The Combined Effects of Obesity and Cardiorespiratory Fitness Are Associated with Response Inhibition: An ERP Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Submaximal Cardiorespiratory Fitness Assessment
2.3. Stop-Signal Task (SST)
2.4. Psychophysiological Recording and Data Analysis
2.5. Experimental Procedure
2.6. Statistical Analysis
3. Results
3.1. Participant Characteristics
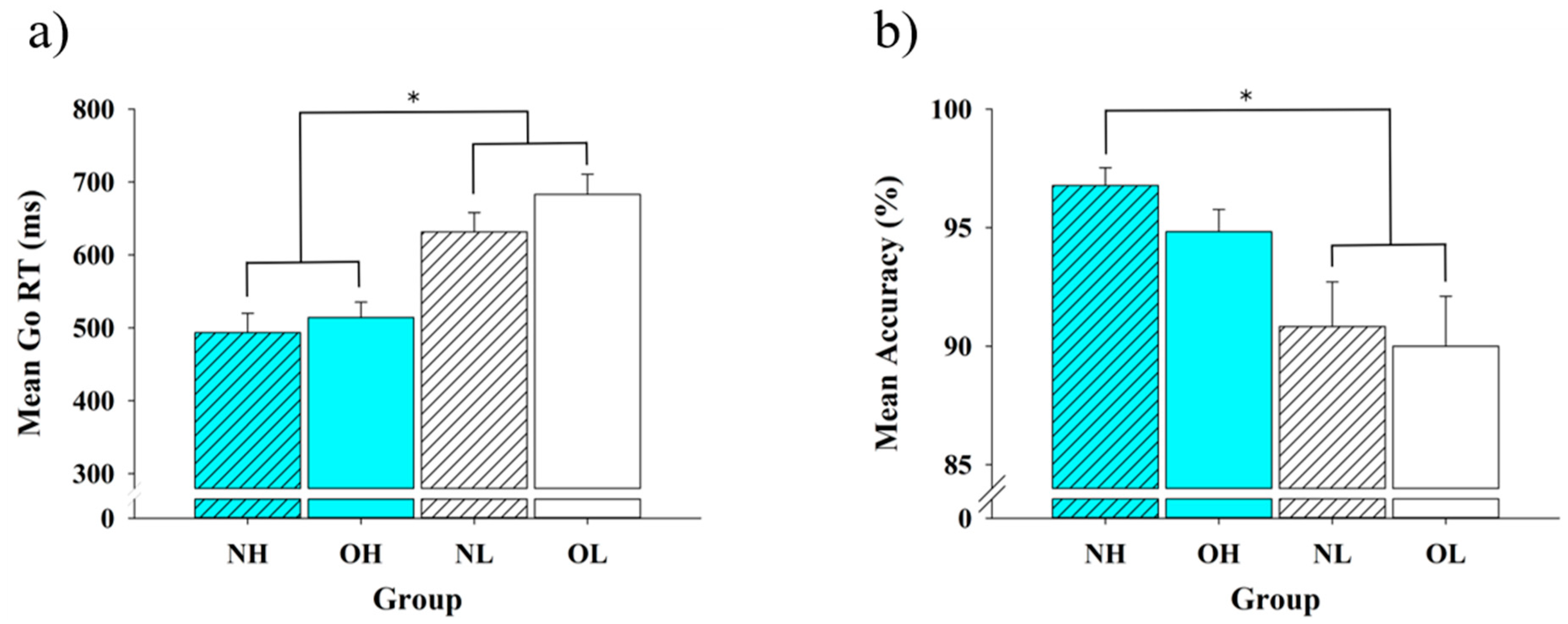
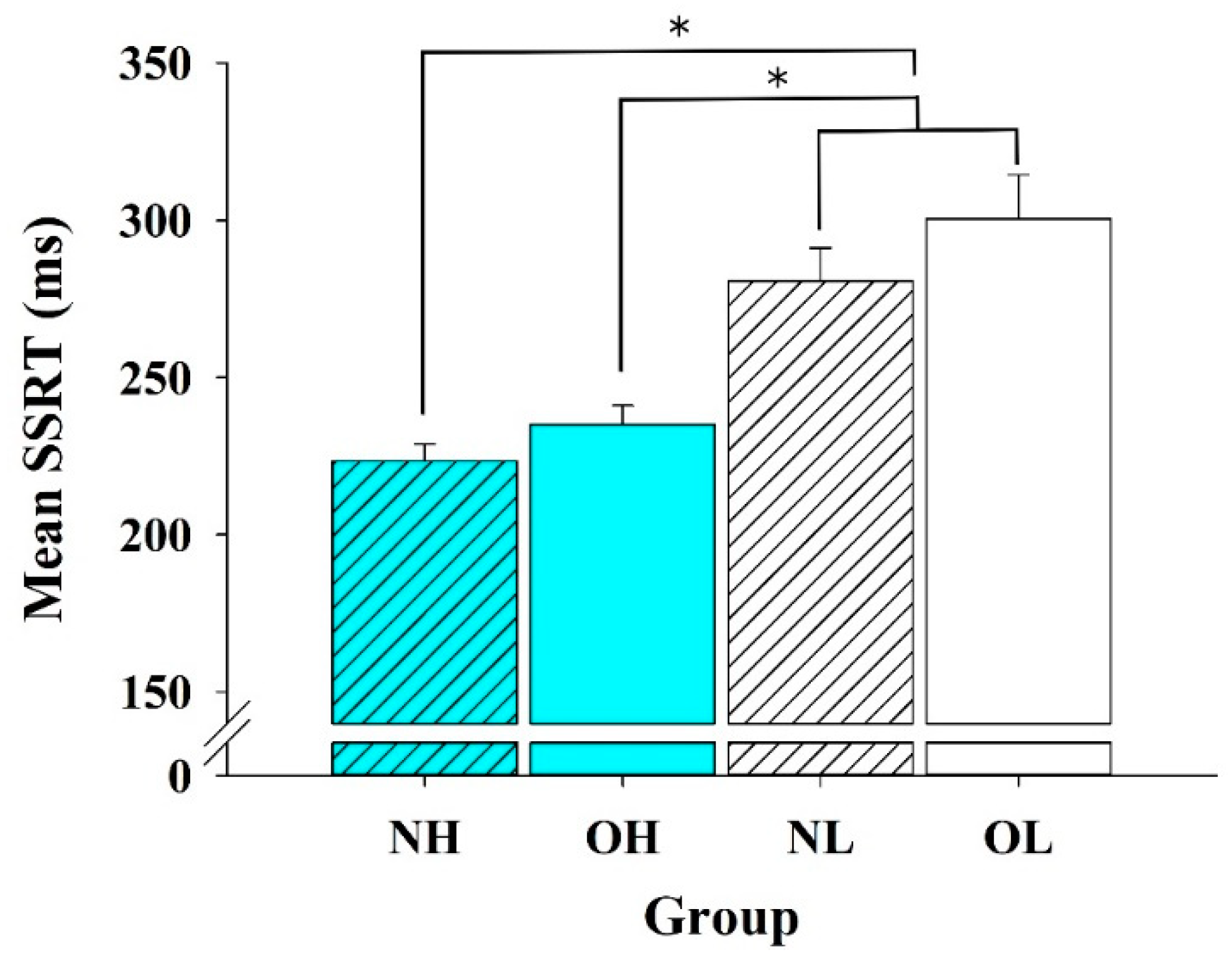
3.2. Behavioral Data
3.3. ERP Data: P3 Amplitudes
4. Discussion
4.1. Behavioral Performance
4.2. ERP
4.3. Limitations and Future Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Obesity and Overweight. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 21 March 2021).
