Reliability of IMU-Derived Static Balance Parameters in Neurological Diseases
Abstract
1. Introduction
2. Materials and Methods
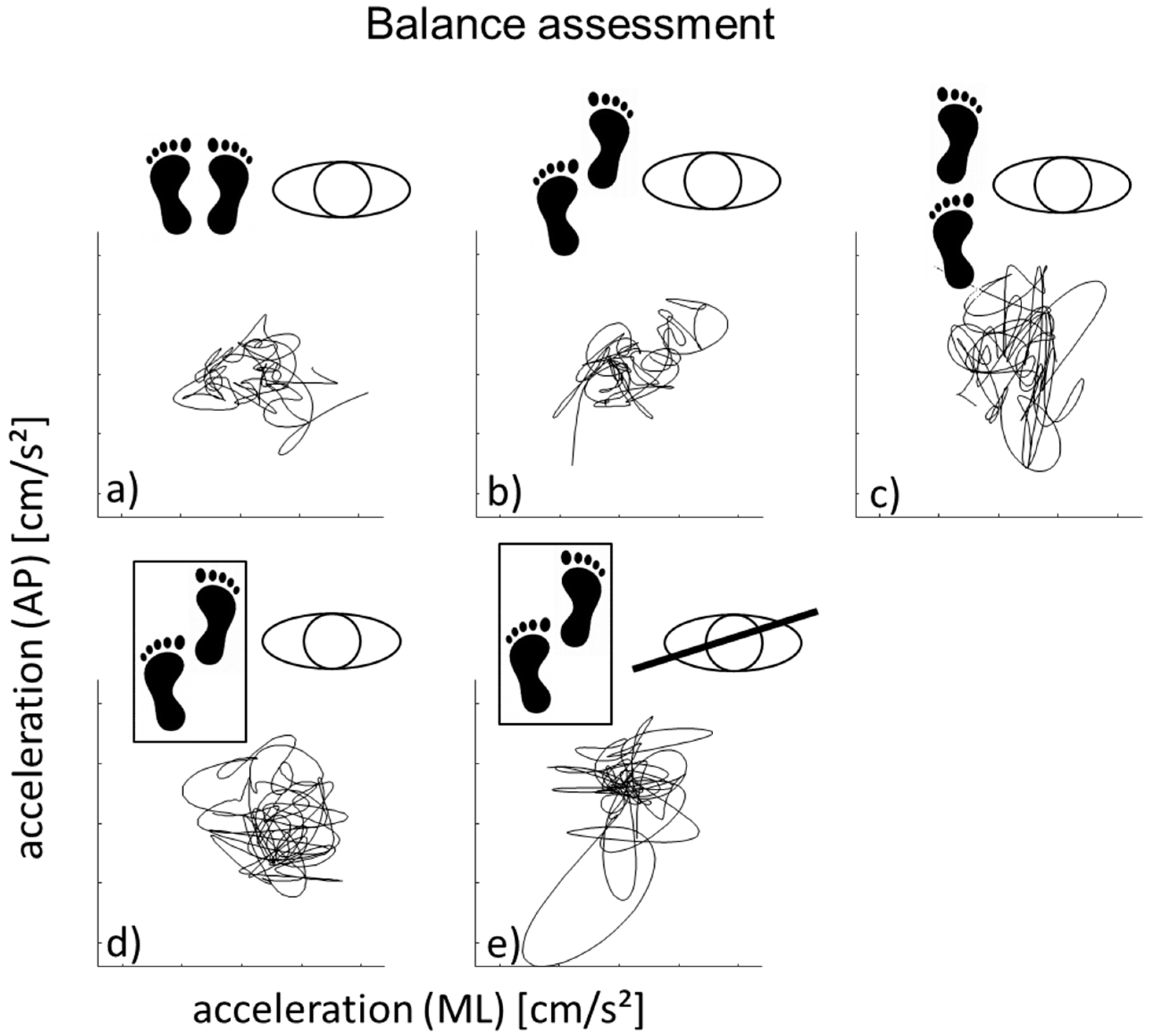
2.1. Quantitative Gait and Balance Assessment
2.2. Sensor Data Processing
2.3. Statistical Analysis
3. Results
3.1. Side-by-Side Stance
3.2. Semi-Tandem Stance on a Hard Surface
3.3. Tandem Stance on a Hard Surface
3.4. Semi Tandem Stance a Soft Surface (Eyes Open)
3.5. Semi Tandem Stance a Soft Surface (Eyes Closed)
3.6. MDC95% Values of All Parameters and Experimental Conditions
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Van Der Kooij, H.; Jacobs, R.; Koopman, B.; Grootenboer, H. A multisensory integration model of human stance control. Biol. Cybern. 1999, 80, 299–308. [Google Scholar] [CrossRef] [PubMed]
- Salzman, B. Gait and balance disorders in older adults. Am. Fam. Physician 2010, 82, 61–68. [Google Scholar]
- Winter, D. Human balance and posture control during standing and walking. Gait Posture 1995, 3, 193–214. [Google Scholar] [CrossRef]
- Trepel, M. Hirnnervenkerne. In Neuroanatomie—Struktur und Funktion, 3rd ed.; Urban & Fischer: Freiburg, Germany, 2004; pp. 117–119, 224–228. [Google Scholar]
- Gang, N.T. Gleichgewicht und stürze—Ursachen und konsequenzen. Dtsch. Med. Wochenschr. 2005, 130, 958–960. [Google Scholar]
- Montemurro, N.; Perrini, P.; Mangini, V.; Galli, M.; Papini, A. The Y-shaped trabecular bone structure in the odontoid process of the axis: A CT scan study in 54 healthy subjects and biomechanical considerations. J. Neurosurg. Spine 2019, 30, 585–592. [Google Scholar] [CrossRef]
- Houlden, H.; Charlton, P.; Singh, D. Neurology and orthopaedics. J. Neurol. Neurosurg. Psychiatry 2006, 78, 224–232. [Google Scholar] [CrossRef]
- Stella, A.B.; Morelli, M.E.; Giudici, F.; Sartori, A.; Manganotti, P.; Di Prampero, P.E. Comfortable walking speed and energy cost of locomotion in patients with multiple sclerosis. Graefe’s Arch. Clin. Exp. Ophthalmol. 2020, 120, 551–566. [Google Scholar]
- Morelli, N.; Morelli, H. Dual task training effects on gait and balance outcomes in multiple sclerosis: A systematic review. Mult. Scler. Relat. Disord. 2021, 49, 102794. [Google Scholar] [CrossRef] [PubMed]
