Influence of ACE Gene I/D Polymorphism on Cardiometabolic Risk, Maximal Fat Oxidation, Cardiorespiratory Fitness, Diet and Physical Activity in Young Adults
Abstract
1. Introduction
2. Materials and Methods
2.1. Design
2.2. Subjects
2.3. Procedure
2.4. Physical Activity
2.5. Dietary Assessment
2.6. Heart Rate and Blood Pressure Measurements
2.7. Blood Extraction
2.8. Genomic Typing of the ACE Gene I/D Polymorphism
2.9. Plasma Biochemical Parameters
2.10. Anthropometry and Body Composition
2.11. Basal Metabolism
2.12. Maximal Fat Oxidation (MFO) and Cardiorespiratory Fitness (VO2max)
2.13. Statistical Analysis
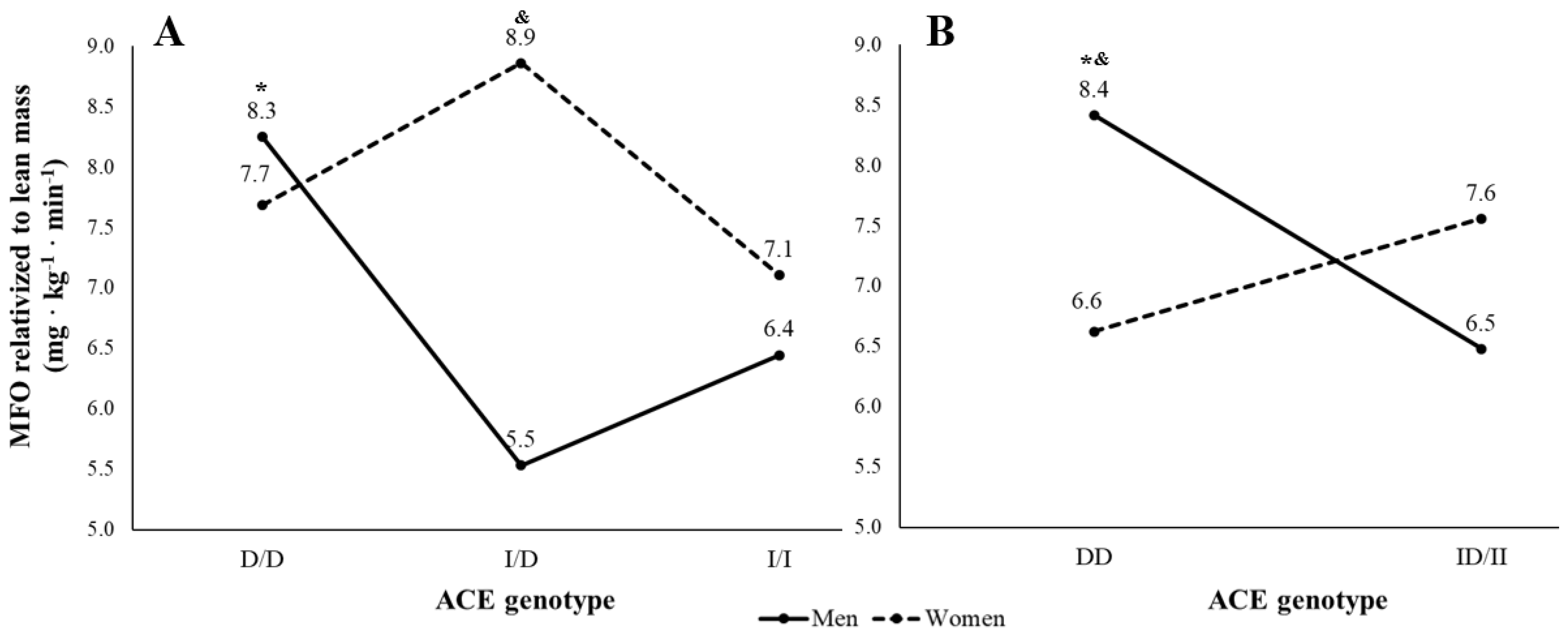
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tikkanen, E.; Gustafsson, S.; Ingelsson, E. Associations of Fitness, Physical Activity, Strength, and Genetic Risk with Cardiovascular Disease: Longitudinal Analyses in the UK Biobank Study. Circulation 2018, 137, 2583–2591. [Google Scholar] [CrossRef]
- Roth, G.A.; Mensah, G.A.; Johnson, C.O.; Addolorato, G.; Ammirati, E.; Baddour, L.M.; Barengo, N.C.; Beaton, A.Z.; Bemjamin, E.J.; Benziger, C.P.; et al. Global Burden of Cardiovascular Diseases and Risk Factors, 1990–2019: Update From the GBD 2019 Study. J. Am. Coll. Cardiol. 2020, 76, 2982–3021. [Google Scholar] [CrossRef] [PubMed]
- Aleksandrova, K.; Mozaffarian, D.; Pischon, T. Addressing the Perfect Storm: Biomarkers in Obesity and Pathophysiology of Cardiometabolic Risk. Clin. Chem. 2018, 64, 142–153. [Google Scholar] [CrossRef]
- Sabbatini, A.R.; Kararigas, G. Estrogen-Related Mechanisms in Sex Differences of Hypertension and Target Organ Damage. Biol. Sex Differ. 2020, 11, 31. [Google Scholar] [CrossRef] [PubMed]
- Dai, S.; Ding, M.; Liang, N.; Li, Z.; Li, D.; Guan, L.; Liu, H. Associations of ACE I/D Polymorphism with the Levels of ACE, Kallikrein, Angiotensin II and Interleukin-6 in STEMI Patients. Sci. Rep. 2019, 9, 19719. [Google Scholar] [CrossRef]
- Dai, S.H.; Li, J.F.; Feng, J.B.; Li, R.J.; Li, C.B.; Li, Z.; Zhang, Y.; Li, D.Q. Association of Serum Levels of AngII, KLK1, and ACE/KLK1 Polymorphisms with Acute Myocardial Infarction Induced by Coronary Artery Stenosis. JRAAS J. Renin Angiotensin Aldosterone Syst. 2016, 17, 1470320316655037. [Google Scholar] [CrossRef]
- Rigat, B.; Hubert, C.; Alhenc-Gelas, F.; Cambien, F.; Corvol, P.; Soubrier, F. An Insertion/Deletion Polymorphism in the Angiotensin I-Converting Enzyme Gene Accounting for Half the Variance of Serum Enzyme Levels. J. Clin. Investig. 1990, 86, 1343–1346. [Google Scholar] [CrossRef]
