Prevalence and Associated Factors of Frailty and Mortality in Patients with End-Stage Renal Disease Undergoing Hemodialysis: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategies
2.2. Study Selection
2.3. Data Extraction and Synthesis
2.4. Data Analysis
2.5. Quality Assessment of the Studies Included
3. Results
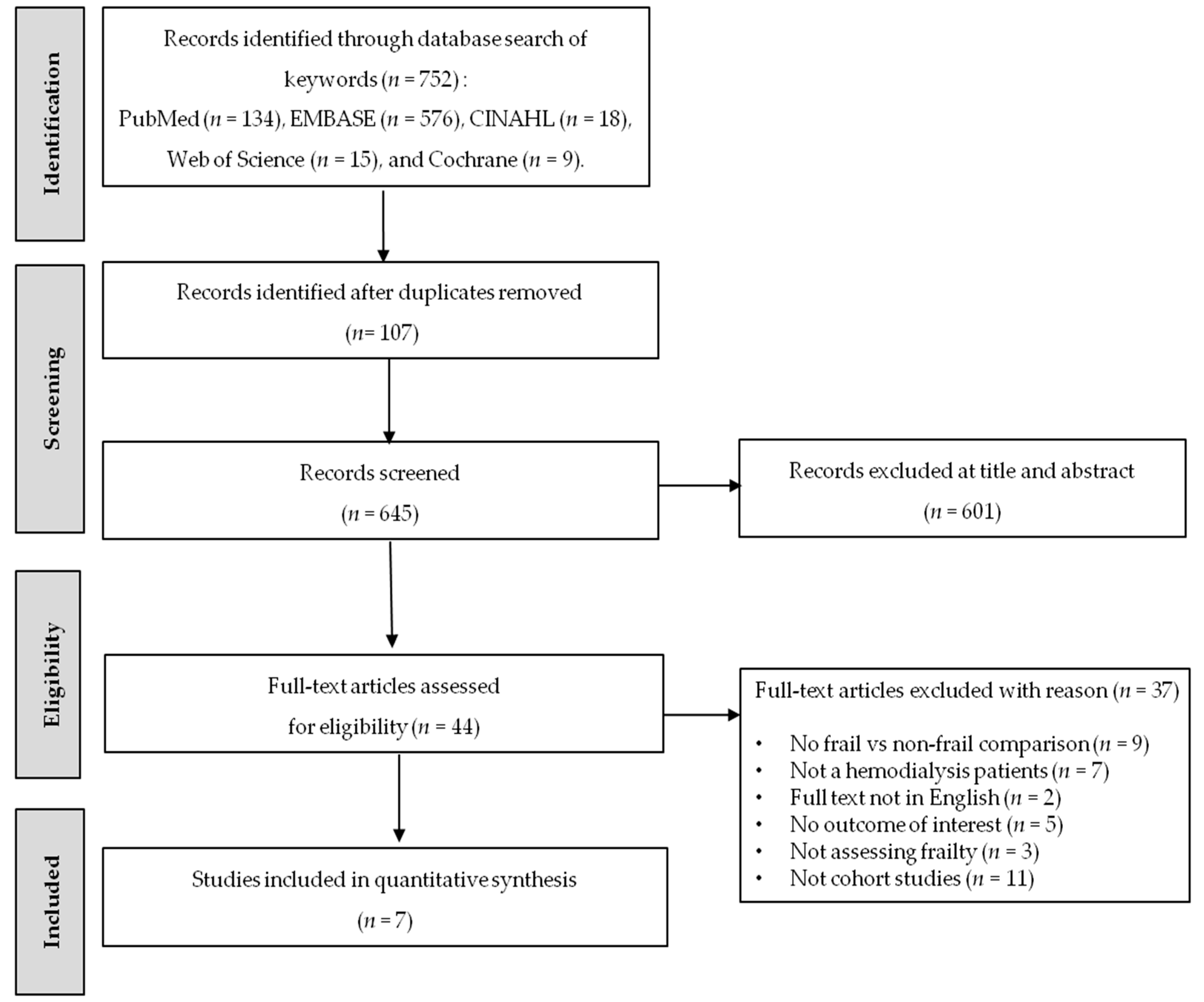
3.1. Study Selection
3.2. Characteristics of the Included Studies
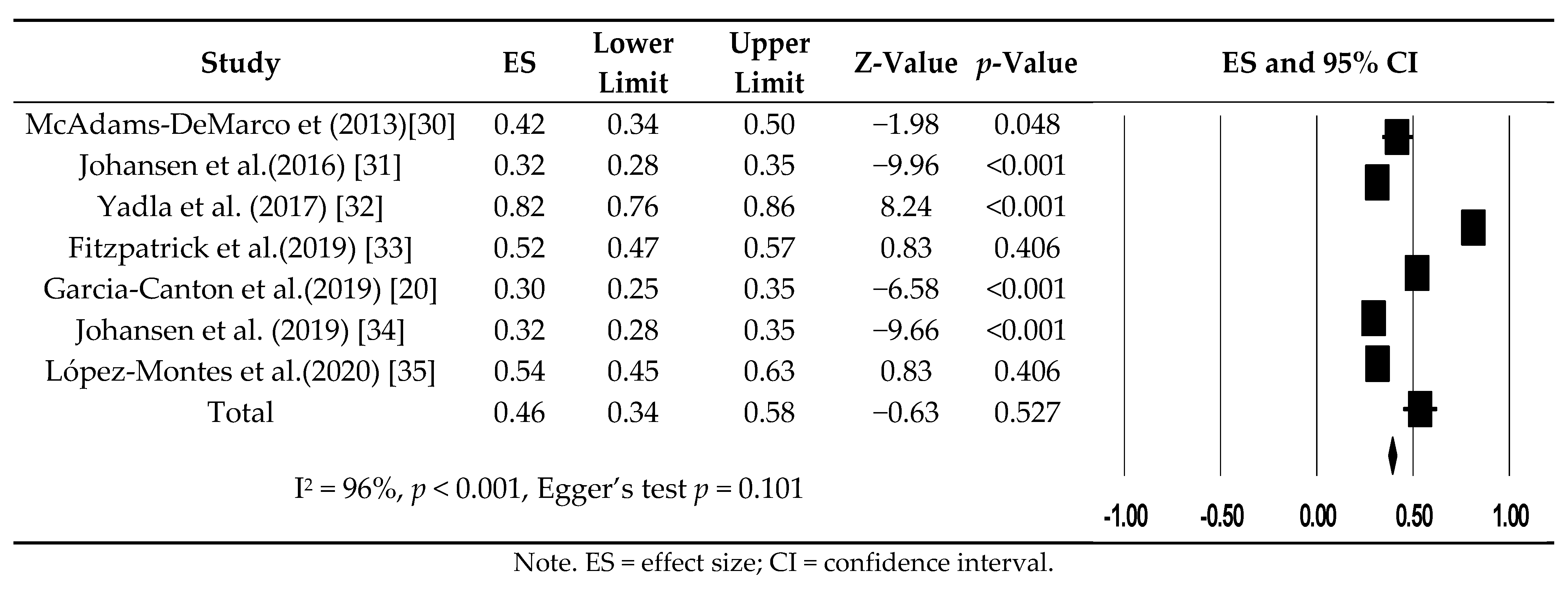
3.3. Prevalence of Frailty in Patients Undergoing Hemodialysis
3.4. Risk Factors for Frailty in Hemodialysis Patients
3.4.1. Age
3.4.2. Female Sex
3.4.3. Diabetes Mellitus
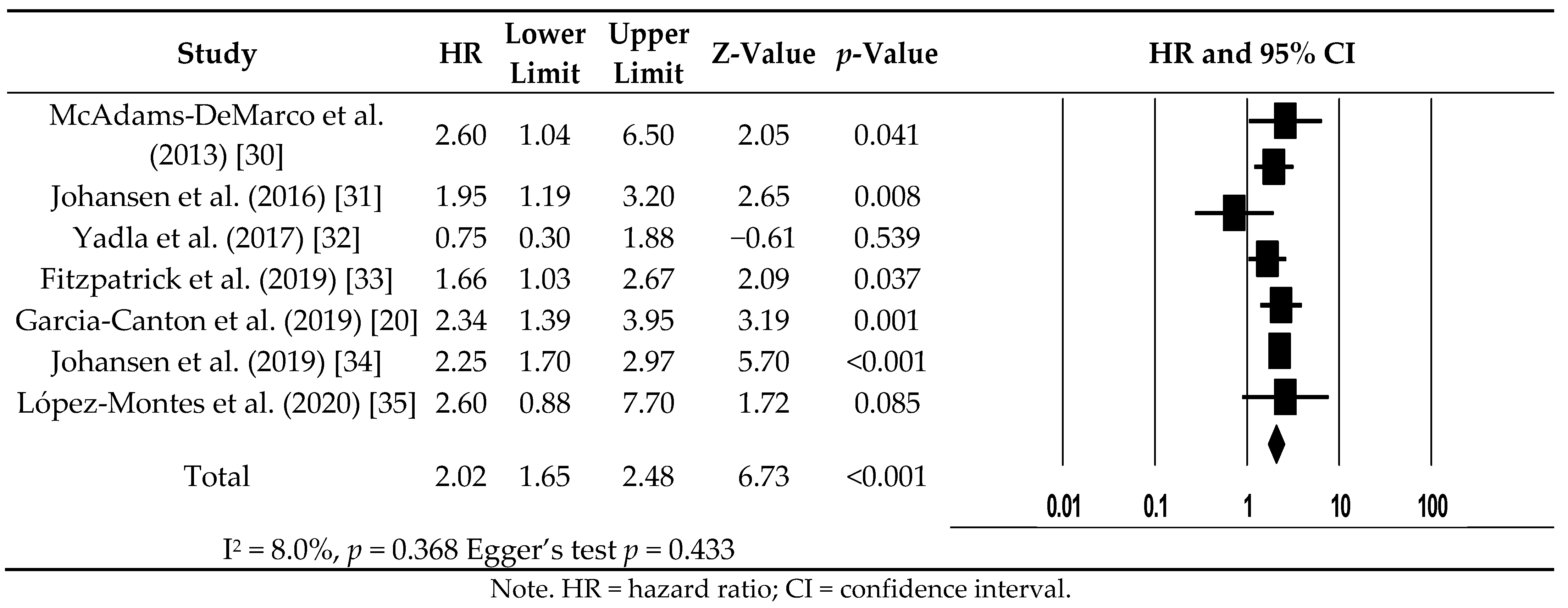
3.5. All-Cause Mortality for Hemodialysis Patients with Frailty
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gaitonde, D.Y.; Cook, D.L.; Rivera, I.M. Chronic kidney disease: Detection and evaluation. Am. Fam. Physician 2017, 96, 776–783. [Google Scholar] [PubMed]
- Ene-Iordache, B.; Perico, N.; Bikbov, B.; Carminati, S.; Remuzzi, A.; Perna, A.; Islam, N.; Bravo, R.F.; Aleckovic-Halilovic, M.; Zou, H.; et al. Chronic kidney disease and cardiovascular risk in six regions of the world (ISN-KDDC): A cross-sectional study. Lancet Glob. Health 2016, 4, 1–13. [Google Scholar] [CrossRef]
- Mills, K.T.; Xu, Y.; Zhang, W.; Bundy, J.D.; Chen, C.S.; Kelly, T.N.; Chen, J.; He, J. A systematic analysis of worldwide population-based data on the global burden of chronic kidney disease in 2010. Kidney Int. 2015, 88, 950–957. [Google Scholar] [CrossRef]
- Fung, E.; Kurella Tamura, M. Epidemiology and public health concerns of CKD in older adults. Adv. Chronic Kidney Dis. 2016, 23, 8–11. [Google Scholar] [CrossRef][Green Version]