- Agurto, C.; Heisig, S.; Abrami, A.; Ho, B.K.; Caggiano, V. Parkinson’s disease medication state and severity assessment based on coordination during walking. PLoS ONE 2021, 16, e0244842. [Google Scholar] [CrossRef] [PubMed]
- Hansen, C.; Wei, Q.; Shieh, J.-S.; Fourcade, P.; Isableu, B.; Majed, L. Sample Entropy, Univariate, and Multivariate Multi-Scale Entropy in Comparison with Classical Postural Sway Parameters in Young Healthy Adults. Front. Hum. Neurosci. 2017, 11, 206. [Google Scholar] [CrossRef]
- Pinsault, N.; Vuillerme, N. Test-retest reliability of centre of foot pressure measures to assess postural control during unperturbed stance. Med. Eng. Phys. 2009, 31, 276–286. [Google Scholar] [CrossRef]
- Golriz, S.; Hebert, J.J.; Foreman, K.B.; Walker, B.F. The reliability of a portable clinical force plate used for the assessment of static postural control: Repeated measures reliability study. Chiropr. Man. Ther. 2012, 20, 14. [Google Scholar] [CrossRef]
- Del Din, S.; Elshehabi, M.; Galna, B.; Hobert, M.A.; Warmerdam, E.; Suenkel, U.; Brockmann, K.; Metzger, F.; Hansen, C.; Berg, D.; et al. Gait analysis with wearables predicts conversion to Parkinson disease. Ann. Neurol. 2019, 86, 357–367. [Google Scholar] [CrossRef]
- Pantall, A.; Del Din, S.; Rochester, L. Longitudinal changes over thirty-six months in postural control dynamics and cognitive function in people with Parkinson’s disease. Gait Posture 2018, 62, 468–474. [Google Scholar] [CrossRef]
- Bernhard, F.P.; Sartor, J.; Bettecken, K.; Hobert, M.A.; Arnold, C.; Weber, Y.G.; Poli, S.; Margraf, N.G.; Schlenstedt, C.; Hansen, C.; et al. Wearables for gait and balance assessment in the neurological ward - study design and first results of a prospective cross-sectional feasibility study with 384 inpatients. BMC Neurol. 2018, 18, 1–8. [Google Scholar] [CrossRef]
- Geritz, J.; Maetzold, S.; Steffen, M.; Pilotto, A.; Corrà, M.F.; Moscovich, M.; Rizzetti, M.C.; Borroni, B.; Padovani, A.; Alpes, A.; et al. Motor, cognitive and mobility deficits in 1000 geriatric patients: Protocol of a quantitative observational study before and after routine clinical geriatric treatment—The ComOn-study. BMC Geriatr. 2020, 20, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Zijlstra, W.; Hof, A.L. Assessment of spatio-temporal gait parameters from trunk accelerations during human walking. Gait Posture 2003, 18, 1–10. [Google Scholar] [CrossRef]
