Attitudes and Knowledge of European Medical Students and Early Graduates about Vaccination and Self-Reported Vaccination Coverage—Multinational Cross-Sectional Survey
Abstract
1. Introduction
2. Materials and Methods
2.1. Questionnaire Description
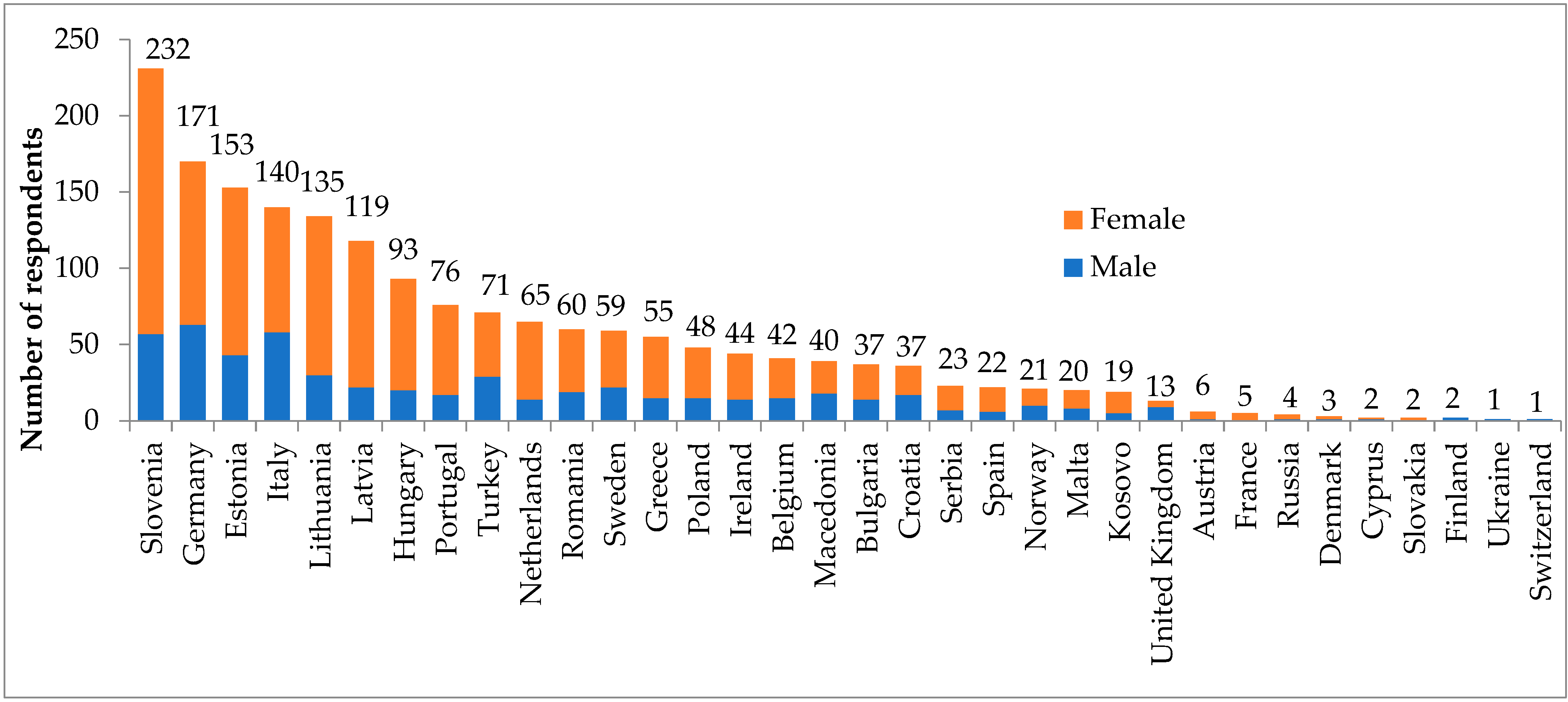
2.2. Dissemination Methods
2.3. Statistical Methods
3. Results
3.1. General Attitude towards Vaccination and Sources of Information
3.2. Knowledge and Practices Regarding Booster Vaccination
3.3. Vaccination Counselling and Attitudes towards Vaccination Programs in Pregnancy
3.4. Attitudes towards Seasonal Influenza Vaccination
3.5. Attitudes towards Mandatory Vaccination of Medical Staff and Medical Students
3.6. Self-Reported Coverage for 19 Selected Vaccines
3.7. Additional Vaccination
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fiore, A.E.; Bridges, C.B.; Cox, N.J. Seasonal influenza vaccines. In Vaccines for Pandemic Influenza; Compans, R.W., Orenstein, W.A., Eds.; Springer: Berlin/Heidelberg, Germany, 2009; pp. 43–82. [Google Scholar] [CrossRef]
- Rémy, V.; Largeron, N.; Quilici, S.; Carroll, S. The economic value of vaccination: Why prevention is wealth. J. Mark. Access. Health Policy 2015, 3. [Google Scholar] [CrossRef]
- Rémy, V.; Zöllner, Y.; Heckmann, U. Vaccination: The cornerstone of an efficient healthcare system. J. Mark. Access. Health Policy 2015, 3. [Google Scholar] [CrossRef]
- Singh, K.; Mehta, S. The clinical development process for a novel preventive vaccine: An overview. J. Postgrad. Med. 2016, 62, 4–11. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Vaccine Hesitancy: A Growing Challenge for Immunization Programs. Available online: https://www.who.int/news/item/18-08-2015-vaccine-hesitancy-a-growing-challenge-for-immunization-programmes (accessed on 16 March 2021).