- Chen, G.; Hu, S.; Lai, Z.; Qiu, B. Association between ACE Gene I/D Polymorphism and Knee Osteoarthritis in a Chinese Population. Biosci. Rep. 2019, 39. [Google Scholar] [CrossRef]
- Ma, Y.; Tong, X.; Liu, Y.; Liu, S.; Xiong, H.; Fan, H. ACE Gene Polymorphism Is Associated with COPD and COPD with Pulmonary Hypertension: A Meta-Analysis. Int. J. Chron. Obstruct. Pulmon. Dis. 2018, 13, 2435–2446. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Meng, L.; Zhou, Y.; Lu, N. Genetic Polymorphism of Angiotensin-Converting Enzyme and Hypertrophic Cardiomyopathy Risk. Medicine 2017, 96. [Google Scholar] [CrossRef]
- Bueno, S.; Pasqua, L.A.; De Araújo, G.; Lima-Silva, A.E.; Bertuzzi, R. The Association of ACE Genotypes on Cardiorespiratory Variables Related to Physical Fitness in Healthy Men. PLoS ONE 2016, 11, e0165310. [Google Scholar] [CrossRef] [PubMed]
- Falahati, A.; Arazi, H. Association of ACE Gene Polymorphism with Cardiovascular Determinants of Trained and Untrained Iranian Men. Genes Environ. 2019, 41, 8. [Google Scholar] [CrossRef] [PubMed]
- Goodpaster, B.H.; Sparks, L.M. Metabolic Flexibility in Health and Disease. Cell Metab. 2017, 25, 1027–1036. [Google Scholar] [CrossRef]
- Montes-de-Oca-García, A.; Perez-Bey, A.; Corral-Pérez, J.; Velázquez-Díaz, D.; Opazo-Díaz, E.; Fernandez-Santos, J.R.; Rebollo-Ramos, M.; Amaro-Gahete, F.J.; Cuenca-García, M.; Ponce-González, J.G. Maximal Fat Oxidation Capacity Is Associated with Cardiometabolic Risk Factors in Healthy Young Adults. Eur. J. Sport Sci. 2020, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Hamada, T.; Kotani, K.; Nagai, N.; Tsuzaki, K.; Sano, Y.; Matsuoka, Y.; Fujibayashi, M.; Kiyohara, N.; Tanaka, S.; Yoshimura, M.; et al. Genetic Polymorphisms of the Renin-Angiotensin System and Obesity-Related Metabolic Changes in Response to Low-Energy Diets in Obese Women. Nutrition 2011, 27, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Fajar, J.K.; Pikir, B.S.; Sidarta, E.P.; Berlinda Saka, P.N.; Akbar, R.R.; Heriansyah, T. The Gene Polymorphism of Angiotensin-Converting Enzyme Intron Deletion and Angiotensin-Converting Enzyme G2350A in Patients With Left Ventricular Hypertrophy: A Meta-Analysis. Indian Heart J. 2019, 71, 199–206. [Google Scholar] [CrossRef]