- Sharief, S.; Hsu, C.Y. The transition from the pre-ESRD to ESRD phase of CKD: Much remains to be learned. Am. J. Kidney Dis. 2017, 69, 8–10. [Google Scholar] [CrossRef] [PubMed]
- Himmelfarb, J.; Vanholder, R.; Mehrotra, R.; Tonelli, M. The current and future landscape of dialysis. Nat. Rev. Nephrol. 2020, 16, 573–585. [Google Scholar] [CrossRef]
- Rodger, R.S. Approach to the management of end stage renal disease. Clin. Med. 2012, 12, 472–475. [Google Scholar] [CrossRef]
- Zhang, L.; Guo, Y.; Ming, H. Effects of hemodialysis, peritoneal dialysis, and renal transplantation on the quality of life of patients with end-stage renal disease. Rev. Assoc. Med. Bras. 2020, 66, 1229–1234. [Google Scholar] [CrossRef]
- Garcia, G.G.; Harden, P.; Chapman, J. The global role of kidney transplantation. Indian J. Nephrol. 2012, 22, 77–82. [Google Scholar] [CrossRef]
- Elshahat, S.; Cockwell, P.; Maxwell, A.P.; Griffin, M.; O’Brien, T.; O’Neill, C. The impact of chronic kidney disease on developed countries from a health economics perspective: A systematic scoping review. PLoS ONE 2020, 15, e0230512. [Google Scholar] [CrossRef]
- Johnson, D.S.; Meyer, K.B. Delaying and averting dialysis treatment: Patient protection or moral hazard? Am. J. Kidney Dis. 2018, 72, 251–254. [Google Scholar] [CrossRef]
- De Rosa, S.; Samoni, S.; Villa, G.; Ronco, C. Management of chronic kidney disease patients in the intensive care unit: Mixing acute and chronic illness. Blood Purif. 2017, 43, 151–162. [Google Scholar] [CrossRef]
