Early Childhood Fat Tissue Changes—Adipocyte Morphometry, Collagen Deposition, and Expression of CD163+ Cells in Subcutaneous and Visceral Adipose Tissue of Male Children
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Tissue Collection
2.2. Anthropometric Measurements
2.3. Blood Samples and Biochemistry
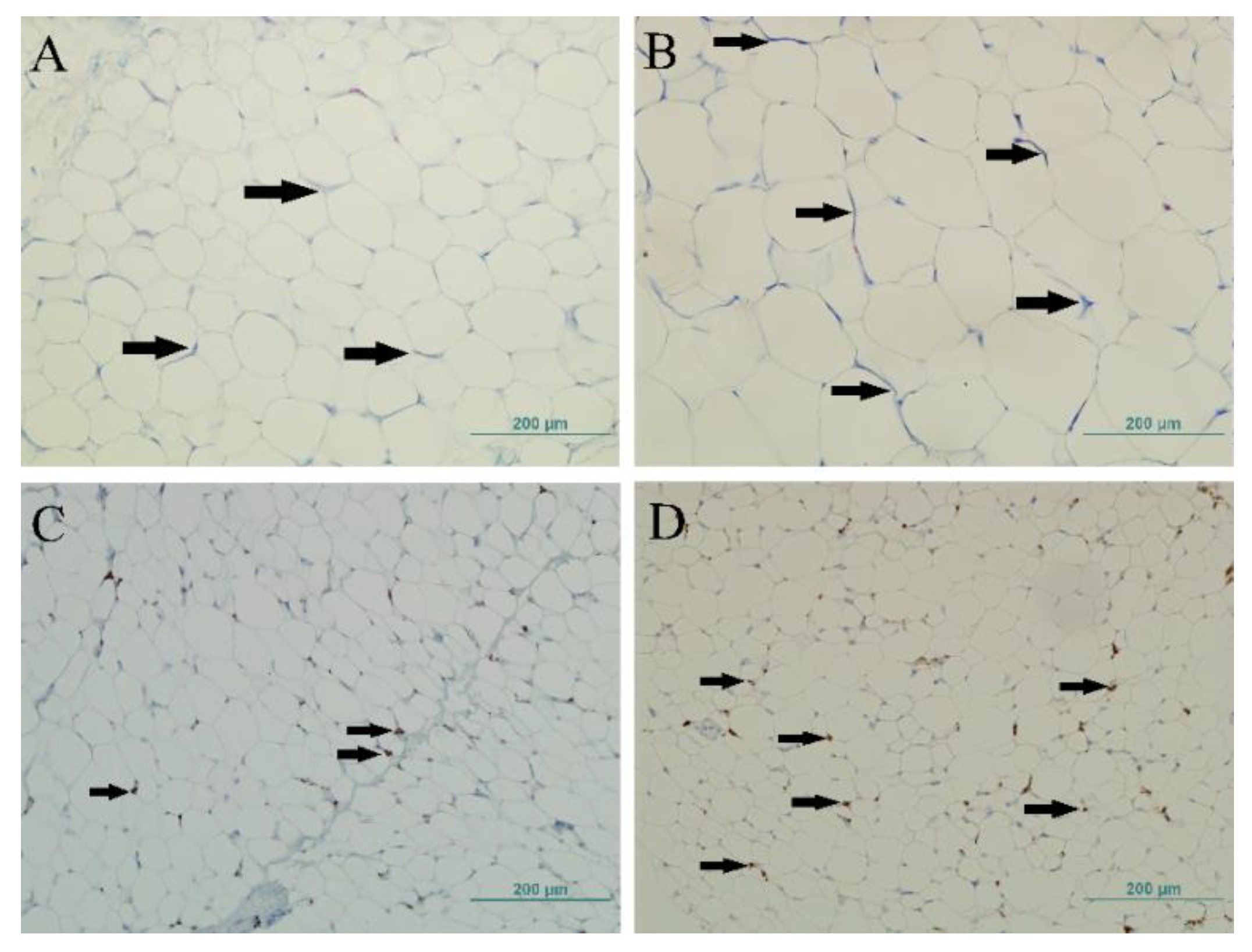
2.4. Adipose Tissue Morphology
2.5. Quantification of Collagen Content
2.6. Immunohistochemistry
2.7. Statistical Analysis
3. Results
3.1. Anthropometric Data
3.2. Biochemical Analysis
3.3. Adipocyte Morphology
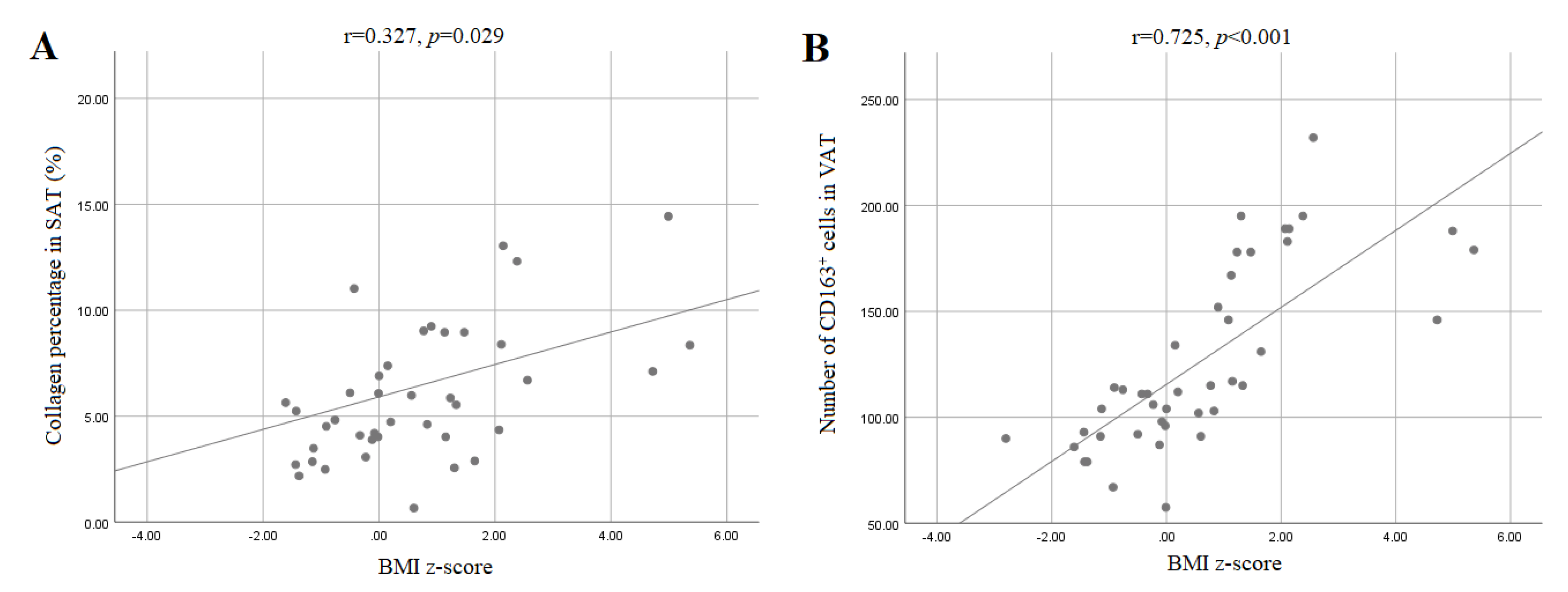
3.4. Collagen Content in Adipose Tissue of Children
3.5. CD163+ Cells and CLS in the Adipose Tissue of Children
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Goyal, A.; Nimmakayala, K.R.; Zonszein, J. Is there a paradox in obesity? Cardiol. Rev. 2014, 22, 163. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Santibañez, G.; Nien-Kai Lumeng, C. Macrophages and the Regulation of Adipose Tissue Remodeling. Annu. Rev. Nutr. 2014, 34, 57–76. [Google Scholar] [CrossRef] [PubMed]
- Chun, T.-H. Peri-adipocyte ECM remodeling in obesity and adipose tissue fibrosis. Adipocyte 2012, 1, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Sun, K.; Tordjman, J.; Clément, K.; Scherer, P.E. Fibrosis and adipose tissue dysfunction. Cell Metab. 2013, 18, 470–477. [Google Scholar] [CrossRef]
- Han, J.; Lee, J.E.; Jin, J.; Lim, J.S.; Oh, N.; Kim, K.; Chang, S.I.; Shibuya, M.; Kim, H.; Koh, G.Y. The spatiotemporal development of adipose tissue. Development 2011, 138, 5027–5037. [Google Scholar] [CrossRef] [PubMed]