- Mancini, M.; Horak, F.B.; Zampieri, C.; Carlson-Kuhta, P.; Nutt, J.G.; Chiari, L. Trunk accelerometry reveals postural instability in untreated Parkinson’s disease. Park. Relat. Disord. 2011, 17, 557–562. [Google Scholar] [CrossRef] [PubMed]
- Mancini, M.; Salarian, A.; Carlson-Kuhta, P.; Zampieri, C.; King, L.; Chiari, L.; Horak, F.B. ISway: A sensitive, valid and reliable measure of postural control. J. Neuroeng. Rehabil. 2012, 9, 59. [Google Scholar] [CrossRef] [PubMed]
- Nasreddine, Z. Montreal Cognitive Assessment (MoCA) Administration, Administration and Scoring Instructions. J. Am. Geriatr. Soc. 2005, 53, 695–699. [Google Scholar] [CrossRef]
- Spain, R.; George, R.S.; Salarian, A.; Mancini, M.; Wagner, J.; Horak, F.; Bourdette, D. Body-worn motion sensors detect balance and gait deficits in people with multiple sclerosis who have normal walking speed. Gait Posture 2012, 35, 573–578. [Google Scholar] [CrossRef]
- Lexell, J.E.; Downham, D.Y. How to Assess the Reliability of Measurements in Rehabilitation. Am. J. Phys. Med. Rehabil. 2005, 84, 719–723. [Google Scholar] [CrossRef]
- Shrout, P.E.; Fleiss, J.L. Intraclass correlations: Uses in assessing rater reliability. Psychol. Bull. 1979, 86, 420–428. [Google Scholar] [CrossRef]
- Koo, T.K.; Li, M.Y. A Guideline of Selecting and Reporting Intraclass Correlation Coefficients for Reliability Research. J. Chiropr. Med. 2016, 15, 155–163. [Google Scholar] [CrossRef]
- Schwenk, M.; Gogulla, S.; Englert, S.; Czempik, A.; Hauer, K. Test-retest reliability and minimal detectable change of repeated sit-to-stand analysis using one body fixed sensor in geriatric patients. Physiol. Meas. 2012, 33, 1931–1946. [Google Scholar] [CrossRef] [PubMed]
- Hollman, J.H.; Beckman, B.A.; Brandt, R.A.; Merriwether, E.N.; Williams, R.T.; Nordrum, J.T. Minimum Detectable Change in Gait Velocity during Acute Rehabilitation following Hip Fracture. J. Geriatr. Phys. Ther. 2008, 31, 53–56. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, A.; Schepens, S. Measurement error and minimum detectable change in 4-meter gait speed in older adults. Aging Clin. Exp. Res. 2011, 23, 406–412. [Google Scholar] [CrossRef]