- Thiery, A.; Séverac, F.; Hannedouche, T.; Couchoud, C.; Do, V.H.; Tiple, A.; Béchade, C.; Sauleau, E.A.; Krummel, T.; REIN Registry. Survival advantage of planned haemodialysis over peritoneal dialysis: A cohort study. Nephrol. Dial. Transplant. 2018, 33, 1411–1419. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.H.; Cai, G.Y.; Xiao, Y.F.; Chen, X.M. Risk factors for mortality in elderly haemodialysis patients: A systematic review and meta-analysis. BMC Nephrol. 2020, 21, 1–10. [Google Scholar] [CrossRef]
- Morfin, J.A.; Fluck, R.J.; Weinhandl, E.D.; Kansal, S.; McCullough, P.A.; Komenda, P. Intensive hemodialysis and treatment complications and tolerability. Am. J. Kidney Dis. 2016, 68, S43–S50. [Google Scholar] [CrossRef] [PubMed]
- Taheri, S.; Baradaran, A.; Aliakbarian, M.; Mortazavi, M. Level of inflammatory factors in chronic hemodialysis patients with and without cardiovascular disease. J. Res. Med. Sci. 2017, 22, 1–5. [Google Scholar]
- Chen, X.; Mao, G.; Leng, S.X. Frailty syndrome: An overview. Clin. Interv. Aging 2014, 9, 433–441. [Google Scholar]
- Zhang, Q.; Ma, Y.; Lin, F.; Zhao, J.; Xiong, J. Frailty and mortality among patients with chronic kidney disease and end-stage renal disease: A systematic review and meta-analysis. Int. Urol. Nephrol. 2020, 52, 363–370. [Google Scholar] [CrossRef]
- Lee, S.W.; Lee, A.; Yu, M.Y.; Kim, S.W.; Kim, K.I.; Na, K.Y.; Chae, D.W.; Kim, C.H.; Chin, H.J. Is frailty a modifiable risk factor of future adverse outcomes in elderly patients with incident end-stage renal disease? J. Korean Med. Sci. 2017, 32, 1800–1806. [Google Scholar] [CrossRef]
- Garcia-Canton, C.; Rodenas, A.; Lopez-Aperador, C.; Rivero, Y.; Anton, G.; Monzon, T.; Diaz, N.; Vega, N.; Loro, J.F.; Santana, A.; et al. Frailty in hemodialysis and prediction of poor short-term outcome: Mortality, hospitalization and visits to hospital emergency services. Ren. Fail. 2019, 41, 567–575. [Google Scholar] [CrossRef]