- Hammarstedt, A.; Graham, T.E.; Kahn, B.B. Adipose tissue dysregulation and reduced insulin sensitivity in non-obese individuals with enlarged abdominal adipose cells. Diabetol. Metab. Syndr. 2012, 4, 1–9. [Google Scholar] [CrossRef]
- Isakson, P.; Hammarstedt, A.; Gustafson, B.; Smith, U. Impaired preadipocyte differentiation in human abdominal obesity: Role of Wnt, tumor necrosis factor-α, and inflammation. Diabetes 2009, 58, 1550–1557. [Google Scholar] [CrossRef]
- Divoux, A.; Tordjman, J.; Lacasa, D.; Veyrie, N.; Hugol, D.; Aissat, A.; Basdevant, A.; Guerre-Millo, M.; Poitou, C.; Zucker, J.D.; et al. Fibrosis in human adipose tissue: Composition, distribution, and link with lipid metabolism and fat mass loss. Diabetes 2010, 59, 2817–2825. [Google Scholar] [CrossRef]
- Ricard-Blum, S. The collagen family. Cold Spring Harb. Perspect. Biol. 2011, 3, a004978. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.; Chun, T.H.; Kang, L. Adipose extracellular matrix remodelling in obesity and insulin resistance. Biochem. Pharmacol. 2016, 119, 8–16. [Google Scholar] [CrossRef]
- Mariman, E.C.M.; Wang, P. Adipocyte extracellular matrix composition, dynamics and role in obesity. Cell. Mol. Life Sci. 2010, 67, 1277–1292. [Google Scholar] [CrossRef]
- Greenberg, A.S.; Obin, M.S. Obesity and the role of adipose tissue in inflammation and metabolism. Am. J. Clin. Nutr. 2006, 83, 461S–465S. [Google Scholar] [CrossRef]
- Gordon, S.; Taylor, P.R. Monocyte and macrophage heterogeneity. Nat. Rev. Immunol. 2005, 5, 953–964. [Google Scholar] [CrossRef]