- Pantalone, K.M.; Hobbs, T.M.; Chagin, K.M.; Kong, S.X.; Wells, B.J.; Kattan, M.W.; Bouchard, J.; Sakurada, B.; Milinovich, A.; Weng, W.; et al. Prevalence and recognition of obesity and its associated comorbidities: Cross-sectional analysis of electronic health record data from a large US integrated health system. BMJ Open 2017, 7, e017583. [Google Scholar] [CrossRef]
- Moral-García, J.E.; Agraso-López, A.D.; Ramos-Morcillo, A.J.; Jiménez, A.; Jiménez-Eguizábal, A. The influence of physical activity, diet, weight status and substance abuse on students’ self-perceived health. Int. J. Environ. Res. Public Health 2020, 17, 1387. [Google Scholar] [CrossRef]
- Pereira-Miranda, E.; Costa, P.R.F.; Queiroz, V.A.O.; Pereira-Santos, M.; Santana, M.L.P. Overweight and obesity associated with higher depression prevalence in adults: A systematic review and meta-analysis. J. Am. Coll. Nutr. 2017, 36, 223–233. [Google Scholar] [CrossRef]
- Favieri, F.; Forte, G.; Casagrande, M. The executive functions in overweight and obesity: A systematic review of neuropsychological cross-sectional and longitudinal studies. Front. Psychol. 2019, 10, 2126. [Google Scholar] [CrossRef]
- Nigg, J.T. Annual research review: On the relations among self-regulation, self-control, executive functioning, effortful control, cognitive control, impulsivity, risk-taking, and inhibition for developmental psychopathology. J. Child Psychol. Psychiatry 2017, 58, 361–383. [Google Scholar] [CrossRef] [PubMed]
- Logan, G.D.; Cowan, W.B.; Davis, K.A. On the ability to inhibit simple and choice reaction time responses: A model and a method. J. Exp. Psychol. Hum. Percept. Perform. 1984, 10, 276–291. [Google Scholar] [CrossRef]
- Yang, Y.; Shields, G.S.; Guo, C.; Liu, Y. Executive function performance in obesity and overweight individuals: A meta-analysis and review. Neurosci. Biobehav. Rev. 2018, 84, 225–244. [Google Scholar] [CrossRef]
- Sellaro, R.; Colzato, L.S. High body mass index is associated with impaired cognitive control. Appetite 2017, 113, 301–309. [Google Scholar] [CrossRef]
- Chamberlain, S.R.; Derbyshire, K.L.; Leppink, E.; Grant, J.E. Obesity and dissociable forms of impulsivity in young adults. CNS Spectr. 2015, 20, 500–507. [Google Scholar] [CrossRef]
- Zhan, Z.; Ai, J.; Ren, F.; Li, L.; Chu, C.H.; Chang, Y.K. Cardiorespiratory fitness, age, and multiple aspects of executive function among preadolescent children. Front. Psychol. 2020, 11, 1198. [Google Scholar] [CrossRef]
- Chen, J.; Li, Y.; Zhang, G.; Jin, X.; Lu, Y.; Zhou, C. Enhanced inhibitory control during re-engagement processing in badminton athletes: An event- related potential study. J. Sport Health Sci. 2019, 8, 585–594. [Google Scholar] [CrossRef] [PubMed]
- Chu, C.H.; Yang, K.T.; Song, T.F.; Liu, J.H.; Hung, T.M.; Chang, Y.K. Cardiorespiratory fitness is associated with executive control in late-middle-aged adults: An event-related (De) sychronization (ERD/ERS) study. Front. Psychol. 2016, 7, 1135. [Google Scholar] [CrossRef]