- Msaad, R.; Essadik, R.; Mohtadi, K.; Meftah, H.; Lebrazi, H.; Taki, H.; Kettani, A.; Madkouri, G.; Ramdani, B.; Saïle, R. Predictors of mortality in hemodialysis patients. Pan. Afr. Med. J. 2019, 33, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Sy, J.; Johansen, K.L. The impact of frailty on outcomes in dialysis. Curr. Opin. Nephrol. Hypertens. 2017, 26, 537–542. [Google Scholar] [CrossRef] [PubMed]
- Walker, S.R.; Gill, K.; Macdonald, K.; Komenda, P.; Rigatto, C.; Sood, M.M.; Bohm, C.J.; Storsley, L.J.; Tangri, N. Association of frailty and physical function in patients with non-dialysis CKD: A systematic review. BMC Nephrol. 2013, 14, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Shamseer, L.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A.; PRISMA Group. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst. Rev. 2015, 4, 1–9. [Google Scholar] [CrossRef]
- Haynes, R.B.; Sacket, D.L.; Guyatt, G.H.; Tugwell, P. Clinical Epidemiology: How Clinical Practice Research, 3rd ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2006; pp. 273–322. [Google Scholar]
- Higgins, J.P.T.; Thompson, S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002, 21, 1539–1558. [Google Scholar] [CrossRef]
- Higgins, J.P.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef]
- Egger, M.; Davey Smith, G.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef]
- Wells, G.A.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. Available online: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 20 June 2019).