- Lumeng, C.N.; Bodzin, J.L.; Saltiel, A.R. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J. Clin. Investig. 2007, 117, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, M.T.A.; Favelyukis, S.; Nguyen, A.K.; Reichart, D.; Scott, P.A.; Jenn, A.; Liu-Bryan, R.; Glass, C.K.; Neels, J.G.; Olefsky, J.M. A subpopulation of macrophages infiltrates hypertrophic adipose tissue and is activated by free fatty acids via toll-like receptors 2 and 4 and JNK-dependent pathways. J. Biol. Chem. 2007, 282, 35279–35292. [Google Scholar] [CrossRef] [PubMed]
- Iyengar, N.M.; Morris, P.G.; Zhou, X.K.; Gucalp, A.; Giri, D.; Harbus, M.D.; Falcone, D.J.; Krasne, M.D.; Vahdat, L.T.; Subbaramaiah, K.; et al. Menopause is a Determinant of Breast Adipose Inflammation HHS Public Access. Cancer Prev. Res. 2015, 8, 349–358. [Google Scholar] [CrossRef] [PubMed]
- Iyengar, N.M.; Ghossein, R.A.; Morris, L.G.; Zhou, X.K.; Kochhar, A.; Morris, P.G.; Pfister, D.G.; Patel, S.G.; Boyle, J.O.; Hudis, C.A.; et al. White adipose tissue inflammation and cancer-specific survival in patients with squamous cell carcinoma of the oral tongue. Cancer 2016, 122, 3794–3802. [Google Scholar] [CrossRef]
- Sbarbati, A.; Osculati, F.; Silvagni, D.; Benati, D.; Galiè, M.; Camoglio, F.S.; Rigotti, G.; Maffeis, C. Obesity and inflammation: Evidence for an elementary lesion. Pediatrics 2006, 117, 220–223. [Google Scholar] [CrossRef]
- Tam, C.S.; Tordjman, J.; Divoux, A.; Baur, L.A.; Clément, K. Adipose tissue remodeling in children: The link between collagen deposition and age-related adipocyte growth. J. Clin. Endocrinol. Metab. 2012, 97, 1320–1327. [Google Scholar] [CrossRef]
- WHO|What Is Overweight and Obesity? Available online: https://www.who.int/dietphysicalactivity/childhood_what/en/ (accessed on 30 August 2020).