- Dupuy, O.; Gauthier, C.J.; Fraser, S.A.; Desjardins-Crèpeau, L.; Desjardins, M.; Mekary, S.; Lesage, F.; Hoge, R.D.; Pouliot, P.; Bherer, L. Higher levels of cardiovascular fitness are associated with better executive function and prefrontal oxygenation in younger and older women. Front. Hum. Neurosci. 2015, 9, 66. [Google Scholar] [CrossRef] [PubMed]
- Hogan, M.; O’Hora, D.; Kiefer, M.; Kubesch, S.; Kilmartin, L.; Collins, P.; Dimitrova, J. The effects of cardiorespiratory fitness and acute aerobic exercise on executive functioning and EEG entropy in adolescents. Front. Hum. Neurosci. 2015, 9, 538. [Google Scholar] [CrossRef]
- Padilla, C.; Perez, L.; Andres, P.; Parmentier, F.B.R. Exercise improves cognitive control: Evidence from the stop signal task. Appl. Cogn. Psychol. 2013, 27, 505–511. [Google Scholar] [CrossRef]
- Padilla, C.; Perez, L.; Andres, P. Chronic exercise keeps working memory and inhibitory capacities fit. Front. Behav. Neurosci. 2014, 8, 49. [Google Scholar] [CrossRef]
- Scott, S.P.; De Souza, M.J.; Koehler, K.; Petkus, D.L.; Murray-Kolb, L.E. Cardiorespiratory fitness is associated with better executive function in young women. Med. Sci. Sports Exerc. 2016, 48, 1994–2002. [Google Scholar] [CrossRef][Green Version]
- Scudder, M.R.; Lambourne, K.; Drollette, E.S.; Herrmann, S.D.; Washburn, R.A.; Donnelly, J.E.; Hillman, C.H. Aerobic capacity and cognitive control in elementary school-age children. Med. Sci. Sports Exerc. 2014, 46, 1025–1035. [Google Scholar] [CrossRef]
- Barry, V.W.; Baruth, M.; Beets, M.W.; Durstine, J.L.; Liu, J.; Blair, S.N. Fitness vs. fatness on all-cause mortality: A meta-analysis. Prog. Cardiovasc. Dis. 2014, 56, 382–390. [Google Scholar] [CrossRef] [PubMed]
- Edwards, M.K.; Dankel, S.J.; Loenneke, J.P.; Loprinzi, P.D. The association between weight status, weight history, physical activity, and cognitive task performance. Int. J. Behav. Med. 2016. [Google Scholar] [CrossRef]
- Song, T.F.; Chi, L.; Chu, C.H.; Chen, F.T.; Zhou, C.; Chang, Y.K. Obesity, cardiovascular fitness, and inhibition function: An electrophysiological study. Front. Psychol. 2016, 7, 1124. [Google Scholar] [CrossRef] [PubMed]
- Ross, N.; Yau, P.L.; Convit, A. Obesity, fitness, and brain integrity in adolescence. Appetite 2015, 93, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Pindus, D.M.; Drollette, E.S.; Raine, L.B.; Kao, S.C.; Khan, N.; Westfall, D.R.; Hamill, M.; Shorin, R.; Calobrisi, E.; John, D.; et al. Moving fast, thinking fast: The relations of physical activity levels and bouts to neuroelectric indices of inhibitory control in preadolescents. J. Sport Health Sci. 2019, 8, 301–314. [Google Scholar] [CrossRef]
- Pontifex, M.B.; Raine, L.B.; Johnson, C.R.; Chaddock, L.; Voss, M.W.; Cohen, N.J.; Kramer, A.F.; Hillman, C.H. Cardiorespiratory fitness and the flexible modulation of cognitive control in preadolescent children. J. Cogn. Neurosci. 2011, 23, 1332–1345. [Google Scholar] [CrossRef]
- Hillman, C.H.; Buck, S.M.; Themanson, J.R.; Pontifex, M.B.; Castelli, D.M. Aerobic fitness and cognitive development: Event-related brain potential and task performance indices of executive control in preadolescent children. Dev. Psychol. 2009, 45, 114–129. [Google Scholar] [CrossRef]