- Smith, T.C. Vaccine Rejection and Hesitancy: A Review and Call to Action. Open Forum Infect. Dis. 2017, 4, ofx146. [Google Scholar] [CrossRef] [PubMed]
- Fournet, N.; Mollema, L.; Ruijs, W.L.; Harmsen, I.A.; Keck, F.; Durand, J.Y.; Cunha, M.P.; Wamsiedel, M.; Reis, R.; French, J.; et al. Under-vaccinated groups in Europe and their beliefs, attitudes and reasons for non-vaccination; two systematic reviews. BMC Public Health 2018, 18, 196. [Google Scholar] [CrossRef] [PubMed]
- Shetty, S.; Prabhu, S.; Shetty, V.; Shetty, A.K. Knowledge, attitudes and factors associated with acceptability of human papillomavirus vaccination among undergraduate medical, dental and nursing students in South India. Hum. Vaccin. Immunother. 2019, 15, 1656–1665. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention (CDC). Measles (Rubeola): Measles Cases and Outbreaks. Available online: https://www.cdc.gov/measles/cases-outbreaks.html (accessed on 16 March 2021).
- World Health Organization (WHO). More than 150 Countries Engaged in COVID-19 Vaccine Global Access Facility. Available online: https://www.who.int/news/item/15-07-2020-more-than-150-countries-engaged-in-covid-19-vaccine-global-access-facility (accessed on 16 March 2021).
- Schwarzinger, M.; Watson, V.; Arwidson, P.; Alla, F.; Luchini, S. COVID-19 vaccine hesitancy in a representative working-age population in France: A survey experiment based on vaccine characteristics. Lancet Public Health 2021, 6, e210–e221. [Google Scholar] [CrossRef]
- Afonso, N.; Kavanagh, M.; Swanberg, S. Improvement in attitudes toward influenza vaccination in medical students following an integrated curricular intervention. Vaccine 2014, 32, 502–506. [Google Scholar] [CrossRef] [PubMed]
- Loulergue, P.; Fonteneau, L.; Armengaud, J.-B.; Momcilovic, S.; Levy-Brühl, D.; Launay, O.; Guthmann, J.-P.; Studyvax survey, g. Vaccine coverage of healthcare students in hospitals of the Paris region in 2009: The Studyvax survey. Vaccine 2013, 31, 2835–2838. [Google Scholar] [CrossRef] [PubMed]
- Walker, L.; Newall, A.; Heywood, A.E. Knowledge, attitudes and practices of Australian medical students towards influenza vaccination. Vaccine 2016, 34, 6193–6199. [Google Scholar] [CrossRef]
- Loulergue, P.; Pulcini, C.; Massin, S.; Bernhard, M.; Fonteneau, L.; Levy-Brühl, D.; Guthmann, J.P.; Launay, O. Validity of self-reported vaccination status among French healthcare students. Clin. Microbiol. Infect. 2014, 20, O1152–O1154. [Google Scholar] [CrossRef][Green Version]
- Lamberti, M.; De Rosa, A.; Garzillo, E.M.; Corvino, A.R.; Sannolo, N.; De Pascalis, S.; Di Fiore, E.; Westermann, C.; Arnese, A.; Gabriella, D.G.; et al. Vaccination against hepatitis b virus: Are Italian medical students sufficiently protected after the public vaccination programme? J. Occup. Med. Toxicol. 2015, 10, 1–6. [Google Scholar] [CrossRef] [PubMed]
- De Figueiredo, A.; Simas, C.; Karafillakis, E.; Paterson, P.; Larson, H.J. Mapping global trends in vaccine confidence and investigating barriers to vaccine uptake: A large-scale retrospective temporal modelling study. Lancet 2020, 396, 898–908. [Google Scholar] [CrossRef]
- Wicker, S.; Rabenau, H.F.; von Gierke, L.; François, G.; Hambach, R.; De Schryver, A. Hepatitis B and influenza vaccines: Important occupational vaccines differently perceived among medical students. Vaccine 2013, 31, 5111–5117. [Google Scholar] [CrossRef]
- Ghomraoui, F.A.; Alfaqeeh, F.A.; Algadheeb, A.S.; Al-Alsheikh, A.S.; Al-Hamoudi, W.K.; Alswat, K.A. Medical students’ awareness of and compliance with the hepatitis B vaccine in a tertiary care academic hospital: An epidemiological study. J. Infect. Public Health 2016, 9, 60–65. [Google Scholar] [CrossRef] [PubMed]
- Souza, E.P.; Teixeira, M.D.S. Hepatitis B vaccination coverage and postvaccination serologic testing among medical students at a public university in Brazil. Rev. Inst. Med. Trop. Sao Paulo 2014, 56, 307–311. [Google Scholar] [CrossRef]
- Tuohetamu, S.; Pang, M.; Nuer, X.; Mahemuti; Mohemaiti, P.; Qin, Y.; Peng, Z.; Zheng, J.; Yu, H.; Feng, L.; et al. The knowledge, attitudes and practices on influenza among medical college students in Northwest China. Hum. Vaccines Immunother. 2017, 13, 1688–1692. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zahumensky, J.; Psenkova, P.; Nadzamova, A.; Drabiscakova, P.; Hruban, L.; Weinberger, V.; Kacerovsky, M.; Dosedla, E. Comparison of opinions of Slovak and Czech female medical students on HPV vaccination. Cent. Eur. J. Public Health 2020, 28, 178–186. [Google Scholar] [CrossRef]
- European Vaccination Information Portal. Vaccination Schedules in the EU/EEA. Available online: https://vaccination-info.eu/en/vaccination/when-vaccinate/vaccination-schedules-eueea (accessed on 16 March 2021).