- McAdams-DeMarco, M.A.; Law, A.; Salter, M.L.; Boyarsky, B.; Gimenez, L.; Jaar, B.G.; Walston, J.D.; Segev, D.L. Frailty as a novel predictor of mortality and hospitalization in individuals of all ages undergoing hemodialysis. J. Am. Geriatr. Soc. 2013, 61, 896–901. [Google Scholar] [CrossRef]
- Johansen, K.L.; Dalrymple, L.S.; Glidden, D.; Delgado, C.; Kaysen, G.A.; Grimes, B.; Chertow, G.M. Association of performance-based and self-reported function-based definitions of frailty with mortality among patients receiving hemodialysis. Clin. J. Am. Soc. Nephrol. 2016, 11, 626–632. [Google Scholar] [CrossRef]
- Yadla, M.; John, J.P.; Mummadi, M. A study of clinical assessment of frailty in patients on maintenance hemodialysis supported by cashless government scheme. Saudi J. Kidney Dis. Transpl. 2017, 28, 15–22. [Google Scholar] [CrossRef]
- Fitzpatrick, J.; Sozio, S.M.; Jaar, B.G.; Estrella, M.M.; Segev, D.L.; Parekh, R.S.; McAdams-DeMarco, M.A. Frailty, body composition and the risk of mortality in incident hemodialysis patients: The predictors of arrhythmic and cardiovascular risk in end stage renal disease study. Nephrol. Dial. Transplant. 2019, 34, 346–354. [Google Scholar] [CrossRef]
- Johansen, K.L.; Delgado, C.; Kaysen, G.A.; Chertow, G.M.; Chiang, J.; Dalrymple, L.S.; Segal, M.R.; Grimes, B.A. Frailty among patients receiving hemodialysis: Evolution of components and associations with mortality. J. Gerontol. A Biol. Sci. Med. Sci. 2019, 74, 380–386. [Google Scholar] [CrossRef]
- López-Montes, A.; Martínez-Villaescusa, M.; Pérez-Rodríguez, A.; Andrés-Monpeán, E.; Martínez-Díaz, M.; Masiá, J.; Giménez-Bachs, J.M.; Abizanda, P. Frailty, physical function and affective status in elderly patients on hemodialysis. Arch. Gerontol. Geriatr. 2020, 87, 1–7. [Google Scholar] [CrossRef]
- Mei, F.; Gao, Q.; Chen, F.; Zhao, L.; Shang, Y.; Hu, K.; Zhang, W.; Zhao, B.; Ma, B. Frailty as a predictor of negative health outcomes in chronic kidney disease: A systematic review and meta-analysis. J. Am. Med. Dir. Assoc. 2021, 22, 535–543.e7. [Google Scholar] [CrossRef]
- Moorthi, R.N.; Avin, K.G. Clinical relevance of sarcopenia in chronic kidney disease. Curr. Opin. Nephrol. Hypertens. 2017, 26, 219–228. [Google Scholar] [CrossRef]
- Carrero, J.J.; Stenvinkel, P.; Cuppari, L.; Ikizler, T.A.; Kalantar-Zadeh, K.; Kaysen, G.; Mitch, W.E.; Price, S.R.; Wanner, C.; Wang, A.Y.; et al. Etiology of the protein-energy wasting syndrome in chronic kidney disease: A consensus statement from the International Society of Renal Nutrition and Metabolism (ISRNM). J. Ren. Nutr. 2013, 23, 77–90. [Google Scholar] [CrossRef] [PubMed]
- Ballew, S.H.; Chen, Y.; Daya, N.R.; Godino, J.G.; Windham, B.G.; McAdams-DeMarco, M.; Coresh, J.; Selvin, E.; Grams, M.E. Frailty, kidney function, and polypharmacy: The Atherosclerosis Risk in Communities (ARIC) Study. Am. J. Kidney Dis. 2017, 69, 228–236. [Google Scholar] [CrossRef] [PubMed]
- Cobo, G.; Lindholm, B.; Stenvinkel, P. Chronic inflammation in end-stage renal disease and dialysis. Nephrol. Dial. Transplant. 2018, 33, 1–6. [Google Scholar] [CrossRef]