- Polich, J. Updating P300: An integrative theory of P3a and P3b. Clin. Neurophysiol. 2007, 118, 2128–2148. [Google Scholar] [CrossRef]
- Tascilar, M.E.; Turkkahraman, D.; Oz, O.; Yucel, M.; Taskesen, M.; Eker, I.; Abaci, A.; Dundaroz, R.; Ulas, U.H. P300 auditory event-related potentials in children with obesity: Is childhood obesity related to impairment in cognitive functions? Pediatr. Diabetes 2011, 12, 589–595. [Google Scholar] [CrossRef]
- Reyes, S.; Peirano, P.; Peigneux, P.; Lozoff, B.; Algarin, C. Inhibitory control in otherwise healthy overweight 10-year-old children. Int. J. Obes. 2015, 39, 1230–1235. [Google Scholar] [CrossRef] [PubMed]
- American College of Sports Medicine. ACSM’s Guidelines for Exercise Testing and Prescription, 11th ed.; Lippincott Williams and Wilkins: New York, NY, USA, 2021. [Google Scholar]
- Wechsler, D. WAIS-III, Wechsler Adult Iintelligence Scale: Administration and Scoring Manual; Psychological Corporation: New York, NY, USA, 1997. [Google Scholar]
- Golding, L.A.; Myers, C.R.; Sinning, W.E. The Y’s Way to Physical Fitness; Human Kinetics Publishers: Champaign, IL, USA, 1989. [Google Scholar]
- Beekley, M.D.; Brechue, W.F.; Dehoyos, D.V.; Garzarella, L.; Werber-Zion, G.; Pollock, M.L. Cross-validation of the YMCA submaximal cycle ergometer test to predict VO2max. Res. Q. Exerc. Sport 2004, 75, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Borg, G.A. Psychophysical bases of perceived exertion. Med. Sci. Sports Exerc. 1982, 14, 377–381. [Google Scholar] [CrossRef]
- Johnstone, S.J.; Dimoska, A.; Smith, J.L.; Barry, R.J.; Pleffer, C.B.; Chiswick, D.; Clarke, A.R. The development of stop-signal and go/nogo response inhibition in children aged 7–12 years: Performance and event-related potential indices. Int. J. Psychophysiol. 2007, 63, 25–38. [Google Scholar] [CrossRef]
- Semlitsch, H.V.; Anderer, P.; Schuster, P.; Presslich, O. A solution for reliable and valid reduction of ocular artifacts, applied to the P300 ERP. Psychophysiology 1986, 23, 695–703. [Google Scholar] [CrossRef] [PubMed]
- Alderman, B.L.; Olson, R.L. The relation of aerobic fitness to cognitive control and heart rate variability: A neurovisceral integration study. Biol. Psychol. 2014, 99, 26–33. [Google Scholar] [CrossRef]
- Schall, J.D. On building a bridge between brain and behavior. Annu. Rev. Psychol. 2004, 55, 23–50. [Google Scholar] [CrossRef]
- Hillman, C.H.; Motl, R.W.; Pontifex, M.B.; Posthuma, D.; Stubbe, J.H.; Boomsma, D.I.; de Geus, E.J. Physical activity and cognitive function in a cross-section of younger and older community-dwelling individuals. Health Psychol. 2006, 25, 678–687. [Google Scholar] [CrossRef] [PubMed]
- Colcombe, S.J.; Kramer, A.F.; Erickson, K.I.; Scalf, P.; McAuley, E.; Cohen, N.J.; Webb, A.; Jerome, G.J.; Marquez, D.X.; Elavsky, S. Cardiovascular fitness, cortical plasticity, and aging. Proc. Natl. Acad. Sci. USA 2004, 101, 3316–3321. [Google Scholar] [CrossRef]
