; Lord, S.R.; Cameron, I.D.; Sherrington, C. Most functional outcomes are similar for men and women after hip fracture: A secondary analysis of the enhancing mobility after hip fracture trial. BMC Geriatr. 2014, 14, 140. [Google Scholar] [CrossRef]
- Kim, J.L.; Jung, J.S.; Kim, S.J. Prediction of Ambulatory Status after Hip Fracture Surgery in Patients Over 60 Years Old. Ann. Rehabil. Med. 2016, 40, 666–674. [Google Scholar] [CrossRef]
- Ariza-Vega, P.; Jiménez-Moleón, J.J.; Kristensen, M.T. Non-Weight-Bearing Status Compromises the Functional Level Up to 1 yr after Hip Fracture Surgery. Am. J. Phys. Med. Rehabil. 2014, 93, 641–648. [Google Scholar] [CrossRef] [PubMed]
- Baer, M.; Neuhaus, V.; Pape, H.C.; Ciritsis, B. Influence of mobilization and weight bearing on in-hospital outcome in geriatric patients with hip fractures. SICOT-J 2019, 5, 4. [Google Scholar] [CrossRef]
- Siu, A.L.; Penrod, J.D.; Boockvar, K.S.; Koval, K.; Strauss, E.; Morrison, R.S. Early Ambulation after Hip Fracture. Arch. Intern. Med. 2006, 166, 766–771. [Google Scholar] [CrossRef]
- Cohn, M.R.; Cong, G.-T.; Nwachukwu, B.U.; Patt, M.L.; Desai, P.; Zambrana, L.; Lane, J.M. Factors Associated With Early Functional Outcome after Hip Fracture Surgery. Geriatr. Orthop. Surg. Rehabil. 2016, 7, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Al-Ani, A.N.; Samuelsson, B.; Tidermark, J.; Norling, Å.; Ekström, W.; Cederholm, T.; Hedström, M. Early Operation on Patients with a Hip Fracture Improved the Ability to Return to Independent Living. J. Bone Jt. Surg. Am. 2008, 90, 1436–1442. [Google Scholar] [CrossRef] [PubMed]
- Magny, E.; Vallet, H.; Cohen-Bittan, J.; Raux, M.; Meziere, A.; Verny, M.; Riou, B.; Khiami, F.; Boddaert, J. Pressure ulcers are associated with 6-month mortality in elderly patients with hip fracture managed in orthogeriatric care pathway. Arch. Osteoporos. 2017, 12, 77. [Google Scholar] [CrossRef]
- Bellelli, G.; Noale, M.; Guerini, F.; Turco, R.; Maggi, S.; Crepaldi, G.; Trabucchi, M. A prognostic model predicting recovery of walking independence of elderly patients after hip-fracture surgery. An experiment in a rehabilitation unit in Northern Italy. Osteoporos. Int. 2012, 23, 2189–2200. [Google Scholar] [CrossRef]
- Zuckerman, J.D.; Koval, K.J.; Aharonoff, G.B.; Hiebert, R.; Skovron, M.L. A Functional Recovery Score for Elderly Hip Fracture Patients: I. Development. J. Orthop. Trauma 2000, 14, 20–25. [Google Scholar] [CrossRef]
- Hirose, J.; Ide, J.; Yakushiji, T.; Abe, Y.; Nishida, K.; Maeda, S.; Anraku, Y.; Usuku, K.; Mizuta, H. Prediction of Postoperative Ambulatory Status 1 Year after Hip Fracture Surgery. Arch. Phys. Med. Rehabil. 2010, 91, 67–72. [Google Scholar] [CrossRef]
- Cancio, J.M.; Vela, E.; Santaeugènia, S.; Clèries, M.; Inzitari, M.; Ruiz, D. Long-term Impact of Hip Fracture on the Use of Healthcare Resources: A Population-Based Study. J. Am. Med Dir. Assoc. 2019, 20, 456–461. [Google Scholar] [CrossRef]
- Hernández, M.J.M.; De Villaumbrosia, C.G.; Murga, E.M.D.F.D.; Alarcón, T.A.; Montero-Fernández, N.; Illán, J.; Bielza, R.; Mora-Fernández, J. Registro de fracturas de cadera multicéntrico de unidades de Ortogeriatría de la Comunidad Autónoma de Madrid. Rev. Española Geriatría Gerontol. 2019, 54, 5–11. [Google Scholar] [CrossRef]
- Muñoz-Pascual, A.; Sáez-López, P.; Jiménez-Mola, S.; Sánchez-Hernández, N.; Alonso-García, N.; Andrés-Sainz, A.I.; Macias-Montero, M.C.; Vázquez-Pedrezuela, C.; Juez, N.P.D.C.; Del Pozo-Tagarro, P.; et al. Ortogeriatría: Primer registro multicéntrico autonómico de fracturas de cadera en Castilla y León (España). Rev. Española Geriatría Gerontol. 2017, 52, 242–248. [Google Scholar] [CrossRef] [PubMed]