- Dybsand, L.L.; Hall, K.J.; Carson, P.J. Immunization attitudes, opinions, and knowledge of healthcare professional students at two Midwestern universities in the United States. BMC Med. Educ. 2019, 19, 242. [Google Scholar] [CrossRef] [PubMed]
- Berera, D.; Thompson, K.M. Medical Student Knowledge, Attitudes, and Practices Regarding Immunization. J. Vaccines Vaccin. 2012, 6. [Google Scholar] [CrossRef]
- Kernéis, S.; Jacquet, C.; Bannay, A.; May, T.; Launay, O.; Verger, P.; Pulcini, C. Vaccine Education of Medical Students: A Nationwide Cross-sectional Survey. Am. J. Prev. Med. 2017, 53, e97–e104. [Google Scholar] [CrossRef] [PubMed]
- Banaszkiewicz, A.; Talarek, E.; Śliwka, J.; Kazubski, F.; Małecka, I.; Stryczyńska-Kazubska, J.; Dziubak, W.; Kuchar, E. Awareness of Influenza and Attitude Toward Influenza Vaccination Among Medical Students. Adv. Exp. Med. Biol. 2016, 934, 83–88. [Google Scholar] [CrossRef]
- Vorsters, A.; Tack, S.; Hendrickx, G.; Vladimirova, N.; Bonanni, P.; Pistol, A.; Metlicar, T.; Pasquin, M.J.; Mayer, M.A.; Aronsson, B.; et al. A summer school on vaccinology: Responding to identified gaps in pre-service immunisation training of future health care workers. Vaccine 2010, 28, 2053–2059. [Google Scholar] [CrossRef] [PubMed]
- Burgess, A.; McGregor, D.; Mellis, C. Medical students as peer tutors: A systematic review. BMC Med. Educ. 2014, 14, 115. [Google Scholar] [CrossRef] [PubMed]
- Betsch, C.; Wicker, S. E-health use, vaccination knowledge and perception of own risk: Drivers of vaccination uptake in medical students. Vaccine 2012, 30, 1143–1148. [Google Scholar] [CrossRef] [PubMed]
- Arif, M.; Daud, S. Immunization in medical students: Knowledge and practice. Pak. J. Med. Health Sci. 2017, 11, 1501–1504. [Google Scholar]
- World Health Organization (WHO). Position Papers—Immunization of Health Care Worker. Available online: https://www.who.int/immunization/policy/Immunization_routine_table4.pdf (accessed on 16 March 2021).
- Centers for Disease Control and Prevention (CDC). Maternal Vaccines: Part of a Healthy Pregnancy; Centers for Disease Control and Prevention (CDC): Atlanta, GA, USA. Available online: https://orwh.od.nih.gov/research/maternal-morbidity-and-mortality/information-for-women/pregnancy-and-vaccination (accessed on 16 March 2021).
- World Health Organization (WHO). Safety of Immunization during Pregnancy Safety of Immunization. Available online: https://www.who.int/vaccine_safety/publications/safety_pregnancy_nov2014.pdf?ua=1 (accessed on 16 March 2021).
- Swamy, G.K.; Heine, R.P. Vaccinations for pregnant women. Obstet. Gynecol. 2015, 125, 212–226. [Google Scholar] [CrossRef]
- Zikmund-Fisher, B.J.; Sarr, B.; Fagerlin, A.; Ubel, P.A. A matter of perspective: Choosing for others differs from choosing for yourself in making treatment decisions. J. Gen. Intern. Med. 2006, 21, 618–622. [Google Scholar] [CrossRef]
- Chamberlain, A.T.; Seib, K.; Ault, K.A.; Orenstein, W.A.; Frew, P.M.; Malik, F.; Cortés, M.; Cota, P.; Whitney, E.A.; Flowers, L.C.; et al. Factors Associated with Intention to Receive Influenza and Tetanus, Diphtheria, and Acellular Pertussis (Tdap) Vaccines during Pregnancy: A Focus on Vaccine Hesitancy and Perceptions of Disease Severity and Vaccine Safety. PLoS Curr. 2015, 7. [Google Scholar] [CrossRef]
- Corben, P.; Leask, J. Vaccination hesitancy in the antenatal period: A cross-sectional survey. BMC Public Health 2018, 18, 566. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention (CDC). Influenza Vaccination Information for Health Care Workers. Available online: https://www.cdc.gov/flu/professionals/healthcareworkers.htm (accessed on 16 March 2021).
- World Health Organization (WHO). How to Implement Seasonal Influenza Vaccination of Health Workers: An Introduction Manual for National Immunization Programme Managers and Policy Makers; WHO: Geneva, Switzerland, 2019. [Google Scholar]
- Lorenc, T.; Marshall, D.; Wright, K.; Sutcliffe, K.; Sowden, A. Seasonal influenza vaccination of healthcare workers: Systematic review of qualitative evidence. BMC Health Serv. Res. 2017, 17, 732. [Google Scholar] [CrossRef] [PubMed]
- Marcu, A.; Rubinstein, H.; Michie, S.; Yardley, L. Accounting for personal and professional choices for pandemic influenza vaccination amongst English healthcare workers. Vaccine 2015, 33, 2267–2272. [Google Scholar] [CrossRef] [PubMed]
- Lytras, T.; Kopsachilis, F.; Mouratidou, E.; Papamichail, D.; Bonovas, S. Interventions to increase seasonal influenza vaccine coverage in healthcare workers: A systematic review and meta-regression analysis. Hum. Vaccin. Immunother. 2016, 12, 671–681. [Google Scholar] [CrossRef] [PubMed]
- European Centre for Disease Prevention and Control (ECDC). Vaccination Schedules in Europe. Available online: https://www.ecdc.europa.eu/en/immunisation-vaccines/EU-vaccination-schedules (accessed on 16 March 2021).