- Wu, C.T.; Pontifex, M.B.; Raine, L.B.; Chaddock, L.; Voss, M.W.; Kramer, A.F.; Hillman, C.H. Aerobic fitness and response variability in preadolescent children performing a cognitive control task. Neuropsychology 2011, 25, 333–341. [Google Scholar] [CrossRef]
- Smiley-Oyen, A.L.; Lowry, K.A.; Francois, S.J.; Kohut, M.L.; Ekkekakis, P. Exercise, fitness, and neurocognitive function in older adults: The “selective improvement” and “cardiovascular fitness” hypotheses. Ann. Behav. Med. 2008, 36, 280–291. [Google Scholar] [CrossRef] [PubMed]
- Verbruggen, F.; Logan, G.D. Models of response inhibition in the stop-signal and stop-change paradigms. Neurosci. Biobehav. Rev. 2009, 33, 647–661. [Google Scholar] [CrossRef]
- Grant, J.E.; Derbyshire, K.; Leppink, E.; Chamberlain, S.R. Obesity and gambling: Neurocognitive and clinical associations. Acta Psychiatr. Scand. 2015, 131, 379–386. [Google Scholar] [CrossRef] [PubMed]
- Verbeken, S.; Braet, C.; Claus, L.; Nederkoorn, C.; Oosterlaan, J. Childhood obesity and impulsivity: An investigation with performance-based measures. Behav. Chang. 2012, 26, 153–167. [Google Scholar] [CrossRef]
- Nederkoorn, C.; Jansen, E.; Mulkens, S.; Jansen, A. Impulsivity predicts treatment outcome in obese children. Behav. Res. Ther. 2007, 45, 1071–1075. [Google Scholar] [CrossRef] [PubMed]
- Kulendran, M.; Vlaev, I.; Sugden, C.; King, D.; Ashrafian, H.; Gately, P.; Darzi, A. Neuropsychological assessment as a predictor of weight loss in obese adolescents. Int. J. Obes. 2014, 38, 507–512. [Google Scholar] [CrossRef]
- Nederkoorn, C.; Smulders, F.T.Y.; Havermans, R.C.; Roefs, A.; Jansen, A. Impulsivity in obese women. Appetite 2006, 47, 253–256. [Google Scholar] [CrossRef] [PubMed]
- Hendrick, O.M.; Luo, X.; Zhang, S.; Li, C.S. Saliency processing and obesity: A preliminary imaging study of the stop signal task. Obesity 2012, 20, 1796–1802. [Google Scholar] [CrossRef] [PubMed]
- Menzies, L.; Achard, S.; Chamberlain, S.R.; Fineberg, N.; Chen, C.H.; del Campo, N.; Sahakian, B.J.; Robbins, T.W.; Bullmore, E. Neurocognitive endophenotypes of obsessive-compulsive disorder. Brain 2007, 130 Pt 12, 3223–3236. [Google Scholar] [CrossRef]
- Bartholdy, S.; Dalton, B.; O’Daly, O.G.; Campbell, I.C.; Schmidt, U. A systematic review of the relationship between eating, weight and inhibitory control using the stop signal task. Neurosci. Biobehav. Rev. 2016, 64, 35–62. [Google Scholar] [CrossRef] [PubMed]
- Kok, A.; Ramautar, J.R.; De Ruiter, M.B.; Band, G.P.; Ridderinkhof, K.R. ERP components associated with successful and unsuccessful stopping in a stop-signal task. Psychophysiology 2004, 41, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Ramautar, J.R.; Kok, A.; Ridderinkhof, K.R. Effects of stop-signal probability in the stop-signal paradigm: The N2/P3 complex further validated. Brain Cogn. 2004, 56, 234–252. [Google Scholar] [CrossRef]
- Tsai, Y.J.; Hung, C.L.; Tsai, C.L.; Chang, Y.K.; Huang, C.J.; Hung, T.M. The relationship between physical fitness and inhibitory ability in children with attention deficit hyperactivity disorder: An event-related potential study. Psychol. Sport Exerc. 2017, 31, 149–157. [Google Scholar] [CrossRef]