- Prieto-Alhambra, D.; Reyes, C.; Sainz, M.S.; González-Macías, J.; Delgado, L.G.; Bouzón, C.A.; Gañan, S.M.; Miedes, D.M.; Vaquero-Cervino, E.; Bardaji, M.F.B.; et al. In-hospital care, complications, and 4-month mortality following a hip or proximal femur fracture: The Spanish registry of osteoporotic femur fractures prospective cohort study. Arch. Osteoporos. 2018, 13, 1–11. [Google Scholar] [CrossRef]
- Caeiro, J.R.; Bartra, A.; Mesa-Ramos, M.; Etxebarría, Í.; Montejo, J.; Carpintero, P.; Sorio, F.; Gatell, S.; Farré, A.; Canals, L.; et al. Burden of First Osteoporotic Hip Fracture in Spain: A Prospective, 12-Month, Observational Study. Calcif. Tissue Int. 2016, 100, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Sáez-López, P.; Ojeda-Thies, C.; Alarcón, T.; Pascual, A.M.; Mora-Fernández, J.; De Villaumbrosia, C.G.; Hernández, M.J.M.; Montero-Fernández, N.; Trujillo, J.M.C.; Pérez, A.D.; et al. Spanish National Hip Fracture Registry (RNFC): First-year results and comparison with other registries and prospective multi-centric studies from Spain. Rev. Esp. Salud. Publica. 2019, 93, 201910072. [Google Scholar]
- Podsiadlo, D.; Richardson, S. The Timed “Up & Go”: A Test of Basic Functional Mobility for Frail Elderly Persons. J. Am. Geriatr. Soc. 1991, 39, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Guralnik, J.M.; Simonsick, E.M.; Ferrucci, L.; Glynn, R.J.; Berkman, L.F.; Blazer, D.G.; Scherr, P.A.; Wallace, R.B. A Short Physical Performance Battery Assessing Lower Extremity Function: Association with Self-Reported Disability and Prediction of Mortality and Nursing Home Admission. J. Gerontol. 1994, 49, M85–M94. [Google Scholar] [CrossRef]
| Functional Ambulation Category | Description |
|---|---|
| 5 | Independent, all surfaces |
| 4 | Independent, level surfaces only |
| 3 | Dependent for supervision |
| 2 | Dependent for physical assistance—level I (light touch) |
| 1 | Dependent for physical assistance—level II (support body weight) |
| 0 | Nonambulator |
| All Patients n = 25,607 | Patients Recovering Ambulation n = 16,839 (65.8) | Patients Not Recovering Ambulation n = 8768 (34.2) | p Value | ||
|---|---|---|---|---|---|
| Age (years) Median ± IQR | 87 (83–90) | 86 (82–90) | 88 (84–91) | <0.001 | |
| Gender (Female) n (%) | 19,753 (77.1) | 13,024 (77.4) | 6729 (76.8) | 0.278 | |
| Place of residence: nursing home n (%) | 5404 (21.1) | 2585 (15.4) | 2819 (32.2) | <0.001 | |
| Prefracture ambulation n (%) | FAC 1 | 1073 (4.2) | 514 (3.1) | 559 (6.4) | <0.001 |
| FAC 2 | 1072 (4.2) | 659 (3.9) | 6413 (4.7) | ||
| FAC 3 | 908 (3.5) | 616 (3.7) | 292 (3.3) | ||
| FAC 4 | 7588 (29.6) | 3662 (21.7) | 3926 (44.8) | ||
| FAC 5 | 14,966 (58.4) | 11,388 (67.6) | 3578 (40.8) | ||
| Cognitive impairment n (%) | 8701 (40.4) | 4589 (32.1) | 4112 (57.0) | <0.001 | |
| ASA n (%) | I | 295 (1.2) | 253 (1.6) | 42 (0.5) | <0.001 |
| II | 7063 (28.6) | 5224 (32.2) | 1839 (21.8) | ||
| III | 15,032 (61) | 9481 (58.4) | 5551 (65.9) | ||
| IV–V | 2266 (9.2) | 1275 (7.9) | 991 (11.8) | ||
| Fracture type n (%) | Intracapsular | 10,005 (39.3) | 7266 (43.4) | 2739 (31.5) | <0.001 |
| Intertrochanteric | 13,496 (53.1) | 8490 (50.8) | 5006 (57.5) | ||
| Subtrochanteric | 1925 (7.6) | 968 (5.8) | 957 (11.0) | ||
| Spinal anesthesia n (%) | 23,948 (93.9) | 15,805 (94.2) | 8143 (93.5) | 0.036 | |
| Peripheral nerve block n (%) | 3534 (16.5) | 2472 (18.0) | 1062 (13.8) | <0.001 | |
| Time to surgery (hours) Median ± IQR | 50.8 (26.1–90) | 49.4 (25.1–89) | 54,5 (28.2–91.6) | <0.001 | |
| Surgery in the first 24 h n (%) | 5788 (22.6) | 3966 (23.6) | 1822 (20.8) | <0.001 | |
| Mobilization on the first postoperative day n (%) | 17,685 (69.1) | 12,354 (73.4) | 5331 (60.9) | <0.001 | |
| Weight bearing not permitted n (%) | 789 (7.9) | 230 (3.6) | 559 (15.8) | <0.001 | |