- Reiter, P.L.; McRee, A.L.; Kadis, J.A.; Brewer, N.T. HPV vaccine and adolescent males. Vaccine 2011, 29, 5595–5602. [Google Scholar] [CrossRef] [PubMed]
- Grandahl, M.; Nevéus, T.; Dalianis, T.; Larsson, M.; Tydén, T.; Stenhammar, C. ‘I also want to be vaccinated!’—Adolescent boys’ awareness and thoughts, perceived benefits, information sources, and intention to be vaccinated against Human papillomavirus (HPV). Hum. Vaccines Immunother. 2019, 15, 1794–1802. [Google Scholar] [CrossRef]
- Hansen, M.N. Social background in recruitment of medical students. Tidsskr Nor Laegeforen 2005, 125, 2213–2215. [Google Scholar]
- O’Neill, L.; Vonsild, M.C.; Wallstedt, B.; Dornan, T. Admission criteria and diversity in medical school. Med. Educ. 2013, 47, 557–561. [Google Scholar] [CrossRef]
- Larson, H.; de Figueiredo, A.; Karafillakis, E.; Rawal, M. State of Vaccine Confidence in the EU 2018; European Commission: Luxembourg, 2018. [Google Scholar]
- Kmietowicz, Z. Boys should be given HPV vaccine, says joint committee. BMJ 2018, 362, k3163. [Google Scholar] [CrossRef]
- European Centre for Disease Prevention and Control (ECDC). Human Papillomavirus (HPV)—Vaccinating Boys and Girls; European Centre for Disease Prevention and Control (ECDC): Stockholm, Sweden, 2019. [Google Scholar]
- Nguyen-Huu, N.H.; Thilly, N.; Derrough, T.; Sdona, E.; Claudot, F.; Pulcini, C.; Agrinier, N. Human papillomavirus vaccination coverage, policies, and practical implementation across Europe. Vaccine 2020, 38, 1315–1331. [Google Scholar] [CrossRef]
- Pebody, R.G.; Leino, T.; Nohynek, H.; Hellenbrand, W.; Salmaso, S.; Ruutu, P. Pneumococcal vaccination policy in Europe. Eurosurveillance 2005, 10, 174–178. [Google Scholar] [CrossRef]
- Trotter, C.L.; Ramsay, M.E. Vaccination against meningococcal disease in Europe: Review and recommendations for the use of conjugate vaccines. FEMS Microbiol. Rev. 2007, 31, 101–107. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention (CDC). Typhoid Fever. Available online: https://wwwnc.cdc.gov/travel/diseases/typhoid (accessed on 16 March 2021).
- Centers for Disease Control and Prevention (CDC). Cholera. Available online: https://wwwnc.cdc.gov/travel/diseases/cholera (accessed on 16 March 2021).
- Centers for Disease Control and Prevention (CDC). Hepatitis A. Available online: https://wwwnc.cdc.gov/travel/diseases/hepatitis-a (accessed on 16 March 2021).
- Haddouche, H.; Salomone, C. Generation Z and the tourist experience: Tourist stories and use of social networks. J. Tour. Futur. 2018, 4, 69–79. [Google Scholar] [CrossRef]
- Ketter, E. Millennial travel: Tourism micro-trends of European Generation Y. J. Tour. Futur. 2020. [Google Scholar] [CrossRef]
- Shouval, D. The History of Hepatitis A. Clin. Liver. Dis. (Hoboken) 2020, 16, 12–23. [Google Scholar] [CrossRef]
- Deleanu, D.; Petricau, C.; Leru, P.; Chiorean, I.; Muntean, A.; Dumitrascu, D.; Nedelea, I. Knowledge influences attitudes toward vaccination in Romania. Exp. Ther. Med. 2019, 18, 5088–5094. [Google Scholar] [CrossRef]
- Hall, A. The Global Eradication of Smallpox. Final Report of the Global Commission for the Certification of Smallpox Eradication. Postgrad. Med. J. 1982, 58, 734–735. [Google Scholar] [CrossRef]
- Rolnick, S.J.; Parker, E.D.; Nordin, J.D.; Hedblom, B.D.; Wei, F.; Kerby, T.; Jackson, J.M.; Crain, A.L.; Euler, G. Self-report compared to electronic medical record across eight adult vaccines: Do results vary by demographic factors? Vaccine 2013, 31, 3928–3935. [Google Scholar] [CrossRef] [PubMed]
- Verger, P.; Scronias, D.; Dauby, N.; Adedzi, K.A.; Gobert, C.; Bergeat, M.; Gagneur, A.; Dubé, E. Attitudes of healthcare workers towards COVID-19 vaccination: A survey in France and French-speaking parts of Belgium and Canada, 2020. Eurosurveillance 2021, 26, 2002047. [Google Scholar] [CrossRef] [PubMed]