- Rebollo-Ramos, M.; Velázquez-Díaz, D.; Corral-Pérez, J.; Barany-Ruiz, A.; Pérez-Bey, A.; Fernández-Ponce, C.; García-Cózar, F.J.; Ponce-González, J.G.; Cuenca-García, M. Aerobic Fitness, Mediterranean Diet and Cardiometabolic Risk Factors in Adults. Endocrinol. Diabetes y Nutr. 2019. [Google Scholar] [CrossRef]
- García-Conesa, M.T.; Philippou, E.; Pafilas, C.; Massaro, M.; Quarta, S.; Andrade, V.; Jorge, R.; Chervenkov, M.; Ivanova, T.; Dimitrova, D.; et al. Exploring the Validity of the 14-Item Mediterranean Diet Adherence Screener (Medas): A Cross-National Study in Seven European Countries around the Mediterranean Region. Nutrients 2020, 12, 2960. [Google Scholar] [CrossRef]
- Michaelides, A.P.; Liakos, C.I.; Vyssoulis, G.P.; Chatzistamatiou, E.I.; Markou, M.I.; Tzamou, V.; Stefanadis, C.I. The Interplay of Exercise Heart Rate and Blood Pressure as a Predictor of Coronary Artery Disease and Arterial Hypertension. J. Clin. Hypertens. 2013, 15, 162–170. [Google Scholar] [CrossRef]
- Rigat, B.; Hubert, C.; Corvol, P.; Soubrier, R. PCR Detection of the Insertion/Deletion Polymorphism of the Human Angiotensin Converting Enzyme Gene (DCP1) (Dipeptidyl Carboxypeptidase 1). Nucleic Acids Res. 1992, 20, 1433. [Google Scholar] [CrossRef]
- Odawara, M.; Matsunuma, A.; Yamashita, K. Mistyping Frequency of the Angiotensin-Converting Enzyme Gene Polymorphism and an Improved Method for Its Avoidance. Hum. Genet. 1997, 100, 163–166. [Google Scholar] [CrossRef]
- Frayn, K.N. Calculation of Substrate Oxidation Rates in Vivo from Gaseous Exchange. J. Appl. Physiol. 1983, 55, 628–634. [Google Scholar] [CrossRef] [PubMed]
- Achten, J.; Gleeson, M.; Jeukendrup, A. Determination of the Exercise Intensity That Elicits Maximal Fat Oxidation Shifts in Energy Substrate Mobilization and Utilization. Med. Sci. Sport. Exerc. 2002, 34, 92–97. [Google Scholar] [CrossRef]
- Mittal, G.; Gupta, V.; Haque, S.F.; Khan, A.S. Effect of Angiotensin Converting Enzyme Gene I/D Polymorphism in Patients with Metabolic Syndrome in North Indian Population. Chin. Med. J. Engl. 2011, 124, 45–48. [Google Scholar] [CrossRef] [PubMed]
- Oegowska, K.; Bogacz, A.; Bartkowiak-Wieczorek, J.; Seremak-Mrozikiewicz, A.; Pawelczyk, L. Association between the Angiotensin Converting Enzyme Gene Insertion/Deletion Polymorphism and Metabolic Disturbances in Women with Polycystic Ovary Syndrome. Mol. Med. Rep. 2016, 14, 5401–5407. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ned, R.M.; Yesupriya, A.; Imperatore, G.; Smelser, D.T.; Moonesinghe, R.; Chang, M.H.; Dowling, N.F. The ACE I/D Polymorphism in US Adults: Limited Evidence of Association with Hypertension-Related Traits and Sex-Specific Effects by Race/Ethnicity. Am. J. Hypertens. 2012, 25, 209–215. [Google Scholar] [CrossRef]
