Primary Care Records of Chronic-Disease Patient Adherence to Treatment
Abstract
1. Introduction
2. Materials and Methods
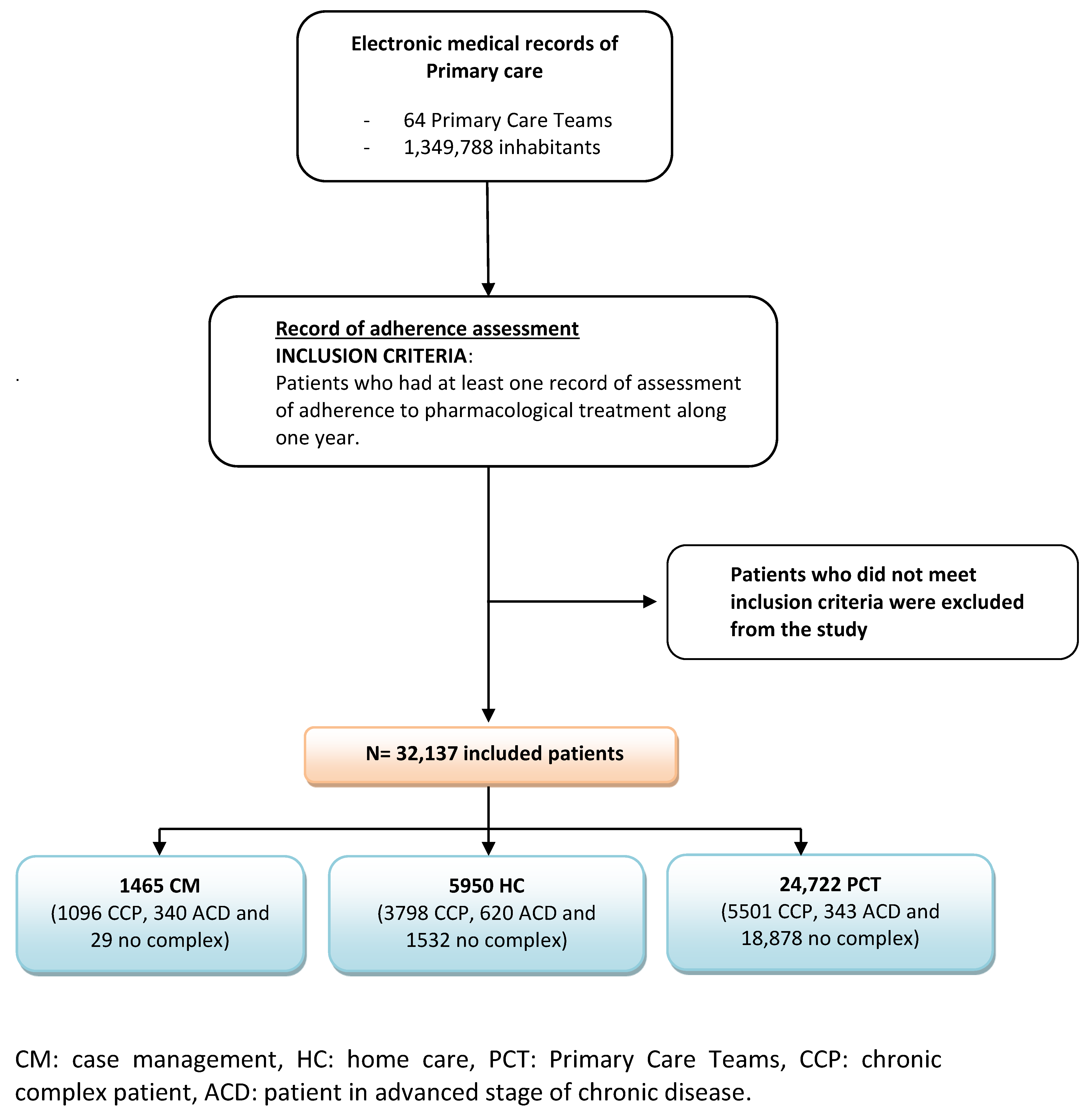
2.1. Study Cohort
2.2. Adherence Measures
2.3. Covariables
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Brown, M.T.; Bussell, J.K. Medication adherence: WHO cares? Mayo Clin. Proc. 2011, 86, 304–314. [Google Scholar] [CrossRef]
- Johnson, M.J.; Williams, M.; Marshall, E.S. Adherent and Nonadherent Medication-Taking in Elderly Hypertensive Patients. Clin. Nurs. Res. 1999, 8, 318–335. [Google Scholar] [CrossRef]
- Iuga, A.O.; McGuire, M.J. Adherence and health care costs. Risk Manag. Healthc. Policy 2014, 7, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Ho, M.; Rumsfeld, J.S.; Masoudi, F.A.; McClure, D.L.; Plomondon, M.E.; Steiner, J.F.; Magid, D.J. Effect of medication nonadherence on hospitalization and mortality among patients with diabetes mellitus. Arch. Intern. Med. 2006, 166, 1836–1841. [Google Scholar] [CrossRef] [PubMed]
- Simpson, S.H.; Eurich, D.T.; Majumdar, S.R.; Padwal, R.S.; Tsuyuki, R.T.; Varney, J.; Johnson, J.A. A meta-analysis of the association between adherence to drug therapy and mortality. BMJ 2006, 333, 15. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Adherence to Long-Term Therapies: Evidence for Action; World Health Organization: Geneva, Switzerland, 2003. [Google Scholar]
- McDonnell, P.J.; Jacobs, M.R. Hospital Admissions Resulting from Preventable Adverse Drug Reactions. Ann. Pharmacother. 2002, 36, 1331–1336. [Google Scholar] [CrossRef] [PubMed]
- al Hamid, A.; Ghaleb, M.; Aljadhey, H.; Aslanpour, Z. A systematic review of hospitalization resulting from medicine-related problems in adult patients. Br. J. Clin. Pharmacol. 2014, 78, 202–217. [Google Scholar] [CrossRef] [PubMed]
- Rand, C.S. Measuring adherence with therapy for chronic diseases: Implications for the treatment of heterozygous familial hypercholesterolemia. Am. J. Cardiol. 1993, 72, D68–D74. [Google Scholar] [CrossRef]
- Vrijens, B.; de Geest, S.; Hughes, D.A.; Przemyslaw, K.; Demonceau, J.; Ruppar, T.; Dobbels, F.; Fargher, E.; Morrison, V.; Lewek, P.; et al. A new taxonomy for describing and defining adherence to medications. Br. J. Clin. Pharmacol. 2012, 73, 691–705. [Google Scholar] [CrossRef]
- Nunes, V.; Neilson, J.; O’Flynn, N.; Calvert, N.; Kuntze, S.; Smithson, H.; Benson, J.; Blair, J.; Bowser, A.; Clyne, W.; et al. Clinical Guidelines and Evidence Review for Medicines Adherence: Involving Patients in Decisions about Prescribed Medicines and Supporting Adherence; National Collaborating Centre for Primary Care and Royal College of General Practitioners: London, UK, 2009. [Google Scholar]
- Osterberg, L.; Blaschke, T. Drug Therapy: Adherence to Medication. N. Engl. J. Med. 2005, 353, 487–497. [Google Scholar] [CrossRef]
- Dilla, T.; Valladares, A.; Lizán, L.; Sacristán, J.A. Treatment adherence and persistence: Causes, consequences and improvement strategies. Atención Primaria 2009, 41, 342–348. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.M.U.; la Caze, A.; Cottrell, N. What are validated self-report adherence scales really measuring?: A systematic review. Br. J. Clin. Pharmacol. 2014, 77, 427–445. [Google Scholar] [CrossRef]