- Haley, S.M.; A Fragala-Pinkham, M. Interpreting Change Scores of Tests and Measures Used in Physical Therapy. Phys. Ther. 2006, 86, 735–743. [Google Scholar] [CrossRef]
- Goldberg, A.; Casby, A.; Wasielewski, M. Minimum detectable change for single-leg-stance-time in older adults. Gait Posture 2011, 33, 737–739. [Google Scholar] [CrossRef]
- Hiengkaew, V.; Jitaree, K.; Chaiyawat, P. Minimal Detectable Changes of the Berg Balance Scale, Fugl-Meyer Assessment Scale, Timed “Up & Go” Test, Gait Speeds, and 2-Minute Walk Test in Individuals With Chronic Stroke With Different Degrees of Ankle Plantarflexor Tone. Arch. Phys. Med. Rehabil. 2012, 93, 1201–1208. [Google Scholar] [PubMed]
- Steffen, T.; Seney, M. Test-Retest Reliability and Minimal Detectable Change on Balance and Ambulation Tests, the 36-Item Short-Form Health Survey, and the Unified Parkinson Disease Rating Scale in People With Parkinsonism. Phys. Ther. 2008, 88, 733–746. [Google Scholar] [CrossRef] [PubMed]
- Najafi, B.; Horn, D.; Marclay, S.; Crews, R.T.; Wu, S.; Wrobel, J.S. Assessing Postural Control and Postural Control Strategy in Diabetes Patients Using Innovative and Wearable Technology. J. Diabetes Sci. Technol. 2010, 4, 780–791. [Google Scholar] [CrossRef] [PubMed]
- Nashner, L.M.; Mccollum, G. The organization of human postural movements: A formal basis and experimental synthesis. Behav. Brain Sci. 1985, 8, 135–150. [Google Scholar] [CrossRef]
- PGatev, P.; Tankov, N.; Gantchev, G.; Krizkova, M. Postural adjustments upon arm movement during sinusoidal induced body oscillations. Acta Physiol. Pharmacol. Bulg. 1991, 17, 3–6. [Google Scholar]
- Kuo, A. An optimal control model for analyzing human postural balance. IEEE Trans. Biomed. Eng. 1995, 42, 87–101. [Google Scholar] [CrossRef]
- Runge, C.; Shupert, C.; Horak, F.; Zajac, F. Ankle and hip postural strategies defined by joint torques. Gait Posture 1999, 10, 161–170. [Google Scholar] [CrossRef]