- Kooman, J.P.; Dekker, M.J.; Usvyat, L.A.; Kotanko, P.; van der Sande, F.M.; Schalkwijk, C.G.; Shiels, P.G.; Stenvinkel, P. Inflammation and premature aging in advanced chronic kidney disease. Am. J. Physiol. Renal. Physiol. 2017, 313, 938–950. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Liu, Q.; Ji, J. The prevalence of frailty in patients on hemodialysis: A systematic review and meta-analysis. Int. Urol. Nephrol. 2020, 52, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, R.; Peel, N.M.; Krosch, M.; Hubbard, R.E. Frailty and chronic kidney disease: A systematic review. Arch. Gerontol. Geriatr. 2017, 68, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Clegg, A.; Young, J.; Iliffe, S.; Rikkert, M.O.; Rockwood, K. Frailty in elderly people. Lancet 2013, 381, 752–762. [Google Scholar] [CrossRef]
- Rockwood, K.; Song, X.; MacKnight, C.; Bergman, H.; Hogan, D.B.; McDowell, I.; Mitnitski, A. A global clinical measure of fitness and frailty in elderly people. CMAJ 2005, 173, 489–495. [Google Scholar] [CrossRef]
- Rolfson, D.B.; Majumdar, S.R.; Tsuyuki, R.T.; Tahir, A.; Rockwood, K. Validity and reliability of the Edmonton frail scale. Age Ageing 2006, 35, 526–529. [Google Scholar] [CrossRef]
- Chao, C.T.; Hsu, Y.H.; Chang, P.Y.; He, Y.T.; Ueng, R.S.; Lai, C.F.; Chiang, C.K.; Huang, J.W.; Huang, S.J. Simple self-report FRAIL scale might be more closely associated with dialysis complications than other frailty screening instruments in rural chronic dialysis patients. Nephrology 2015, 20, 321–328. [Google Scholar] [CrossRef]
- Orlandi, F.S.; Gesualdo, G.D. Assessment of the frailty level of elderly people with chronic kidney disease undergoing hemodialysis. Acta Paul. Enferm. 2014, 27, 29–34. [Google Scholar] [CrossRef]
- Lee, S.Y.; Yang, D.H.; Hwang, E.; Kang, S.H.; Park, S.H.; Kim, T.W.; Lee, D.H.; Park, K.; Kim, J.C. The Prevalence, association, and clinical outcomes of frailty in maintenance dialysis patients. J. Ren. Nutr. 2017, 27, 106–112. [Google Scholar] [CrossRef]
- Takeuchi, H.; Uchida, H.A.; Kakio, Y.; Okuyama, Y.; Okuyama, M.; Umebayashi, R.; Wada, K.; Sugiyama, H.; Sugimoto, K.; Rakugi, H.; et al. The prevalence of frailty and its associated factors in Japanese hemodialysis patients. Aging Dis. 2018, 9, 192–207. [Google Scholar] [CrossRef]
- Nitta, K.; Hanafusa, N.; Tsuchiya, K. Frailty and mortality among dialysis patients. Ren. Replace Ther. 2017, 3, 1–6. [Google Scholar] [CrossRef]
- Nitta, K.; Tsuchiya, K. Recent advances in the pathophysiology and management of protein-energy wasting in chronic kidney disease. Ren. Replace Ther. 2016, 2, 1–12. [Google Scholar] [CrossRef]
- Gordon, E.H.; Peel, N.M.; Samanta, M.; Theou, O.; Howlett, S.E.; Hubbard, R.E. Sex differences in frailty: A systematic review and meta-analysis. Exp. Gerontol. 2017, 89, 30–40. [Google Scholar] [CrossRef] [PubMed]