- Garfield, S.; Clifford, S.; Eliasson, L.; Barber, N.; Willson, A. Suitability of measures of self-reported medication adherence for routine clinical use: A systematic review. BMC Med. Res. Methodol. 2011, 11, 149. [Google Scholar] [CrossRef] [PubMed]
- Chamorro, M.Á.R.; García-Jiménez, E.; Amariles, P.; Chamorro, A.R.; Faus, M.J. Revisión de tests de medición del cumplimiento terapéutico utilizados en la práctica clínica. Atención Primaria 2008, 40, 413–417. [Google Scholar] [CrossRef] [PubMed]
- McHorney, C.A.; Gadkari, A.S. Individual patients hold different beliefs to prescription medications to which they persist vs nonpersist and persist vs nonfulfill. Patient Prefer. Adherence 2010, 4, 187–195. [Google Scholar] [CrossRef][Green Version]
- van Dulmen, S.; Sluijs, E.; van Dijk, L.; de Ridder, D.; Heerdink, R.; Bensing, J. Patient adherence to medical treatment: A review of reviews. BMC Health Serv. Res. 2007, 7, 55. [Google Scholar] [CrossRef]
- Helmy, R.; Zullig, L.L.; Dunbar-Jacob, J.; Hughes, D.A.; Vrijens, B.; Wilson, I.B.; de Geest, S. ESPACOMP Medication Adherence Reporting Guidelines (EMERGE): A reactive-Delphi study protocol. BMJ Open 2017, 7. [Google Scholar] [CrossRef]
- Costa, E.; Giardini, A.; Savin, M.; Menditto, E.; Lehane, E.; Laosa, O.; Pecorelli, S.; Monaco, A.; Marengoni, A. Interventional tools to improve medication adherence: Review of literature. Patient Prefer. Adherence 2015, 9, 1303–1314. [Google Scholar] [CrossRef]
- Figueiredo, D.; Teixeira, L.; Poveda, V.; Paúl, C.; Santos-Silva, A.; Costa, E. Predictors of difficulty in medication intake in Europe: A cross-country analysis based on SHARE. Aging Dis. 2016, 7, 246–253. [Google Scholar] [CrossRef]
- Jankowska-Polańska, B.; Chudiak, A.; Uchmanowicz, I.; Dudek, K.; Mazur, G. Selected factors affecting adherence in the pharmacological treatment of arterial hypertension. Patient Prefer. Adherence 2017, 11, 363–371. [Google Scholar] [CrossRef]
- Cabezas, C.L.; Salvador, C.F.; Quadrada, D.C.; Bartés, A.A.; Boré, M.Y.; Perea, N.M.; Peipoch, E.H. Randomized clinical trial of a postdischarge pharmaceutical care program vs. regular follow-up in patients with heart failure. Farm. Hosp. 2006, 30, 328–342. [Google Scholar] [CrossRef]
- Wu, J.-R.; Moser, D.K.; Chung, M.L.; Lennie, T.A. Objectively Measured, but Not Self-Reported, Medication Adherence Independently Predicts Event-Free Survival in Patients with Heart Failure. J. Card. Fail. 2008, 14, 203–210. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Programa de Prevenció i Atenció a la Cronicitat. Departament de Salut, Generalitat de Catalunya. Direcció General de Regulació, Planificació i Recursos Sanitaris. 2018. Available online: https://salutweb.gencat.cat/web/.content/_ambits-actuacio/Linies-dactuacio/Estrategies-de-salut/Cronicitat/Documentacio-cronicitat/arxius/Programa.pdf (accessed on 1 April 2021).