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]
- Galarraga, M.; Campión, J.; Munõz-Barrutia, A.; Boqué, N.; Moreno, H.; Martínez, J.A.; Milagro, F.; Ortiz-de-Solórzano, C. Adiposoft: Automated software for the analysis of white adipose tissue cellularity in histological sections. J. Lipid Res. 2012, 53, 2791–2796. [Google Scholar] [CrossRef]
- Chen, Y.; Yu, Q.; Xu, C.B. A convenient method for quantifying collagen fibers in atherosclerotic lesions by imagej software. Int. J. Clin. Exp. Med. 2017, 10, 14904–14910. [Google Scholar]
- Van Steenberghe, M.; Schubert, T.; Guiot, Y.; Bouzin, C.; Bollen, X.; Gianello, P. Enhanced vascular biocompatibility of decellularized xeno-/allogeneic matrices in a rodent model. Cell Tissue Bank. 2017, 18, 249–262. [Google Scholar] [CrossRef]
- Fischer, I.P.; Irmler, M.; Meyer, C.W.; Sachs, S.J.; Neff, F.; Hrabe De Angelis, M.; Beckers, J.; Tschöp, M.H.; Hofmann, S.M.; Ussar, S. A history of obesity leaves an inflammatory fingerprint in liver and adipose tissue. Int. J. Obes. 2018, 42, 507–517. [Google Scholar] [CrossRef]
- Wood, C.L.; Lane, L.C.; Cheetham, T. Puberty: Normal physiology (brief overview). Best Pract. Res. Clin. Endocrinol. Metab. 2019, 33, 101265. [Google Scholar] [CrossRef]
- Wang, Q.A.; Tao, C.; Gupta, R.K.; Scherer, P.E. Tracking adipogenesis during white adipose tissue development, expansion and regeneration. Nat. Med. 2013, 19, 1338–1344. [Google Scholar] [CrossRef]
- Poissonnet, C.M.; Burdi, A.R.; Garn, S.M. The chronology of adipose tissue appearance and distribution in the human fetus. Early Hum. Dev. 1984, 10, 1–11. [Google Scholar] [CrossRef]
- Knittle, J.L.; Timmers, K.; Ginsberg-Fellner, F.; Brown, R.E.; Katz, D.P. The growth of adipose tissue in children and adolescents. Cross-sectional and longitudinal studies of adipose cell number and size. J. Clin. Investig. 1979, 63, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Siegel, M.J.; Hildebolt, C.F.; Bae, K.T.; Hong, C.; White, N.H. Total and Intraabdominal Fat Distribution in Preadolescents and Adolescents: Measurement with MR Imaging. Radiology 2007, 242, 846–856. [Google Scholar] [CrossRef] [PubMed]
- Landgraf, K.; Rockstroh, D.; Wagner, I.V.; Weise, S.; Tauscher, R.; Schwartze, J.T.; Löffler, D.; Bühligen, U.; Wojan, M.; Till, H.; et al. Evidence of early alterations in adipose tissue biology and function and its association with obesity-related inflammation and insulin resistance in children. Diabetes 2015, 64, 1249–1261. [Google Scholar] [CrossRef] [PubMed]
- Choe, S.S.; Huh, J.Y.; Hwang, I.J.; Kim, J.I.; Kim, J.B. Adipose tissue remodeling: Its role in energy metabolism and metabolic disorders. Front. Endocrinol. (Lausanne) 2016, 7, 1. [Google Scholar] [CrossRef]
- Arner, E.; Westermark, P.O.; Spalding, K.L.; Britton, T.; Rydén, M.; Frisén, J.; Bernard, S.; Arner, P. Adipocyte turnover: Relevance to human adipose tissue morphology. Diabetes 2010, 59, 105–109. [Google Scholar] [CrossRef]