- Grech, V.; Gauci, C.; Agius, S. Withdrawn: Vaccine hesitancy among Maltese Healthcare workers toward influenza and novel COVID-19 vaccination. Early Hum. Dev. 2020. [Google Scholar] [CrossRef]
- Barello, S.; Nania, T.; Dellafiore, F.; Graffigna, G.; Caruso, R. ‘Vaccine hesitancy’ among university students in Italy during the COVID-19 pandemic. Eur. J. Epidemiol. 2020, 35, 781–783. [Google Scholar] [CrossRef]
- Lucia, V.C.; Kelekar, A.; Afonso, N.M. COVID-19 vaccine hesitancy among medical students. J. Public Health (Oxford) 2020. [Google Scholar] [CrossRef] [PubMed]
- Saied, S.M.; Saied, E.M.; Kabbash, I.A.; Abdo, S.A.E. Vaccine Hesitancy: Beliefs and Barriers Associated with COVID-19 Vaccination among Egyptian Medical Students. J. Med. Virol. 2021. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | Category | n (%) |
|---|---|---|
| Year of birth | up to 1991 | 571 (31.4) |
| 1992–1993 | 604 (33.2) | |
| 1994 or higher | 646 (35.4) | |
| Gender | Male | 555 (30.5) |
| Female | 1259 (69.1) | |
| Other | 7 (0.4) | |
| Year of study | 1–2. | 467 (25.6) |
| 3–4. | 645 (35.5) | |
| 5–6. * | 651 (35.7) | |
| JD | 58 (3.2) |
| Question Answer | Total (n = 1821) | Gender | Year of Study | ||||
|---|---|---|---|---|---|---|---|
| Male (n = 555) | Female (n = 1259) | 1–2 (n = 467) | 3–4 (n = 645) | 5–6 (n = 651) | JD (n = 58) | ||
| Q1. Which statement describes your opinion about vaccinations the most? | |||||||
| A. It is useful and safe and I think that everybody should get vaccinated. | 1797 (98.7) | 549 (98.9) | 1241 (98.6) | 451 (96.6) | 640 (99.2) | 649 (99.7) | 57 (98.3) |
| B. There is too little evidence to prove that it is effective. | 16 (0.9) | 4 (0.7) | 12 (1.0) | 12 (2.6) | 3 (0.5) | 0 (0.0) | 1 (1.7) |
| C. There is too little evidence to prove that it is even safe to get vaccinated and I think that nobody should do this. | 8 (0.4) | 2 (0.4) | 6 (0.5) | 4 (0.9) | 2 (0.3) | 2 (0.3) | 0 (0.0) |
| Q2. Do you think that vaccination programs are an effective tool in disease prevention? | |||||||
| A. Yes, I think it is effective. | 1770 (97.2) | 545 (98.2) | 1219 (96.8) | 438 (93.8) | 630 (97.7) | 645 (99.1) | 57 (98.3) |
| B. I don’t think that it makes a difference because I would choose to vaccinate either way. | 35 (1.9) | 8 (1.4) | 26 (2.1) | 18 (3.9) | 13 (2.0) | 4 (0.6) | 0 (0.0) |
| C. No because there is not enough proof that vaccines are effective or even safe. | 6 (0.3) | 2 (0.4) | 4 (0.3) | 2 (0.4) | 2 (0.3) | 2 (0.3) | 0 (0.0) |
| D. No because I don’t think that such things should be forced on. | 10 (0.5) | 0 (0.0) | 10 (0.8) | 9 (1.9) | 0 (0.0) | 0 (0.0) | 1 (1.7) |
| Q3: What influences your opinion about vaccinations the most? | |||||||
| A. Scientific facts | 1610 (88.4) | 506 (91.2) | 1099 (87.3) | 388 (83.1) | 573 (88.8) | 593 (91.1) | 56 (96.6) |
| B. Social Media | 19 (1.0) | 4 (0.7) | 15 (1.2) | 9 (1.9) | 3 (0.5) | 7 (1.1) | 0 (0.0) |
| C. Senior physicians, professors | 159 (8.7) | 35 (6.3) | 122 (9.7) | 48 (10.3) | 66 (10.2) | 44 (6.8) | 1 (1.7) |
| D. My relatives | 26 (1.4) | 7 (1.3) | 19 (1.5) | 20 (4.3) | 2 (0.3) | 3 (0.5) | 1 (1.7) |
| E. Religious beliefs | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| F. My friends, colleagues | 7 (0.4) | 3 (0.5) | 4 (0.3) | 2 (0.4) | 1 (0.2) | 4 (0.6) | 0 (0.0) |
| Question Answer | Total (n = 1821) | Gender | Year of Study | ||||
|---|---|---|---|---|---|---|---|
| Male (n = 555) | Female (n = 1259) | 1–2(n = 467) | 3–4 (n = 645) | 5–6 (n = 651) | JD (n = 58) | ||
| Q4. Did you get vaccinated as part of the vaccination program of your country? | |||||||
| A. Yes | 1765 (96.9) | 537 (96.8) | 1221 (97.0) | 438 (93.8) | 629 (97.5) | 640 (98.3) | 58 (100) |
| B. No | 25 (1.4) | 6 (1.1) | 19 (1.5) | 13 (2.8) | 7 (1.1) | 5 (0.8) | 0 (0.0) |
| C. I don’t know | 31 (1.7) | 12 (2.2) | 19 (1.5) | 16 (3.4) | 9 (1.4) | 6 (0.9) | 0 (0.0) |