- Projecte de Programa D’atenció al Pacient Crònic Complex Versió 1.1. Agost de 2012. Programa de Prevenció i Atenció a la Cronicitat. Departament de Salut. Generalitat de Catalunya. Barcelona. 2012. Available online: https://salutweb.gencat.cat/web/.content/_ambits-actuacio/Linies-dactuacio/Estrategies-de-salut/Cronicitat/Documentacio-cronicitat/arxius/pcc__juny_2012.pdf (accessed on 1 April 2021).
- Recomanacions Pràctiques per a la Identificació i la Millora de L’atenció de Persones amb Malalties Cròniques Avançades (MACA) amb Necessitat D’atenció pal•Liativa en Territoris i Serveis de Salut i Socials. Versió 1.1. Agost de 2012. Programa de Prevenció i Atenció a la Cronicitat. Departament de Salut. Generalitat de Catalunya. Barcelona. 2012. Available online: https://salutweb.gencat.cat/web/.content/_ambits-actuacio/Linies-dactuacio/Estrategies-de-salut/Cronicitat/Documentacio-cronicitat/arxius/metodologia_maca.pdf (accessed on 1 April 2021).
- Monterde, D.; Vela, E.; Clèries, M. Los grupos de morbilidad ajustados: Nuevo agrupador de morbilidad poblacional de utilidad en el ámbito de la atención primaria. Atención Primaria 2016, 48, 674–682. [Google Scholar] [CrossRef] [PubMed]
- Turner, A.; Hochschild, A.; Burnett, J.; Zulfiqar, A.; Dyer, C.B. High prevalence of medication non-adherence in a sample of community-dwelling older adults with adult protective services-validated self-neglect. Drugs Aging 2012, 29, 741–749. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Sklar, G.E.; Oh, V.M.S.; Li, S.C. Factors affecting therapeutic compliance: A review from the patient’s perspective. Ther. Clin. Risk Manag. 2008, 4, 269–286. [Google Scholar] [CrossRef]
- du Vaure, C.B.; Ravaud, P.; Baron, G.; Barnes, C.; Gilberg, S.; Boutron, I. Potential workload in applying clinical practice guidelines for patients with chronic conditions and multimorbidity: A systematic analysis. BMJ Open 2016, 6, e010119. [Google Scholar] [CrossRef]
- Ridgeway, J.L.; Egginton, J.S.; Tiedje, K.; Linzer, M.; Boehm, D.; Poplau, S.; de Oliveira, D.R.; Odell, L.; Montori, V.M.; Eton, D.T. Factors that lessen the burden of treatment in complex patients with chronic conditions: A qualitative study. Patient Prefer. Adherence 2014, 8, 339–351. [Google Scholar] [CrossRef]
- Alves-Conceiçao, V.; Rocha, K.S.S.; Silva, F.V.N.; Silva, R.O.S.; da Silva, D.T.; de Lyra, D.P., Jr. Medication Regimen Complexity Measured by MRCI: A systematic review to identify Health Outcomes. Ann. Pharmacother. 2018, 52, 1117–1134. [Google Scholar] [CrossRef]
- Marcum, Z.A.; Zheng, Y.; Perera, S.; Strotmeyer, E.; Newman, A.; Simonsick, E.M.; Shorr, R.I.; Bauer, D.C.; Donohue, J.M.; Hanlon, J.T.; et al. Prevalence and correlates of self-reported medication non-adherence among older adults with coronary heart disease, diabetes mellitus, and/or hypertension. Res. Soc. Adm. Pharm. 2013, 9, 817–827. [Google Scholar] [CrossRef]
- Shi, L.; Liu, J.; Koleva, Y.; Fonseca, V.; Kalsekar, A.; Pawaskar, M. Concordance of Adherence Measurement Using Self-Reported Adherence Questionnaires and Medication Monitoring Devices. Pharmacoeconomics 2010, 28, 1097–1107. [Google Scholar] [CrossRef]