- Linsel-Nitschke, P.; Götz, A.; Erdmann, J.; Braenne, I.; Braund, P.; Hengstenberg, C.; Stark, K.; Fischer, M.; Schreiber, S.; El Mokhtari, N.E.; et al. Lifelong reduction of LDL-cholesterol related to a common varriant in the LDL-receptor gene decreases the risk of coronary artery disease—A Mendelian randomisation study. PLoS ONE 2008, 3, e2986. [Google Scholar] [CrossRef]
- Yoo, D.-Y.; Kang, Y.S.; Kwon, E.B.; Yoo, E.-G. The triglyceride-to-high density lipoprotein cholesterol ratio in overweight Korean children and adolescents. Ann. Pediatr. Endocrinol. Metab. 2017, 22, 158–163. [Google Scholar] [CrossRef]
- Pasarica, M.; Gowronska-Kozak, B.; Burk, D.; Remedios, I.; Hymel, D.; Gimble, J.; Ravussin, E.; Bray, G.A.; Smith, S.R. Adipose tissue collagen VI in obesity. J. Clin. Endocrinol. Metab. 2009, 94, 5155–5162. [Google Scholar] [CrossRef]
- Khan, T.; Muise, E.S.; Iyengar, P.; Wang, Z.V.; Chandalia, M.; Abate, N.; Zhang, B.B.; Bonaldo, P.; Chua, S.; Scherer, P.E. Metabolic Dysregulation and Adipose Tissue Fibrosis: Role of Collagen VI. Mol. Cell. Biol. 2009, 29, 1575–1591. [Google Scholar] [CrossRef]
- Datta, R.; Podolsky, M.J.; Atabai, K.; Datta, R.; Podolsky, M.J.; Atabai, K. Fat fibrosis: Friend or foe? JCI Insight 2018, 3, e122289. [Google Scholar] [CrossRef]
- Zöller, N.; Schreiner, S.; Petry, L.; Hoffmann, S.; Steinhorst, K.; Kleemann, J.; Jäger, M.; Kaufmann, R.; Meissner, M.; Kippenberger, S. Collagen I Promotes Adipocytogenesis in Adipose-Derived Stem Cells In Vitro. Cells 2019, 8, 302. [Google Scholar] [CrossRef]
- Ruiz-Ojeda, F.J.; Méndez-Gutiérrez, A.; Aguilera, C.M.; Plaza-Díaz, J. Extracellular matrix remodeling of adipose tissue in obesity and metabolic diseases. Int. J. Mol. Sci. 2019, 20, 4888. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zitsman, J.L.; Hou, J.; Fennoy, I.; Guo, K.; Feinberg, J.; Leibel, R.L. Fat cell size and adipokine expression in relation to gender, depot, and metabolic risk factors in morbidly obese adolescents. Obesity 2014, 22, 691–697. [Google Scholar] [CrossRef]
- Cha, Y.J.; Kim, E.S.; Koo, J.S. Tumor-associated macrophages and crown-like structures in adipose tissue in breast cancer. Breast Cancer Res. Treat. 2018, 170, 15–25. [Google Scholar] [CrossRef]
- Murano, I.; Barbatelli, G.; Parisani, V.; Latini, C.; Muzzonigro, G.; Castellucci, M.; Cinti, S. Dead adipocytes, detected as crown-like structures, are prevalent in visceral fat depots of genetically obese mice. J. Lipid Res. 2008, 49, 1562–1568. [Google Scholar] [CrossRef]
- Sun, K.; Kusminski, C.M.; Scherer, P.E. Adipose tissue remodeling and obesity. J. Clin. Investig. 2011, 121, 2094–2101. [Google Scholar] [CrossRef]
| Group | Normal Weight | Overweight/Obese | p-Value |
|---|---|---|---|
| n | 28 | 17 | |
| Age (years) | 4.47 ± 2.64 | 7.29 ± 4.23 | 0.009 * |
| BMI z-score | −0.37 ± 0.89 | 2.25 ± 1.40 | <0.001 * |
| Thigh circumference (cm) | 32.00 (28.00–36.88) | 44.00 (35.00–56.00) | 0.002 † |