- Wilson, G.C.; Mavros, Y.; Tajouri, L.; Singh, M.F. The Role of Genetic Profile in Functional Performance Adaptations to Exercise Training or Physical Activity: A Systematic Review of the Literature. J. Aging Phys. Act. 2019, 27, 594–616. [Google Scholar] [CrossRef] [PubMed]
- Ponce-González, J.G.; Rodríguez-Garcia, L.; Losa-Reyna, J.; Guadalupe-Grau, A.; Rodriguez-Gonzalez, F.G.; Díaz-Chico, B.N.; Dorado, C.; Serrano-Sanchez, J.A.; Calbet, J.A.L. Androgen Receptor Gene Polymorphism Influence Fat Accumulation: A Longitudinal Study from Adolescence to Adult Age. Scand. J. Med. Sci. Sport. 2016, 26, 1313–1320. [Google Scholar] [CrossRef]
- Brosnihan, K.B.; Li, P.; Ganten, D.; Ferrario, C.M. Estrogen Protects Transgenic Hypertensive Rats by Shifting the Vasoconstrictor-Vasodilator Balance of RAS. Am. J. Physiol. 1997, 273, R1908-15. [Google Scholar] [CrossRef] [PubMed]
- Haynes, M.P.; Russell, K.S.; Bender, J.R. Molecular Mechanisms of Estrogen Actions on the Vasculature. J. Nucl. Cardiol. Off. Publ. Am. Soc. Nucl. Cardiol. 2000, 7, 500–508. [Google Scholar] [CrossRef]
- Gupte, M.; Thatcher, S.E.; Boustany-Kari, C.M.; Shoemaker, R.; Yiannikouris, F.; Zhang, X.; Karounos, M.; Cassis, L.A. Angiotensin Converting Enzyme 2 Contributes to Sex Differences in the Development of Obesity Hypertension in C57BL/6 Mice. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 1392–1399. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Engel, A.; Morcillo, L.; Aranda, F.J.; Ruiz, M.; Gaitan, M.J.; Mayor-Olea, A.; Aranda, P.; Ferrario, C.M. Influence of Gender and Genetic Variability on Plasma Angiotensin Peptides. J. Renin. Angiotensin. Aldosterone. Syst. 2006, 7, 92–97. [Google Scholar] [CrossRef] [PubMed]
- Tobina, T.; Michishita, R.; Yamasawa, F.; Zhang, B.; Sasaki, H.; Tanaka, H.; Saku, K.; Kiyonaga, A. Association between the Angiotensin I-Converting Enzyme Gene Insertion/Deletion Polymorphism and Endurance Running Speed in Japanese Runners. J. Physiol. Sci. 2010, 60, 325–330. [Google Scholar] [CrossRef] [PubMed]