- Liotti, M.; Pliszka, S.R.; Higgins, K.; Perez, R., 3rd; Semrud-Clikeman, M. Evidence for specificity of ERP abnormalities during response inhibition in ADHD children: A comparison with reading disorder children without ADHD. Brain Cogn. 2010, 72, 228–237. [Google Scholar] [CrossRef] [PubMed]
- Hayes, S.M.; Hayes, J.P.; Cadden, M.; Verfaellie, M. A review of cardiorespiratory fitness-related neuroplasticity in the aging brain. Front. Aging Neurosci. 2013, 5, 31. [Google Scholar] [CrossRef] [PubMed]
- Selim, M.; Jones, R.; Novak, P.; Zhao, P.; Novak, V. The effects of body mass index on cerebral blood flow velocity. Clin. Auton. Res. 2008, 18, 331. [Google Scholar] [CrossRef]
- Guiney, H.; Lucas, S.J.; Cotter, J.D.; Machado, L. Evidence cerebral blood-flow regulation mediates exercise–cognition links in healthy young adults. Neuropsychology 2015, 29, 1. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.D.; McMorris, C.A.; Longman, R.S.; Leigh, R.; Hill, M.D.; Friedenreich, C.M.; Poulin, M.J. Effects of cardiorespiratory fitness and cerebral blood flow on cognitive outcomes in older women. Neurobiol. Aging 2010, 13, 2047–2057. [Google Scholar] [CrossRef]
- Hong, S.; Dimitrov, S.; Pruitt, C.; Shaikh, F.; Beg, N. Benefit of physical fitness against inflammation in obesity: Role of beta adrenergic receptors. Brain Behav. Immun. 2014, 39, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Tong, L.; Balazs, R.; Soiampornkul, R.; Thangnipon, W.; Cotman, C.W. Interleukin-1 beta impairs brain derived neurotrophic factor-induced signal transduction. Neurobiol. Aging 2008, 29, 1380–1393. [Google Scholar] [CrossRef]
- Voss, M.W.; Vivar, C.; Kramer, A.F.; van Praag, H. Bridging animal and human models of exercise-induced brain plasticity. Trends Cogn. Sci. 2013, 17, 525–544. [Google Scholar] [CrossRef]
- Hwang, J.; Castelli, D.M.; Gonzalez-Lima, F. The positive cognitive impact of aerobic fitness is associated with peripheral inflammatory and brain-derived neurotrophic biomarkers in young adults. Physiol. Behav. 2017, 179, 75–89. [Google Scholar] [CrossRef]
- Weinstein, A.M.; Voss, M.W.; Prakash, R.S.; Chaddock, L.; Szabo, A.; White, S.M.; Wojcicki, T.R.; Mailey, E.; McAuley, E.; Kramer, A.F. The association between aerobic fitness and executive function is mediated by prefrontal cortex volume. Brain Behav. Immun. 2012, 26, 811–819. [Google Scholar] [CrossRef] [PubMed]
- Chaddock, L.; Erickson, K.I.; Prakash, R.S.; VanPatter, M.; Voss, M.W.; Pontifex, M.B.; Raine, L.B.; Hillman, C.H.; Kramer, A.F. Basal ganglia volume is associated with aerobic fitness in preadolescent children. Dev. Neurosci. 2010, 32, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Verstynen, T.D.; Lynch, B.; Miller, D.L.; Voss, M.W.; Prakash, R.S.; Chaddock, L.; Basak, C.; Szabo, A.; Olson, E.A.; Wojcicki, T.R.; et al. Caudate nucleus volume mediates the link between cardiorespiratory fitness and cognitive flexibility in older adults. J. Aging Res. 2012, 2012, 939285. [Google Scholar] [CrossRef] [PubMed]
- Ward, M.A.; Carlsson, C.M.; Trivedi, M.A.; Sager, M.A.; Johnson, S.C. The effect of body mass index on global brain volume in middle-aged adults: A cross sectional study. BMC Neurol. 2005, 5, 23. [Google Scholar] [CrossRef]