| Waist circumference (cm) | 49.36 ± 11.96 | 61.29 ± 17.61 | 0.010 * |
| Waist/hip ratio | 1.54 ± 0.41 | 1.40 ± 0.47 | 0.325 * |
| Glucose (mmol/L) | 5.0 (4.40–5.80) | 5.20 (4.60–6.10) | 0.378 † |
| Triglycerides (mmol/L) | 0.84 ± 0.35 | 1.11 ± 0.37 | 0.021 * |
| Cholesterol (mmol/L) | 4.22 ± 0.70 | 4.02 ± 0.86 | 0.418 * |
| HDL cholesterol (mmol/L) | 1.45 ± 0.30 | 1.31 ± 0.24 | 0.087 * |
| LDL cholesterol (mmol/L) | 2.56 ± 0.55 | 2.33 ± 0.71 | 0.236 * |
| HDL/total cholesterol ratio (%) | 33.96 ± 5.56 | 35.18 ± 6.64 | 0.517 * |
| Testosterone (nmol/L) | 0.23 (0.10–0.35) | 0.34 (0.20–0.38) | 0.171 † |
| Adipocyte surface area (µm2) | |||
| Subcutaneous AT | 576.21 ± 191.95 | 1031.15 ± 327.95 | <0.001 * |
| Visceral AT | 354.52 ± 139.59 | 407.87 ± 153.69 | 0.238 * |
| Collagen percentage (%) | |||
| Subcutaneous AT | 5.46 ± 3.29 | 7.82 ± 4.21 | 0.042 * |
| Visceral AT | 9.92 ± 3.78 | 10.76 ± 4.48 | 0.504 * |
| Number of CD163+ cells | |||
| Subcutaneous AT | 75.36 ± 34.26 | 84.29 ± 34.60 | 0.403 * |
| Visceral AT | 98.57 ± 20.74 | 168.18 ± 33.13 | <0.001 * |
| Number of CLS | |||
| Subcutaneous AT | 0.00 (0.00–1.00) | 1.00 (0.00–2.50) | 0.002 † |
| Visceral AT | 2.50 (2.00–4.00) | 6.00 (4.00–7.00) | <0.001 † |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mujkić, R.; Šnajder Mujkić, D.; Ilić, I.; Rođak, E.; Šumanovac, A.; Grgić, A.; Divković, D.; Selthofer-Relatić, K. Early Childhood Fat Tissue Changes—Adipocyte Morphometry, Collagen Deposition, and Expression of CD163+ Cells in Subcutaneous and Visceral Adipose Tissue of Male Children. Int. J. Environ. Res. Public Health 2021, 18, 3627. https://doi.org/10.3390/ijerph18073627
Mujkić R, Šnajder Mujkić D, Ilić I, Rođak E, Šumanovac A, Grgić A, Divković D, Selthofer-Relatić K. Early Childhood Fat Tissue Changes—Adipocyte Morphometry, Collagen Deposition, and Expression of CD163+ Cells in Subcutaneous and Visceral Adipose Tissue of Male Children. International Journal of Environmental Research and Public Health. 2021; 18(7):3627. https://doi.org/10.3390/ijerph18073627
Chicago/Turabian StyleMujkić, Robert, Darija Šnajder Mujkić, Ivana Ilić, Edi Rođak, Antun Šumanovac, Anđela Grgić, Dalibor Divković, and Kristina Selthofer-Relatić. 2021. "Early Childhood Fat Tissue Changes—Adipocyte Morphometry, Collagen Deposition, and Expression of CD163+ Cells in Subcutaneous and Visceral Adipose Tissue of Male Children" International Journal of Environmental Research and Public Health 18, no. 7: 3627. https://doi.org/10.3390/ijerph18073627
APA StyleMujkić, R., Šnajder Mujkić, D., Ilić, I., Rođak, E., Šumanovac, A., Grgić, A., Divković, D., & Selthofer-Relatić, K. (2021). Early Childhood Fat Tissue Changes—Adipocyte Morphometry, Collagen Deposition, and Expression of CD163+ Cells in Subcutaneous and Visceral Adipose Tissue of Male Children. International Journal of Environmental Research and Public Health, 18(7), 3627. https://doi.org/10.3390/ijerph18073627