- Horak, F.B.; Nashner, L.M. Central programming of postural movements: Adaptation to altered support-surface configurations. J. Neurophysiol. 1986, 55, 1369–1381. [Google Scholar] [CrossRef]
- Beyea, J.; McGibbon, C.A.; Sexton, A.; Noble, J.; O’Connell, C. Convergent validity of a wearable sensor system for measuring sub-task performance during the timed up-and-go test. Sensors 2017, 17, 934. [Google Scholar] [CrossRef] [PubMed]
- Washabaugh, E.P.; Kalyanaraman, T.; Adamczyk, P.G.; Claflin, E.S.; Krishnan, C. Validity and repeatability of inertial measurement units for measuring gait parameters. Gait Posture 2017, 55, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Donath, L.; Faude, O.; Lichtenstein, E.; Pagenstert, G.; Nüesch, C.; Mündermann, A.; Muendermann, A. Mobile inertial sensor based gait analysis: Validity and reliability of spatiotemporal gait characteristics in healthy seniors. Gait Posture 2016, 49, 371–374. [Google Scholar] [CrossRef]
- Pham, M.H.; Warmerdam, E.; Elshehabi, M.; Schlenstedt, C.; Bergeest, L.-M.; Heller, M.; Haertner, L.; Ferreira, J.J.; Berg, D.; Schmidt, G.; et al. Validation of a Lower Back “Wearable”-Based Sit-to-Stand and Stand-to-Sit Algorithm for Patients With Parkinson’s Disease and Older Adults in a Home-Like Environment. Front. Neurol. 2018, 9, 652. [Google Scholar] [CrossRef]
- Horak, F.B.; Mancini, M. Objective biomarkers of balance and gait for Parkinson’s disease using body-worn sensors. Mov. Disord. 2013, 28, 1544–1551. [Google Scholar] [CrossRef] [PubMed]
- Quinn, N.P. Classification of fluctuations in patients with Parkinson’s disease. Neurology 1998, 51, S25–S29. [Google Scholar] [CrossRef]
- Mehta, S.; Bastero-Caballero, R.F.; Sun, Y.; Zhu, R.; Murphy, D.K.; Hardas, B.; Koch, G. Performance of intraclass correlation coefficient (ICC) as a reliability index under various distributions in scale reliability studies. Stat. Med. 2018, 37, 2734–2752. [Google Scholar] [CrossRef] [PubMed]
- Weir, J.P. Quantifying test-retest reliability using the intraclass correlation coefficient and the SEM. J. Strength Cond. Res. 2005, 19, 231–240. [Google Scholar] [PubMed]
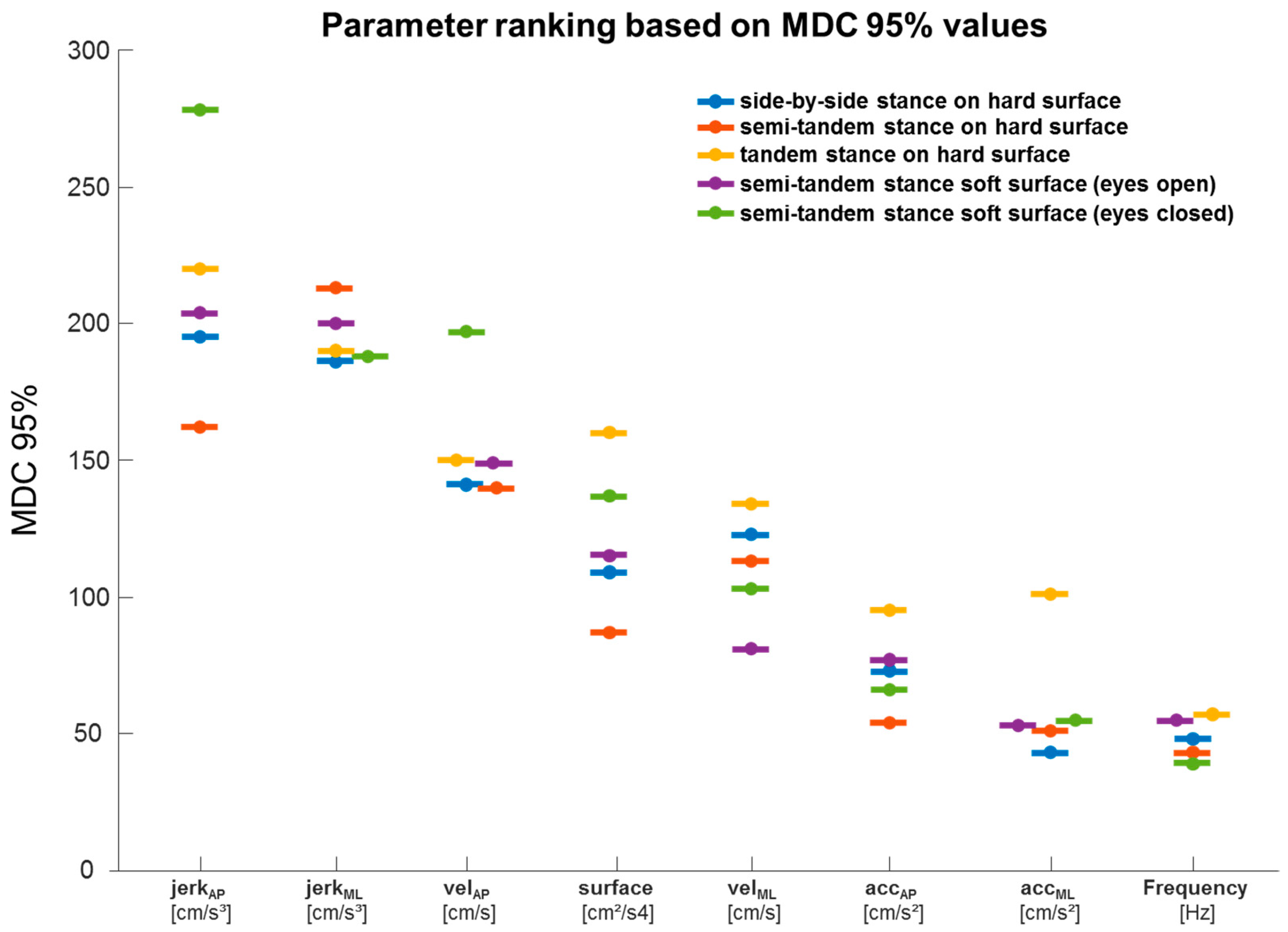
| Parameter | Meant1 | SDt1 | Meant2 | SDt2 | pt1-T2 | ICC | MDC95% |