| Q25. Do you know that in order to be protected properly you need to get revaccinated for several vaccines? | |||||||
| A. Yes, I am aware it and doing it properly. | 1239 (68.0) | 375 (67.6) | 860 (68.3) | 279 (59.7) | 420 (65.1) | 495 (76.0) | 45 (77.6) |
| B. Yes, I am aware of it, but I am not sure if I have full vaccination. | 568 (31.2) | 173 (31.2) | 392 (31.1) | 184 (39.4) | 220 (34.1) | 151 (23.2) | 13 (22.4) |
| C. No, this is the first time I hear about that. | 12 (0.7) | 6 (1.1) | 6 (0.5) | 4 (0.9) | 5 (0.8) | 3 (0.5) | 0 (0.0) |
| D. No, there is no need because vaccination is always life-long protection | 2 (0.1) | 1 (0.2) | 1 (0.1) | 0 (0.0) | 0 (0.0) | 2 (0.3) | 0 (0.0) |
| Question Answer | Total (n = 1821) | Gender | Year of Study | ||||
|---|---|---|---|---|---|---|---|
| Male (n = 555) | Female (n = 1259) | 1–2(n = 467) | 3–4 (n = 645) | 5–6 (n = 651) | JD (n = 58) | ||
| Q26. Do you advise your relatives, friends, colleagues etc. to get vaccinated? | |||||||
| A. Yes | 1629 (89.5) | 477 (85.9) | 1146 (91.0) | 366 (78.4) | 586 (90.9) | 622 (95.5) | 55 (94.8) |
| B. No | 52 (2.9) | 22 (4.0) | 30 (2.4) | 26 (5.6) | 11 (1.7) | 13 (2.0) | 2 (3.4) |
| C. Never thought about that | 140 (7.7) | 56 (10.1) | 83 (6.6) | 75 (16.1) | 48 (7.4) | 16 (2.5) | 1 (1.7) |
| Q27. Do you think that a more specific vaccination program should be available to pregnant women (e.g., seasonal flu, mumps, rubella)? | |||||||
| A. Yes, because that way the fetus will be protected against inborn anomalies and fewer miscarriages will occur | 1507 (82.8) | 478 (86.1) | 1025 (81.4) | 358 (76.7) | 536 (83.1) | 565 (86.8) | 48 (82.8) |
| B. No, because a specific programme is not safe to a pregnant woman or the fetus | 194 (10.7) | 46 (8.3) | 145 (11.5) | 67 (14.3) | 70 (10.9) | 55 (8.4) | 2 (3.4) |
| C. No, because the vaccine is not effective for the pregnant woman or the fetus. | 12 (0.7) | 4 (0.7) | 8 (0.6) | 6 (1.3) | 3 (0.5) | 3 (0.5) | 0 (0.0) |
| D. No, because everyone should have the right to choose. | 108 (5.9) | 27 (4.9) | 81 (6.4) | 36 (7.7) | 36 (5.6) | 28 (4.3) | 8 (13.8) |
| Question Answer | Total (n = 1821) | Gender | Year of Study | ||||
|---|---|---|---|---|---|---|---|
| Male (n = 555) | Female (n = 1259) | 1–2 (n = 467) | 3–4 (n = 645) | 5–6 (n = 651) | JD (n = 58) | ||
| Q28: What is your opinion about the seasonal flu vaccine? | |||||||
| A. It is an almost 100% protection against seasonal flu. | 126 (6.9) | 54 (9.7) | 72 (5.7) | 32 (6.9) | 48 (7.4) | 45 (6.9) | 1 (1.7) |
| B. It is not useful because the seasonal flu virus mutates constantly and there is a different type every year. | 310 (17.0) | 94 (16.9) | 214 (17.0) | 120 (25.7) | 109 (16.9) | 75 (11.5) | 6 (10.3) |
| C. It won’t necessary prevent you from contracting the seasonal flu, but the disease will be less serious. | 1379 (75.7) | 405 (73.0) | 969 (77.0) | 313 (67.0) | 486 (75.3) | 529 (81.3) | 51 (87.9) |
| D. Vaccines in general are not effective and the seasonal flu vaccine is not an exception. | 6 (0.3) | 2 (0.4) | 4 (0.3) | 2 (0.4) | 2 (0.3) | 2 (0.3) | 0 (0.0) |
| Q29: How often do you get vaccinated against seasonal flu? | |||||||
| A. Every other season. | 73 (4.0) | 23 (4.1) | 50 (4.0) | 17 (3.6) | 21 (3.3) | 29 (4.5) | 6 (10.3) |
| B. Every season. | 329 (18.1) | 96 (17.3) | 232 (18.4) | 57 (12.2) | 88 (13.6) | 167 (25.7) | 17 (29.3) |
| C. I have never been vaccinated against seasonal flu. | 836 (45.9) | 234 (42.2) | 599 (47.6) | 233 (49.9) | 315 (48.8) | 271 (41.6) | 17 (29.3) |
| D. I haven only been vaccinated once. | 263 (14.4) | 80 (14.4) | 181 (14.4) | 69 (14.8) | 108 (16.7) | 81 (12.4) | 5 (8.6) |
| E. Not regularly. | 320 (17.6) | 122 (22.0) | 197 (15.6) | 91 (19.5) | 113 (17.5) | 103 (15.8) | 13 (22.4) |
| Question Answer | Total (n = 1821) | Gender | Year of Study | ||||
|---|---|---|---|---|---|---|---|
| Male (n = 555) | Female (n = 1259) | 1–2 (n = 467) | 3–4 (n = 645) | 5–6 (n = 651) | JD (n = 58) | ||