- Varadhan, R.; Chaves, P.H.; Lipsitz, L.A.; Stein, P.K.; Tian, J.; Windham, B.G.; Berger, R.D.; Fried, L.P. Frailty and impaired cardiac autonomic control: New insights from principal components aggregation of traditional heart rate variability indices. J. Gerontol. A Biol. Sci. Med. Sci. 2009, 64, 682–687. [Google Scholar] [CrossRef] [PubMed]
- Hyde, Z.; Flicker, L.; Almeida, O.P.; Hankey, G.J.; McCaul, K.A.; Chubb, S.A.; Yeap, B.B. Low free testosterone predicts frailty in older men: The health in men study. J. Clin. Endocrinol. Metab. 2010, 95, 3165–3172. [Google Scholar] [CrossRef]
- Walker, S.R.; Wagner, M.; Tangri, N. Chronic kidney disease, frailty, and unsuccessful aging: A review. J. Ren. Nutr. 2014, 24, 364–370. [Google Scholar] [CrossRef] [PubMed]
- Kakio, Y.; Uchida, H.A.; Takeuchi, H.; Okuyama, Y.; Okuyama, M.; Umebayashi, R.; Wada, K.; Sugiyama, H.; Sugimoto, K.; Rakugi, H.; et al. Diabetic nephropathy is associated with frailty in patients with chronic hemodialysis. Geriatr. Gerontol. Int. 2018, 18, 1597–1602. [Google Scholar] [CrossRef]
- Angulo, J.; El Assar, M.; Rodríguez-Mañas, L. Frailty and sarcopenia as the basis for the phenotypic manifestation of chronic diseases in older adults. Mol. Asp. Med. 2016, 50, 1–32. [Google Scholar] [CrossRef]
- Yanase, T.; Yanagita, I.; Muta, K.; Nawata, H. Frailty in elderly diabetes patients. Endocr. J. 2018, 65, 1–11. [Google Scholar] [CrossRef]
- Cheng, H.T.; Xu, X.; Lim, P.S.; Hung, K.Y. Worldwide epidemiology of diabetes-related end-stage renal disease, 2000–2015. Diabetes Care 2021, 44, 89–97. [Google Scholar] [CrossRef]
- Prasad, N.; Jha, V. Hemodialysis in Asia. Kidney Dis. 2015, 1, 165–177. [Google Scholar] [CrossRef]
| Author (Year)/ Country | Study Design | Follow- Up Period (Months) | Frailty Scale | Definition of Frailty | Sample Characteristics | Prevalence of Frailty (%) | Outcome | Adjustments for Covariates | Quality Score | |
|---|---|---|---|---|---|---|---|---|---|---|
| Frail | Non-Frail | |||||||||
| McAdams-DeMarco et al. (2013)/USA [30] | Prospective | 36 | Fried phenotype | Components ≥3 | n = 61 Mean age: 62.9 ± 12.9 (years) Male: 28 (45.9%) | n = 85 Mean age: 58.6 ± 13.5 (years) Male: 50 (58.8%) | 41.8 | All-cause mortality | Age, sex, comorbidity, disability | 8 |
| Johansen et al. (2016)/USA [31] | Prospective | 20 | Fried phenotype | Components ≥3 | n = 240 Mean age: 63.5 ± 13.1 (years) Male: 130 (54.2%) | n = 522 Mean age: 54.1 ± 14.4 (years) Male: 322 (61.7%) | 31.0 | All-cause mortality | Age, sex, race, BMI, diabetes, heart failure, coronary artery disease, serum albumin, CRP, dialysis via a catheter | 8 |
| Yadla et al. (2017)/India [32] | Prospective | 12 | Fried phenotype | Components ≥3 | n = 167 Mean age: 50.0 ± 13.3 (years) Male: 112 (67.1%) | n = 38 Mean age: 50.0 ± 13.3 (years) Male: 30 (78.9%) | 81.5 | All-cause mortality | Not adjusted | 8 |
| Fitzpatrick et al. (2019)/USA [33] | Prospective | Unreported | Fried phenotype | Components ≥3 | n = 193 Mean age: 57.2 ± 13.5 (years) Male: 112 (58.0%) | n = 177 Mean age: 52.3 ± 12.1 (years) Male: 104 (58.8%) | 52.0 | All-cause mortality | Age, sex, race, CCI, serum albumin | 7 |