|---|---|---|---|---|---|---|---|
| SURFACE (CM2/S4) | 20.9 | 9.8 | 20.1 | 9.1 | 0.657 | 0.26 | 109 |
| VELAP (CM/S) | 2.62 | 1.51 | 2.34 | 1.62 | 0.336 | 0.35 | 141 |
| VELML (CM/S) | 1.02 | 0.45 | 0.88 | 0.43 | 0.160 | 0.11 | 123 |
| ACCAP (CM/S2) | 1.24 | 0.43 | 1.19 | 0.37 | 0.469 | 0.36 | 73 |
| ACCML (CM/S2) | 0.87 | 0.16 | 0.84 | 0.14 | 0.413 | 0.21 | 43 |
| JERKAP (CM/S3) | 1072 | 832 | 1110 | 765 | 0.827 | 0.06 | 195 |
| JERKML (CM/S3) | 944 | 765 | 987 | 534 | 0.777 | 0.02 | 186 |
| FREQUENCY (HZ) | 1.57 | 0.34 | 1.55 | 0.30 | 0.735 | 0.29 | 48 |
| Parameter | Meant1 | SDt1 | Meant2 | SDt2 | pt1-T2 | ICC | MDC95% |
|---|---|---|---|---|---|---|---|
| SURFACE (CM2/S4) | 23.5 | 10.8 | 22.4 | 9.5 | 0.538 | 0.5 | 87 |
| VELAP (CM/S) | 2.29 | 1.30 | 2.27 | 1.42 | 0.950 | 0.28 | 140 |
| VELML (CM/S) | 1.26 | 0.64 | 1.27 | 0.57 | 0.946 | 0.27 | 113 |
| ACCAP (CM/S) | 1.19 | 0.35 | 1.19 | 0.33 | 0.937 | 0.52 | 54 |
| ACCML (CM/S2) | 1.09 | 0.32 | 1.02 | 0.19 | 0.122 | 0.45 | 51 |
| JERKAP (CM/S3) | 1067 | 586 | 974 | 641 | 0.507 | 0.06 | 162 |
| JERKML (CM/S3) | 1057 | 833 | 976 | 711 | 0.660 | 0.03 | 213 |
| FREQUENCY (HZ) | 1.70 | 0.32 | 1.62 | 0.32 | 0.151 | 0.34 | 43 |
| Parameter | Meant1 | SDt1 | Meant2 | SDt2 | pt1-T2 | ICC | MDC95% |
|---|---|---|---|---|---|---|---|
| SURFACE (CM2/S4) | 43.2 | 25.6 | 46.4 | 34.5 | 0.736 | 0.25 | 160 |
| VELAP (CM/S) | 3.62 | 2.00 | 3.22 | 2.35 | 0.500 | 0.27 | 150 |
| VELML (CM/S) | 1.97 | 0.99 | 1.71 | 0.96 | 0.377 | 0.16 | 134 |
| ACCAP (CM/S2) | 1.48 | 0.60 | 1.69 | 0.88 | 0.238 | 0.48 | 95 |
| ACCML (CM/S2) | 1.83 | 0.71 | 1.65 | 0.78 | 0.397 | 0.26 | 101 |
| JERKAP (CM/S3) | 1632 | 1211 | 964 | 889 | 0.044 | 0.13 | 220 |
| JERKML (CM/S3) | 1265 | 785 | 1230 | 953 | 0.901 | 0.01 | 190 |
| FREQUENCY (HZ) | 1.72 | 0.46 | 1.69 | 0.42 | 0.812 | 0.35 | 57 |
| Parameter | Meant1 | SDt1 | Meant2 | SDt2 | pt1-T2 | ICC | MDC95% |
|---|---|---|---|---|---|---|---|
| SURFACE (CM2/S4) | 49.3 | 35.3 | 49.2 | 35.5 | 0.992 | 0.66 | 115 |
| VELAP (CM/S) | 8.44 | 6.06 | 9.05 | 7.71 | 0.703 | 0.53 | 149 |
| VELML (CM/S) | 3.23 | 1.63 | 2.88 | 1.41 | 0.296 | 0.65 | 81 |
| ACCAP (CM/S2) | 1.76 | 0.79 | 1.82 | 0.72 | 0.704 | 0.55 | 77 |