| Pressure ulcers n (%) | 1233 (4.9) | 592 (3.6) | 641 (7.5) | <0.001 | |
| Clinician in addition to surgeon. n (%) | 24,615 (96.2) | 16,206 (96.3) | 8409 (95.9) | 0.167 | |
| Discharge destination n (%) | Home | 11,345 (44.3) | 8743 (51.9) | 2602 (29.7) | <0.001 |
| Nursing home | 7910 (30.9) | 3928 (23.3) | 3982 (45.4) | ||
| Geriatric rehabilitation unit | 5893 (23.0) | 3985 (23.7) | 1908 (21.8) | ||
| Other | 450 (1.8) | 178 (1.1) | 272 (3.1) | ||
| Length of stay (days) Median ± IQR | 8.9 (6.6–12.1) | 8.7 (6.5–11.8) | 9.2 (6.8–12.9) | <0.001 | |
| Odds Ratio | 95% Confidence Interval | |||
|---|---|---|---|---|
| Lower Margin | Higher Margin | |||
| Age | 0.953 | 0.942 | 0.964 | |
| Prefracture ambulation | Dependent (FAC 1–3) | 1.279 | 1.055 | 1.550 |
| Cognitive impairment | Present | 0.464 | 0.401 | 0.537 |
| Unknown | 0.681 | 0.566 | 0.820 | |
| ASA | I | 2.891 | 1.273 | 6.565 |
| II | 1.214 | 1.043 | 1.413 | |
| IV–V | 0.715 | 0.573 | 0.892 | |
| Unknown | 0.753 | 0.539 | 1.052 | |
| Type of fracture; Subtrochanteric fracture | 0.532 | 0.422 | 0.671 | |
| Surgical delay ≤24 h. | 1.159 | 0.993 | 1.352 | |
| Postoperative mobilization >24 h | 0.644 | 0.562 | 0.737 | |
| Weight bearing Not allowed | 0.149 | 0.098 | 0.224 | |
| Pressure ulcers Present | 0.579 | 0.443 | 0.758 | |
| Discharge destination | Nursing home | 0.373 | 0.321 | 0.434 |
| Geriatric rehabilitation unit | 0.699 | 0.592 | 0.826 | |
| Other | 0.271 | 0.173 | 0.425 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
González de Villaumbrosia, C.; Sáez López, P.; Martín de Diego, I.; Lancho Martín, C.; Cuesta Santa Teresa, M.; Alarcón, T.; Ojeda Thies, C.; Queipo Matas, R.; González-Montalvo, J.I.; on behalf of the Participants in the Spanish National Hip Fracture Registry. Predictive Model of Gait Recovery at One Month after Hip Fracture from a National Cohort of 25,607 Patients: The Hip Fracture Prognosis (HF-Prognosis) Tool. Int. J. Environ. Res. Public Health 2021, 18, 3809. https://doi.org/10.3390/ijerph18073809
González de Villaumbrosia C, Sáez López P, Martín de Diego I, Lancho Martín C, Cuesta Santa Teresa M, Alarcón T, Ojeda Thies C, Queipo Matas R, González-Montalvo JI, on behalf of the Participants in the Spanish National Hip Fracture Registry. Predictive Model of Gait Recovery at One Month after Hip Fracture from a National Cohort of 25,607 Patients: The Hip Fracture Prognosis (HF-Prognosis) Tool. International Journal of Environmental Research and Public Health. 2021; 18(7):3809. https://doi.org/10.3390/ijerph18073809
Chicago/Turabian StyleGonzález de Villaumbrosia, Cristina, Pilar Sáez López, Isaac Martín de Diego, Carmen Lancho Martín, Marina Cuesta Santa Teresa, Teresa Alarcón, Cristina Ojeda Thies, Rocío Queipo Matas, Juan Ignacio González-Montalvo, and on behalf of the Participants in the Spanish National Hip Fracture Registry. 2021. "Predictive Model of Gait Recovery at One Month after Hip Fracture from a National Cohort of 25,607 Patients: The Hip Fracture Prognosis (HF-Prognosis) Tool" International Journal of Environmental Research and Public Health 18, no. 7: 3809. https://doi.org/10.3390/ijerph18073809
APA StyleGonzález de Villaumbrosia, C., Sáez López, P., Martín de Diego, I., Lancho Martín, C., Cuesta Santa Teresa, M., Alarcón, T., Ojeda Thies, C., Queipo Matas, R., González-Montalvo, J. I., & on behalf of the Participants in the Spanish National Hip Fracture Registry. (2021). Predictive Model of Gait Recovery at One Month after Hip Fracture from a National Cohort of 25,607 Patients: The Hip Fracture Prognosis (HF-Prognosis) Tool. International Journal of Environmental Research and Public Health, 18(7), 3809. https://doi.org/10.3390/ijerph18073809