| Garcia-Canton et al. (2019)/Spain [20] | Prospective | 29 | Edmonton Frail Scale | Scoring ≥8 | n = 82 Median age: 71.0 (years) Male: 42 (52.4%) | n = 195 Median age: 63.5 (years) Male: 139 (71.3%) | 29.6 | All-cause mortality | CCI | 8 |
| Johansen et al. (2019)/USA [34] | Prospective | 24 | Fried phenotype | Components ≥3 | n = 230 Mean age: 62.9 ± 12.2 (years) Male: 93 (40.4%) | n = 497 Mean age: 54.6 ± 13.6 (years) Male: 306 (61.6%) | 31.6 | All-cause mortality | Age, sex, race, ethnicity, BMI, diabetes, atherosclerotic heart disease, heart failure, dialysis via a catheter, serum albumin concentration | 8 |
| López-Montes et al. (2020)/Spain [35] | Retrospective | 12 | Fried phenotype | Components ≥3 | n = 63 Mean age: 78.6 ± 3.8 (years) Male: 29 (46.0%) | n = 54 Mean age: 77.4 ± 4.3 (years) Male: 45 (83.3%) | 53.8 | All-cause mortality | Age, sex, BMI, CCI | 8 |
| Risk Factors | No. of Studies | No. of Participants | OR/SMD | 95% CI | I2 (%) | p-Value | Egger’s Test p |
|---|---|---|---|---|---|---|---|
| Demographic characteristics | |||||||
| Age (years) | 6 | 1787 | 0.43 * | 0.24−0.61 | 72 | 0.003 | 0.018 |
| Sex (female) | 7 | 2604 | 1.89 | 1.33 −2.67 | 71 | 0.002 | 0.395 |
| Smoking, yes | 3 | 721 | 1.39 | 0.58−3.32 | 80 | 0.005 | 0.186 |
| Comorbidities | |||||||
| Diabetes mellitus, yes | 7 | 2604 | 2.42 | 1.68−3.49 | 73 | 0.001 | 0.108 |
| Hypertension, yes | 3 | 721 | 2.16 | 0.46−10.04 | 82 | 0.003 | 0.472 |
| CAD, yes | 3 | 1249 | 0.96 | 0.63−1.46 | 57 | 0.098 | 0.668 |
| PVD, yes | 5 | 1600 | 1.87 | 0.81−4.29 | 68 | <0.001 | 0.240 |
| HF, yes | 4 | 1483 | 1.35 | 0.92−2.00 | 57 | 0.070 | 0.588 |
| CVA or TIA, yes | 4 | 1454 | 1.96 | 0.93−4.17 | 73 | 0.011 | 0.161 |
| COPD, yes | 3 | 633 | 1.43 | 0.98−2.09 | 0 | 0.835 | 0.532 |
| Cancer, yes | 2 | 516 | 1.35 | 0.48−3.84 | 68 | 0.077 | NA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, H.-J.; Son, Y.-J. Prevalence and Associated Factors of Frailty and Mortality in Patients with End-Stage Renal Disease Undergoing Hemodialysis: A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2021, 18, 3471. https://doi.org/10.3390/ijerph18073471
Lee H-J, Son Y-J. Prevalence and Associated Factors of Frailty and Mortality in Patients with End-Stage Renal Disease Undergoing Hemodialysis: A Systematic Review and Meta-Analysis. International Journal of Environmental Research and Public Health. 2021; 18(7):3471. https://doi.org/10.3390/ijerph18073471
Chicago/Turabian StyleLee, Hyeon-Ju, and Youn-Jung Son. 2021. "Prevalence and Associated Factors of Frailty and Mortality in Patients with End-Stage Renal Disease Undergoing Hemodialysis: A Systematic Review and Meta-Analysis" International Journal of Environmental Research and Public Health 18, no. 7: 3471. https://doi.org/10.3390/ijerph18073471
APA StyleLee, H.-J., & Son, Y.-J. (2021). Prevalence and Associated Factors of Frailty and Mortality in Patients with End-Stage Renal Disease Undergoing Hemodialysis: A Systematic Review and Meta-Analysis. International Journal of Environmental Research and Public Health, 18(7), 3471. https://doi.org/10.3390/ijerph18073471