- Shi, L.; Liu, J.; Fonseca, V.; Walker, P.; Kalsekar, A.; Pawaskar, M. Correlation between adherence rates measured by MEMS and self-reported questionnaires: A meta-analysis. Health Qual. Life Outcomes 2010, 8, 99. [Google Scholar] [CrossRef] [PubMed]
| Clinical Criteria | Value Indicating Failure to Adhere |
|---|---|
| Medication possession ratio * | Less than 80% |
| Effectiveness of additional medications | Ineffective after adding 1–2 medications to current treatment |
| Clinical interview | Patient confirmation or explanation |
| Medicine cabinet review | High number of full boxes |
| Patient Typology | Total Patients (n) | Patients Included (n) | Adherence Assessment (%) |
|---|---|---|---|
| CCP | 20,131 | 10,395 | 51.54% |
| ACD | 3986 | 1303 | 32.69% |
| Noncomplex | 1323,670 | 20,439 | 1.54% |
| Care Unit | Total (n, Chronic Patients) | n Patients (1) | % Adherence Assessment | % Failure to Adhere |
|---|---|---|---|---|
| HC | 8858 | 5950 | 67.17% + | 31.91% * |
| CCP not CM | 3798 | |||
| ACD not CM | 620 | |||
| Noncomplex | 1532 | |||
| CM | 2101 | 1465 | 69.73% + | 49.3% * |
| CCP | 1096 | |||
| ACD | 340 | |||
| Noncomplex | 29 | |||
| PCT | 1,043,386 | 24,722 | 2.37% + | 17.58% * |
| CCP not CM | 5501 | |||
| ACD not CM | 343 | |||
| Noncomplex | 18,878 |
| Total n of CCPs | No. of Patients with AD Assessment | % | |
|---|---|---|---|
| CM | 1410 | 1096 | 77.73% |
| HC | 5654 | 3798 | 67.17% |
| PCT | 13,067 | 5501 | 42.10% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Massot Mesquida, M.; de la Fuente, J.A.; Andrés Lorca, A.M.; Arteaga Pillasagua, I.; Balboa Blanco, E.; Gracia Vidal, S.; Pablo Reyes, S.; Gómez Iparraguirre, P.; Seda Gombau, G.; Torán-Monserrat, P. Primary Care Records of Chronic-Disease Patient Adherence to Treatment. Int. J. Environ. Res. Public Health 2021, 18, 3710. https://doi.org/10.3390/ijerph18073710
Massot Mesquida M, de la Fuente JA, Andrés Lorca AM, Arteaga Pillasagua I, Balboa Blanco E, Gracia Vidal S, Pablo Reyes S, Gómez Iparraguirre P, Seda Gombau G, Torán-Monserrat P. Primary Care Records of Chronic-Disease Patient Adherence to Treatment. International Journal of Environmental Research and Public Health. 2021; 18(7):3710. https://doi.org/10.3390/ijerph18073710
Chicago/Turabian StyleMassot Mesquida, Mireia, Josep Anton de la Fuente, Anna María Andrés Lorca, Ingrid Arteaga Pillasagua, Edelmiro Balboa Blanco, Sonia Gracia Vidal, Sara Pablo Reyes, Paula Gómez Iparraguirre, Gemma Seda Gombau, and Pere Torán-Monserrat. 2021. "Primary Care Records of Chronic-Disease Patient Adherence to Treatment" International Journal of Environmental Research and Public Health 18, no. 7: 3710. https://doi.org/10.3390/ijerph18073710
APA StyleMassot Mesquida, M., de la Fuente, J. A., Andrés Lorca, A. M., Arteaga Pillasagua, I., Balboa Blanco, E., Gracia Vidal, S., Pablo Reyes, S., Gómez Iparraguirre, P., Seda Gombau, G., & Torán-Monserrat, P. (2021). Primary Care Records of Chronic-Disease Patient Adherence to Treatment. International Journal of Environmental Research and Public Health, 18(7), 3710. https://doi.org/10.3390/ijerph18073710