| ACCML (CM/S2) | 1.41 | 0.51 | 1.36 | 0.56 | 0.564 | 0.75 | 53 |
| JERKAP (CM/S3) | 1339 | 1035 | 1363 | 1009 | 0.941 | 0.03 | 204 |
| JERKML (CM/S3) | 1207 | 1018 | 1119 | 739 | 0.753 | 0.09 | 200 |
| FREQUENCY (HZ) | 1.57 | 0.48 | 1.58 | 0.41 | 0.925 | 0.48 | 55 |
| Parameter | Meant1 | SDt1 | Meant2 | SDt2 | pt1-T2 | ICC | MDC95% |
|---|---|---|---|---|---|---|---|
| SURFACE (CM2/S4) | 105.3 | 74.9 | 71.9 | 43.0 | 0.125 | 0.5 | 137 |
| VELAP (CM/S) | 9.67 | 7.06 | 8.56 | 6.75 | 0.719 | 0.08 | 197 |
| VELML (CM/S) | 6.14 | 3.85 | 4.75 | 2.19 | 0.139 | 0.59 | 103 |
| ACCAP (CM/S2) | 2.55 | 1.00 | 2.01 | 0.62 | 0.025 | 0.6 | 66 |
| ACCML (CM/S2) | 2.14 | 0.71 | 1.93 | 0.76 | 0.302 | 0.69 | 55 |
| JERKAP (CM/S3) | 1067 | 897 | 1055 | 942 | 0.98 | −0.41 | 278 |
| JERKML (CM/S3) | 1171 | 573 | 1025 | 834 | 0.678 | −0.13 | 188 |
| FREQUENCY (HZ) | 1.58 | 0.30 | 1.69 | 0.39 | 0.364 | 0.54 | 39 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hansen, C.; Beckbauer, M.; Romijnders, R.; Warmerdam, E.; Welzel, J.; Geritz, J.; Emmert, K.; Maetzler, W. Reliability of IMU-Derived Static Balance Parameters in Neurological Diseases. Int. J. Environ. Res. Public Health 2021, 18, 3644. https://doi.org/10.3390/ijerph18073644
Hansen C, Beckbauer M, Romijnders R, Warmerdam E, Welzel J, Geritz J, Emmert K, Maetzler W. Reliability of IMU-Derived Static Balance Parameters in Neurological Diseases. International Journal of Environmental Research and Public Health. 2021; 18(7):3644. https://doi.org/10.3390/ijerph18073644
Chicago/Turabian StyleHansen, Clint, Maximilian Beckbauer, Robbin Romijnders, Elke Warmerdam, Julius Welzel, Johanna Geritz, Kirsten Emmert, and Walter Maetzler. 2021. "Reliability of IMU-Derived Static Balance Parameters in Neurological Diseases" International Journal of Environmental Research and Public Health 18, no. 7: 3644. https://doi.org/10.3390/ijerph18073644
APA StyleHansen, C., Beckbauer, M., Romijnders, R., Warmerdam, E., Welzel, J., Geritz, J., Emmert, K., & Maetzler, W. (2021). Reliability of IMU-Derived Static Balance Parameters in Neurological Diseases. International Journal of Environmental Research and Public Health, 18(7), 3644. https://doi.org/10.3390/ijerph18073644