- Goleva-Fjellet, S.; Bjurholt, A.M.; Kure, E.H.; Larsen, I.K.; Støren, Ø.; Sæbø, M. Distribution of Allele Frequencies for Genes Associated with Physical Activity and/or Physical Capacity in a Homogenous Norwegian Cohort- a Cross-Sectional Study. BMC Genet. 2020, 21, 8. [Google Scholar] [CrossRef]
- Wang, G.; Tanaka, M.; Eynon, N.; North, K.N.; Williams, A.G.; Collins, M.; Moran, C.N.; Britton, S.L.; Fuku, N.; Ashley, E.A.; et al. The Future of Genomic Research in Athletic Performance and Adaptation to Training. Med. Sport Sci. 2016, 61, 55–67. [Google Scholar] [CrossRef]
- Georgiades, E.; Klissouras, V.; Baulch, J.; Wang, G.; Pitsiladis, Y. Why Nature Prevails over Nurture in the Making of the Elite Athlete. BMC Genom. 2017, 18 (Suppl. 8), 835. [Google Scholar] [CrossRef] [PubMed]
| Total | Men | Women | p | d | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Age (years) | 22.46 | ± | 4.21 | 22.23 | ± | 3.59 | 22.88 | ± | 5.21 | 0.526 | −0.15 |
| Height (cm) | 172.06 | ± | 8.85 | 176.68 | ± | 6.32 | 163.52 | ± | 6.07 | <0.001 | 2.11 |
| Body Mass (kg) | 74.97 | ± | 15.74 | 77.84 | ± | 14.43 | 69.69 | ± | 16.95 | 0.033 | 0.53 |
| Lean body mass (kg) | 54.38 | ± | 8.92 | 59.26 | ± | 6.41 | 45.37 | ± | 5.00 | <0.001 | 2.35 |
| Body fat (%) | 21.97 | ± | 9.34 | 18.28 | ± | 7.08 | 28.77 | ± | 9.30 | <0.001 | −1.31 |
| Body Mass Index (kg · m−2) | 25.36 | ± | 5.48 | 24.87 | ± | 3.87 | 26.28 | ± | 7.64 | 0.292 | −0.25 |
| Waist circumference (cm) | 82.27 | ± | 14.33 | 83.73 | ± | 12.19 | 79.92 | ± | 17.24 | 0.289 | 0.27 |
| Total MVPA (min/week) | 501.96 | ± | 166.99 | 480.95 | ± | 146.25 | 539.14 | ± | 196.08 | 0.157 | −0.35 |
| Energy balance (kcal/day) | 280.26 | ± | 1008.83 | 303.99 | ± | 1164.61 | 236.45 | ± | 647.19 | 0.785 | 0.07 |
| MD adherence (0–14 range) | 6.99 | ± | 1.78 | 7.21 | ± | 1.92 | 6.58 | ± | 1.45 | 0.146 | 0.36 |
| Systolic Blood Pressure (mmHg) | 114.20 | ± | 10.26 | 116.70 | ± | 7.88 | 109.52 | ± | 12.56 | 0.004 | 0.73 |
| Diastolic Blood Pressure (mmHg) | 68.46 | ± | 9.88 | 66.96 | ± | 9.00 | 71.27 | ± | 10.98 | 0.078 | −0.44 |
| Blood pressure recovery (ratio) | 0.82 | ± | 0.30 | 0.79 | ± | 0.34 | 0.88 | ± | 0.19 | 0.242 | −0.29 |
| Plasma glucose (mg/dL) | 101.76 | ± | 10.02 | 103.90 | ± | 9.55 | 97.83 | ± | 9.84 | 0.016 | 0.63 |
| Plasma triglycerides (mg/dL) | 69.06 | ± | 24.83 | 70.28 | ± | 24.52 | 66.84 | ± | 25.77 | 0.589 | 0.14 |
| Tumor necrosis factor-α (pg/mL) | 8467.15 | ± | 7574.43 | 9934.35 | ± | 8443.06 | 5960.69 | ± | 5027.59 | 0.040 | 0.54 |