| Q30: Do you think that a vaccine against seasonal flu and hepatitis B should be mandatory for medical staff (attending doctors, nurses etc.)? | |||||||
| A. No, because everyone should have the right to choose. | 227 (12.5) | 63 (11.4) | 164 (13.0) | 75 (16.1) | 77 (11.9) | 65 (10.0) | 10 (17.2) |
| B. No, because those vaccines are not effective. | 21 (1.2) | 9 (1.6) | 12 (1.0) | 9 (1.9) | 5 (0.8) | 6 (0.9) | 1 (1.7) |
| C. No, because those vaccines are not safe. | 7 (0.4) | 3 (0.5) | 4 (0.3) | 3 (0.6) | 2 (0.3) | 2 (0.3) | 0 (0.0) |
| D. Yes, because medical staff has a greater chance to get infected and then spread the spread the virus. | 1566 (86.0) | 480 (86.5) | 1079 (85.7) | 380 (81.4) | 561 (87.0) | 578 (88.8) | 47 (81.0) |
| Q31: Do you think that a vaccine against seasonal flu and hepatitis B should be mandatory for medical students? | |||||||
| A. No, because everyone should have the right to choose. | 287 (15.8) | 85 (15.3) | 202 (16.0) | 106 (22.7) | 96 (14.9) | 75 (11.5) | 10 (17.2) |
| B. No, because these vaccines are not safe. | 6 (0.3) | 2 (0.4) | 4 (0.3) | 3 (0.6) | 1 (0.2) | 2 (0.3) | 0 (0.0) |
| C. No, because those vaccines are not effective. | 22 (1.2) | 8 (1.4) | 14 (1.1) | 9 (1.9) | 6 (0.9) | 6 (0.9) | 1 (1.7) |
| D. Yes, because medical students rotate through different departments in a hospital and can spread the virus. | 1506 (82.7) | 460 (82.9) | 1039 (82.5) | 349 (74.7) | 542 (84.0) | 568 (87.3) | 47 (81.0) |
| Types of Vaccination | Vaccine | Total (n = 1821) | Gender | p | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Male (n = 555) | Female (n = 1259) | ||||||||||
| Yes | No | Don’t Know | Yes | No | Don’t Know | Yes | No | Don’t Know | |||
| Childhood schedules in all EU/EEA | Tetanus | 94.2 | 3.0 | 2.7 | 93.9 | 3.1 | 3.1 | 94.4 | 3.0 | 2.6 | 0.848 |
| Diphtheria | 89.0 | 3.6 | 7.4 | 88.8 | 3.4 | 7.7 | 89.0 | 3.7 | 7.3 | 0.908 | |
| Poliomyelitis | 85.7 | 4.9 | 9.4 | 85.4 | 4.5 | 10.1 | 85.9 | 5.1 | 9.1 | 0.704 | |
| Rubella | 83.3 | 6.9 | 9.8 | 79.5 | 8.5 | 12.1 | 85.1 | 6.2 | 8.7 | 0.012 | |
| Measles | 81.0 | 8.7 | 10.3 | 80.5 | 9.2 | 10.3 | 81.3 | 8.6 | 10.2 | 0.898 | |
| Mumps | 80.2 | 8.6 | 11.2 | 77.8 | 9.5 | 12.6 | 81.3 | 8.3 | 10.5 | 0.229 | |
| Pertussis | 79.3 | 8.0 | 12.7 | 79.5 | 8.1 | 12.4 | 79.2 | 7.9 | 12.9 | 0.963 | |
| Haemophilus influenzae type b | 53.8 | 25.7 | 20.5 | 54.2 | 23.6 | 22.2 | 53.7 | 26.6 | 19.7 | 0.287 | |
| Human papillomavirus | 25.0 | 65.4 | 9.6 | 7.2 | 78.4 | 14.4 | 33.0 | 59.7 | 7.4 | <0.001 | |
| Additional childhood vaccines | Tuberculosis | 60.6 | 29.8 | 9.6 | 57.1 | 31.7 | 11.2 | 62.2 | 29.0 | 8.8 | 0.088 |
| Hepatitis A | 40.5 | 47.4 | 12.0 | 42.9 | 43.4 | 13.7 | 39.7 | 49.1 | 11.2 | 0.058 | |
| Meningococci | 32.2 | 46.8 | 20.9 | 33.2 | 42.0 | 24.9 | 32.0 | 48.8 | 19.1 | 0.006 | |
| Pneumococci | 25.6 | 51.8 | 22.6 | 26.7 | 49.9 | 23.4 | 25.2 | 52.6 | 22.2 | 0.573 | |
| Chickenpox | 24.6 | 60.6 | 14.8 | 23.2 | 60.2 | 16.6 | 25.3 | 60.8 | 13.9 | 0.281 | |
| Vaccines recommended both for children and for adults in risk groups | Hepatitis B | 83.4 | 11.3 | 5.3 | 78.9 | 13.3 | 7.7 | 85.5 | 10.2 | 4.3 | 0.001 |
| Influenza/seasonal flu | 32.9 | 62.4 | 4.6 | 34.2 | 58.4 | 7.4 | 32.4 | 64.2 | 3.4 | 0.001 | |
| Vaccines for adults | Typhus | 21.1 | 56.8 | 22.1 | 20.2 | 54.1 | 25.8 | 21.6 | 58.0 | 20.4 | 0.041 |
| Cholera | 12.9 | 67.4 | 19.7 | 14.6 | 62.9 | 22.5 | 12.1 | 69.5 | 18.4 | 0.020 | |
| Smallpox | 24.4 | 53.7 | 22.0 | 25.0 | 50.6 | 24.3 | 24.1 | 55.0 | 20.9 | 0.160 | |
| Types of vaccination | Vaccine | Year of study | p | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1–2 (n = 467) | 3–4 (n = 645) | 5–6 (n = 651) | JD (n = 58) | |||||||||||
| Yes | No | Don’t know | Yes | No | Don’t know | Yes | No | Don’t know | Yes | No | Don’t know | |||