- Raji, C.A.; Ho, A.J.; Parikshak, N.N.; Becker, J.T.; Lopez, O.L.; Kuller, L.H.; Hua, X.; Leow, A.D.; Toga, A.W.; Thompson, P.M. Brain structure and obesity. Hum. Brain Mapp. 2010, 31, 353–364. [Google Scholar] [CrossRef]
- Taki, Y.; Kinomura, S.; Sato, K.; Inoue, K.; Goto, R.; Okada, K.; Uchida, S.; Kawashima, R.; Fukuda, H. Relationship between body mass index and gray matter volume in 1428 healthy individuals. Obesity 2008, 16, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Jepsen, P.; Johnsen, S.P.; Gillman, M.W.; Sørensen, H.T. Interpretation of observational studies. Heart 2004, 90, 956–960. [Google Scholar] [CrossRef]
- Yuan, J.; He, Y.; Qinglin, Z.; Chen, A.; Li, H. Gender differences in behavioral inhibitory control: ERP evidence from a two-choice oddball task. Psychophysiology 2008, 45, 986–993. [Google Scholar] [CrossRef]
- Adab, P.; Pallan, M.; Whincup, P.H. Is BMI the best measure of obesity? BMJ 2018, 360, k1274. [Google Scholar] [CrossRef] [PubMed]

| Variables | NH (n = 23) | OH (n = 23) | NL (n = 23) | OL (n = 23) |
|---|---|---|---|---|
| Age (years) | 20.52 ± 1.65 | 20.70 ± 2.16 | 21.47 ± 2.00 | 21.04 ± 2.16 |
| Height (cm) | 173.43± 4.86 | 177.26 ± 7.63 | 175.91 ± 4.74 | 174.30 ± 6.27 |
| Weight (kg) | 63.91 ± 5.28 | 91.78 ± 14.54 b | 66.65 ± 5.36 | 102.43 ± 19.93 a |
| BMI (kg/m2) | 21.25 ±1.38 | 29.08 ± 2.50 b | 21.53 ±1.23 | 33.63 ± 5.93 a |
| Digit span: Forward | 14.50 ± 1.30 | 14.00 ± 1.43 | 14.68 ± 1.32 | 14.14 ± 1.08 |
| Digit span: Backward | 8.82 ± 3.10 | 8.23 ± 2.10 | 9.60 ± 2.67 | 8.55 ± 2.76 |
| VO2max (mL/kg/min) | 55.19 ± 4.73 a | 53.28 ± 4.29 a | 41.66 ± 9.60 | 36.04 ± 3.63 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chi, L.; Hung, C.-L.; Lin, C.-Y.; Song, T.-F.; Chu, C.-H.; Chang, Y.-K.; Zhou, C. The Combined Effects of Obesity and Cardiorespiratory Fitness Are Associated with Response Inhibition: An ERP Study. Int. J. Environ. Res. Public Health 2021, 18, 3429. https://doi.org/10.3390/ijerph18073429
Chi L, Hung C-L, Lin C-Y, Song T-F, Chu C-H, Chang Y-K, Zhou C. The Combined Effects of Obesity and Cardiorespiratory Fitness Are Associated with Response Inhibition: An ERP Study. International Journal of Environmental Research and Public Health. 2021; 18(7):3429. https://doi.org/10.3390/ijerph18073429
Chicago/Turabian StyleChi, Lin, Chiao-Ling Hung, Chi-Yen Lin, Tai-Fen Song, Chien-Heng Chu, Yu-Kai Chang, and Chenglin Zhou. 2021. "The Combined Effects of Obesity and Cardiorespiratory Fitness Are Associated with Response Inhibition: An ERP Study" International Journal of Environmental Research and Public Health 18, no. 7: 3429. https://doi.org/10.3390/ijerph18073429
APA StyleChi, L., Hung, C.-L., Lin, C.-Y., Song, T.-F., Chu, C.-H., Chang, Y.-K., & Zhou, C. (2021). The Combined Effects of Obesity and Cardiorespiratory Fitness Are Associated with Response Inhibition: An ERP Study. International Journal of Environmental Research and Public Health, 18(7), 3429. https://doi.org/10.3390/ijerph18073429