| Interleukin-6 (pg/mL) | 473.44 | ± | 338.41 | 504.84 | ± | 370.25 | 408.79 | ± | 259.09 | 0.342 | 0.29 |
| BM VO2max (mL · kg−1 · min−1) | 42.10 | ± | 11.49 | 46.07 | ± | 10.11 | 34.93 | ± | 10.42 | <0.001 | 1.09 |
| LM VO2max (mL · kg−1 · min−1) | 18.90 | ± | 4.54 | 18.80 | ± | 4.84 | 19.09 | ± | 4.00 | 0.789 | −0.07 |
| Absolute MFO (g · min−1) | 0.38 | ± | 0.15 | 0.40 | ± | 0.17 | 0.32 | ± | 0.11 | 0.029 | 0.55 |
| R-MFO (mg ·kg−1 · min−1) | 7.03 | ± | 2.86 | 6.95 | ± | 3.05 | 7.16 | ± | 2.57 | 0.768 | −0.07 |
| Clustered CMR (Z-score) | 0.32 | ± | 3.29 | 0.50 | ± | 3.15 | 0.04 | ± | 3.56 | 0.605 | 0.14 |
| INFLAM-Clustered CMR (Z-score) | −0.03 | ± | 3.14 | 0.10 | ± | 3.39 | −0.24 | ± | 2.79 | 0.732 | 0.11 |
| N | 74 | 46 | 28 | ||||||||
| DD | ID | II | p | η2 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of men/women | 11/12 | 23/10 | 12/6 | - | - | |||||||
| Age (years) | 23.57 | ± | 5.29 | 22.33 | ± | 4.29 | 21.83 | ± | 2.07 | 0.390 | 0.03 | |
| Height (cm) | 170.67 | ± | 10.32 | 173.09 | ± | 8.31 | 172.34 | ± | 7.12 | 0.593 | 0.01 | |
| Body Mass (kg) | 79.71 | ± | 17.44 | 76.94 | ± | 16.31 | 70.77 | ± | 11.81 | 0.194 | 0.05 | |
| Lean body mass (kg) | 53.66 | ± | 8.20 | 55.37 | ± | 9.80 | 53.51 | ± | 7.90 | 0.694 | 0.01 | |
| Body fat (%) | 27.29 | ± | 10.82 | 22.55 | ± | 9.72 | 19.31 | ± | 7.13 | 0.030 | * | 0.09 |
| Body Mass Index (kg · m−2) | 27.64 | ± | 7.38 | 25.63 | ± | 4.89 | 23.78 | ± | 3.33 | 0.088 | 0.07 | |
| Waist circumference (cm) | 86.78 | ± | 16.89 | 84.01 | ± | 14.81 | 78.26 | ± | 9.77 | 0.223 | 0.05 | |
| Total MVPA (min/week) | 501.55 | ± | 171.02 | 469.59 | ± | 175.24 | 562.75 | ± | 131.27 | 0.164 | 0.05 | |
| Energy balance (kcal/day) | 159.89 | ± | 854.92 | 529.75 | ± | 1290.38 | 9.12 | ± | 652.53 | 0.187 | 0.05 | |
| MD adherence (0–14 range) | 6.83 | ± | 1.75 | 6.76 | ± | 1.66 | 7.41 | ± | 2.00 | 0.442 | 0.02 | |
| Systolic Blood Pressure (mmHg) | 116.38 | ± | 11.84 | 116.72 | ± | 6.25 | 109.44 | ± | 12.32 | 0.035 | *& | 0.09 |
| Diastolic Blood Pressure (mmHg) | 70.68 | ± | 11.12 | 68.89 | ± | 9.26 | 66.85 | ± | 8.87 | 0.466 | 0.02 | |
| Blood pressure recovery (ratio) | 0.88 | ± | 0.20 | 0.83 | ± | 0.27 | 0.83 | ± | 0.31 | 0.731 | 0.01 | |
| Plasma glucose (mg/dL) | 98.68 | ± | 11.61 | 101.56 | ± | 9.57 | 103.00 | ± | 9.25 | 0.376 | 0.03 | |
| Plasma triglycerides (mg/dL) | 67.20 | ± | 23.09 | 71.36 | ± | 26.29 | 70.54 | ± | 24.94 | 0.822 | 0.01 | |