| Childhood schedules in all EU/EEA | Tetanus | 88.0 | 6.6 | 5.4 | 94.7 | 2.5 | 2.8 | 97.7 | 1.2 | 1.1 | 100.0 | 0.0 | 0.0 | <0.001 |
| Diphtheria | 73.4 | 8.1 | 18.4 | 91.2 | 2.5 | 6.4 | 97.2 | 1.5 | 1.2 | 96.6 | 3.4 | 0.0 | <0.001 | |
| Poliomyelitis | 71.7 | 8.1 | 20.1 | 85.3 | 5.1 | 9.6 | 94.9 | 2.8 | 2.3 | 100.0 | 0.0 | 0.0 | <0.001 | |
| Rubella | 67.2 | 10.1 | 22.7 | 85.4 | 6.7 | 7.9 | 92.3 | 4.5 | 3.2 | 87.9 | 10.3 | 1.7 | <0.001 | |
| Measles | 68.7 | 12.8 | 18.4 | 80.8 | 8.1 | 11.2 | 89.4 | 6.3 | 4.3 | 87.9 | 10.3 | 1.7 | <0.001 | |
| Mumps | 65.7 | 12.6 | 21.6 | 80.0 | 8.2 | 11.8 | 90.3 | 5.7 | 4.0 | 84.5 | 13.8 | 1.7 | <0.001 | |
| Pertussis | 55.5 | 14.8 | 29.8 | 82.3 | 6.4 | 11.3 | 92.6 | 4.5 | 2.9 | 87.9 | 10.3 | 1.7 | <0.001 | |
| Haemophilus influenzae type b | 40.3 | 25.9 | 33.8 | 55.8 | 23.4 | 20.8 | 61.9 | 26.4 | 11.7 | 50.0 | 41.4 | 8.6 | <0.001 | |
| Human papillomavirus | 30.4 | 51.0 | 18.6 | 27.6 | 61.7 | 10.7 | 19.0 | 78.2 | 2.8 | 20.7 | 79.3 | 0.0 | <0.001 | |
| Additional childhood vaccines | Tuberculosis | 49.0 | 33.2 | 17.8 | 58.1 | 31.0 | 10.9 | 70.7 | 26.1 | 3.2 | 69.0 | 31.0 | 0.0 | <0.001 |
| Hepatitis A | 52.2 | 27.8 | 19.9 | 40.6 | 47.3 | 12.1 | 33.0 | 59.9 | 7.1 | 29.3 | 67.2 | 3.4 | <0.001 | |
| Meningococci | 38.5 | 28.7 | 32.8 | 31.0 | 44.2 | 24.8 | 29.3 | 60.2 | 10.4 | 27.6 | 72.4 | 0.0 | <0.001 | |
| Pneumococci | 32.5 | 31.3 | 36.2 | 26.5 | 46.5 | 27.0 | 20.0 | 69.6 | 10.4 | 22.4 | 75.9 | 1.7 | <0.001 | |
| Chickenpox | 26.3 | 51.2 | 22.5 | 27.0 | 56.6 | 16.4 | 20.4 | 70.8 | 8.8 | 31.0 | 67.2 | 1.7 | <0.001 | |
| Vaccines recommended both for children and for adults in risk groups | Hepatitis B | 79.2 | 10.1 | 10.7 | 84.2 | 10.5 | 5.3 | 85.6 | 12.7 | 1.7 | 84.5 | 12.1 | 3.4 | <0.001 |
| Influenza/seasonal flu | 33.6 | 58.0 | 8.4 | 28.4 | 67.6 | 4.0 | 35.6 | 61.4 | 2.9 | 48.3 | 51.7 | 0.0 | <0.001 | |
| Vaccines for adults | Typhus | 24.2 | 38.3 | 37.5 | 21.9 | 53.6 | 24.5 | 18.6 | 71.0 | 10.4 | 17.2 | 81.0 | 1.7 | <0.001 |
| Cholera | 18.2 | 48.2 | 33.6 | 14.4 | 64.3 | 21.2 | 8.1 | 82.0 | 9.8 | 5.2 | 93.1 | 1.7 | <0.001 | |
| Smallpox | 29.3 | 38.3 | 32.3 | 26.0 | 48.2 | 25.7 | 19.7 | 67.9 | 12.4 | 19.0 | 77.6 | 3.4 | <0.001 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rostkowska, O.M.; Peters, A.; Montvidas, J.; Magdas, T.M.; Rensen, L.; Zgliczyński, W.S.; Durlik, M.; Pelzer, B.W. Attitudes and Knowledge of European Medical Students and Early Graduates about Vaccination and Self-Reported Vaccination Coverage—Multinational Cross-Sectional Survey. Int. J. Environ. Res. Public Health 2021, 18, 3595. https://doi.org/10.3390/ijerph18073595
Rostkowska OM, Peters A, Montvidas J, Magdas TM, Rensen L, Zgliczyński WS, Durlik M, Pelzer BW. Attitudes and Knowledge of European Medical Students and Early Graduates about Vaccination and Self-Reported Vaccination Coverage—Multinational Cross-Sectional Survey. International Journal of Environmental Research and Public Health. 2021; 18(7):3595. https://doi.org/10.3390/ijerph18073595
Chicago/Turabian StyleRostkowska, Olga M., Alexandra Peters, Jonas Montvidas, Tudor M. Magdas, Leon Rensen, Wojciech S. Zgliczyński, Magdalena Durlik, and Benedikt W. Pelzer. 2021. "Attitudes and Knowledge of European Medical Students and Early Graduates about Vaccination and Self-Reported Vaccination Coverage—Multinational Cross-Sectional Survey" International Journal of Environmental Research and Public Health 18, no. 7: 3595. https://doi.org/10.3390/ijerph18073595
APA StyleRostkowska, O. M., Peters, A., Montvidas, J., Magdas, T. M., Rensen, L., Zgliczyński, W. S., Durlik, M., & Pelzer, B. W. (2021). Attitudes and Knowledge of European Medical Students and Early Graduates about Vaccination and Self-Reported Vaccination Coverage—Multinational Cross-Sectional Survey. International Journal of Environmental Research and Public Health, 18(7), 3595. https://doi.org/10.3390/ijerph18073595