| Tumor necrosis factor-α (pg/mL) | 8691.86 | ± | 9769.21 | 6489.78 | ± | 5639.46 | 11,644.02 | ± | 6755.80 | 0.083 | 0.07 | |
| Interleukin-6 (pg/mL) | 609.51 | ± | 495.12 | 422.08 | ± | 292.73 | 428.72 | ± | 165.75 | 0.198 | 0.06 | |
| BM VO2max (mL · kg−1 · min−1) | 37.30 | ± | 13.15 | 42.25 | ± | 11.93 | 43.46 | ± | 9.40 | 0.189 | 0.05 | |
| LM VO2max (mL · kg−1 · min−1) | 18.05 | ± | 4.04 | 18.57 | ± | 5.27 | 19.47 | ± | 3.73 | 0.617 | 0.01 | |
| Absolute MFO (g · min−1) | 0.40 | ± | 0.17 | 0.35 | ± | 0.15 | 0.38 | ± | 0.16 | 0.493 | 0.02 | |
| R-MFO (mg ·kg−1 · min−1) | 7.48 | ± | 2.80 | 6.66 | ± | 3.04 | 7.11 | ± | 2.66 | 0.582 | 0.02 | |
| Clustered CMR (Z-score) | 0.89 | ± | 3.64 | 0.73 | ± | 3.47 | −0.65 | ± | 2.09 | 0.323 | 0.03 | |
| INFLAM-Clustered CMR (Z-score) | 1.35 | ± | 3.01 | −0.28 | ± | 3.54 | −0.74 | ± | 1.80 | 0.172 | 0.08 | |
| N | 23 | 33 | 18 | |||||||||
| DD | ID/II | p | d | |||||
|---|---|---|---|---|---|---|---|---|
| Number of men/women | 11/12 | 35/16 | - | - | ||||
| Age (years) | 23.57 | ± | 5.29 | 22.16 | ± | 3.65 | 0.188 | 0.33 |
| Height (cm) | 170.67 | ± | 10.32 | 172.83 | ± | 7.85 | 0.326 | −0.25 |
| Body Mass (kg) | 79.71 | ± | 17.44 | 74.76 | ± | 15.05 | 0.217 | 0.31 |
| Lean body mass (kg) | 53.66 | ± | 8.20 | 54.71 | ± | 9.13 | 0.638 | −0.12 |
| Body fat (%) | 27.29 | ± | 10.82 | 21.41 | ± | 8.96 | 0.017 | 0.61 |
| Body Mass Index (kg · m−2) | 27.64 | ± | 7.38 | 24.98 | ± | 4.46 | 0.058 | 0.48 |
| Waist circumference (cm) | 86.78 | ± | 16.89 | 82.13 | ± | 13.54 | 0.226 | 0.32 |
| Total MVPA (min/week) | 501.55 | ± | 171.02 | 503.13 | ± | 165.67 | 0.971 | −0.01 |
| Energy balance (kcal/day) | 159.89 | ± | 854.92 | 346.00 | ± | 1128.52 | 0.484 | −0.18 |
| MD adherence (0–14 range) | 6.83 | ± | 1.75 | 6.98 | ± | 1.79 | 0.732 | −0.09 |
| Systolic Blood Pressure (mmHg) | 116.38 | ± | 11.84 | 114.10 | ± | 9.47 | 0.382 | 0.22 |
| Diastolic Blood Pressure (mmHg) | 70.68 | ± | 11.12 | 68.15 | ± | 9.09 | 0.308 | 0.26 |
| Blood pressure recovery (ratio) | 0.88 | ± | 0.20 | 0.83 | ± | 0.28 | 0.427 | 0.20 |
| Plasma glucose (mg/dL) | 98.68 | ± | 11.61 | 102.07 | ± | 9.39 | 0.187 | −0.33 |
| Plasma triglycerides (mg/dL) | 67.20 | ± | 23.09 | 71.07 | ± | 25.57 | 0.537 | −0.16 |
| Tumor necrosis factor-α (pg/mL) | 8691.86 | ± | 9769.21 | 8207.86 | ± | 6447.65 | 0.804 | 0.06 |
| Interleukin-6 (pg/mL) | 609.51 | ± | 495.12 | 424.63 | ± | 249.00 | 0.071 | 0.54 |
| BM VO2max (mL · kg−1 · min−1) | 37.30 | ± | 13.15 | 42.69 | ± | 11.01 | 0.072 | −0.46 |
| LM VO2max (mL · kg−1 · min−1) | 18.05 | ± | 4.04 | 18.89 | ± | 4.76 | 0.468 | −0.18 |
| Absolute MFO (g · min−1) | 0.40 | ± | 0.17 | 0.36 | ± | 0.15 | 0.337 | 0.24 |
| R-MFO (mg ·kg−1 · min−1) | 7.48 | ± | 2.80 | 6.83 | ± | 2.89 | 0.367 | 0.32 |
| Clustered CMR (Z-score) | 0.89 | ± | 3.64 | 0.27 | ± | 3.12 | 0.469 | 0.19 |
| INFLAM-Clustered CMR (Z-score) | 1.35 | ± | 3.01 | −0.43 | ± | 3.03 | 0.066 | 0.59 |
| N | 23 | 51 | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Montes-de-Oca-García, A.; Perez-Bey, A.; Velázquez-Díaz, D.; Corral-Pérez, J.; Opazo-Díaz, E.; Rebollo-Ramos, M.; Gómez-Gallego, F.; Cuenca-García, M.; Casals, C.; Ponce-González, J.G. Influence of ACE Gene I/D Polymorphism on Cardiometabolic Risk, Maximal Fat Oxidation, Cardiorespiratory Fitness, Diet and Physical Activity in Young Adults. Int. J. Environ. Res. Public Health 2021, 18, 3443. https://doi.org/10.3390/ijerph18073443
Montes-de-Oca-García A, Perez-Bey A, Velázquez-Díaz D, Corral-Pérez J, Opazo-Díaz E, Rebollo-Ramos M, Gómez-Gallego F, Cuenca-García M, Casals C, Ponce-González JG. Influence of ACE Gene I/D Polymorphism on Cardiometabolic Risk, Maximal Fat Oxidation, Cardiorespiratory Fitness, Diet and Physical Activity in Young Adults. International Journal of Environmental Research and Public Health. 2021; 18(7):3443. https://doi.org/10.3390/ijerph18073443
Chicago/Turabian StyleMontes-de-Oca-García, Adrián, Alejandro Perez-Bey, Daniel Velázquez-Díaz, Juan Corral-Pérez, Edgardo Opazo-Díaz, María Rebollo-Ramos, Félix Gómez-Gallego, Magdalena Cuenca-García, Cristina Casals, and Jesús G. Ponce-González. 2021. "Influence of ACE Gene I/D Polymorphism on Cardiometabolic Risk, Maximal Fat Oxidation, Cardiorespiratory Fitness, Diet and Physical Activity in Young Adults" International Journal of Environmental Research and Public Health 18, no. 7: 3443. https://doi.org/10.3390/ijerph18073443
APA StyleMontes-de-Oca-García, A., Perez-Bey, A., Velázquez-Díaz, D., Corral-Pérez, J., Opazo-Díaz, E., Rebollo-Ramos, M., Gómez-Gallego, F., Cuenca-García, M., Casals, C., & Ponce-González, J. G. (2021). Influence of ACE Gene I/D Polymorphism on Cardiometabolic Risk, Maximal Fat Oxidation, Cardiorespiratory Fitness, Diet and Physical Activity in Young Adults. International Journal of Environmental Research and Public Health, 18(7), 3443. https://doi.org/10.3390/ijerph18073443









