Older Adults Who Spend More Time Outdoors in Summer and Have Higher Dietary Vitamin D Than Younger Adults Can Present at Least as High Vitamin D Status: A Pilot Study
Abstract
:1. Introduction
1.1. Vitamin D Production/Intake
1.2. Population Sunlight Exposure Behaviours and Vitamin D Status
1.3. Dietary Sources of Vitamin D
2. Materials and Methods
2.1. Volunteers
2.2. Skin Type Assessment
2.3. Lifestyle Questionnaires
2.4. Vitamin D Diet Diaries
2.5. Circulating Vitamin D Analysis
2.6. Statistical Software
3. Results
3.1. Volunteers
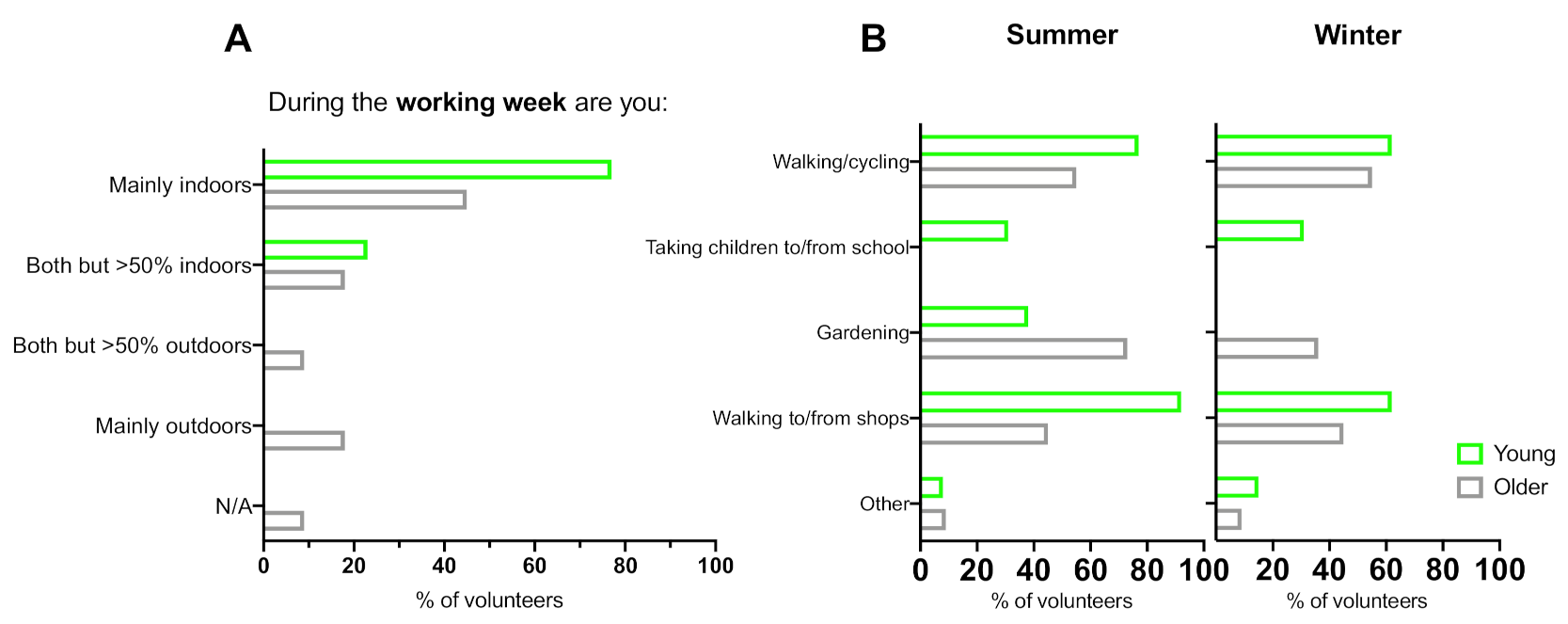
3.2. Occupation
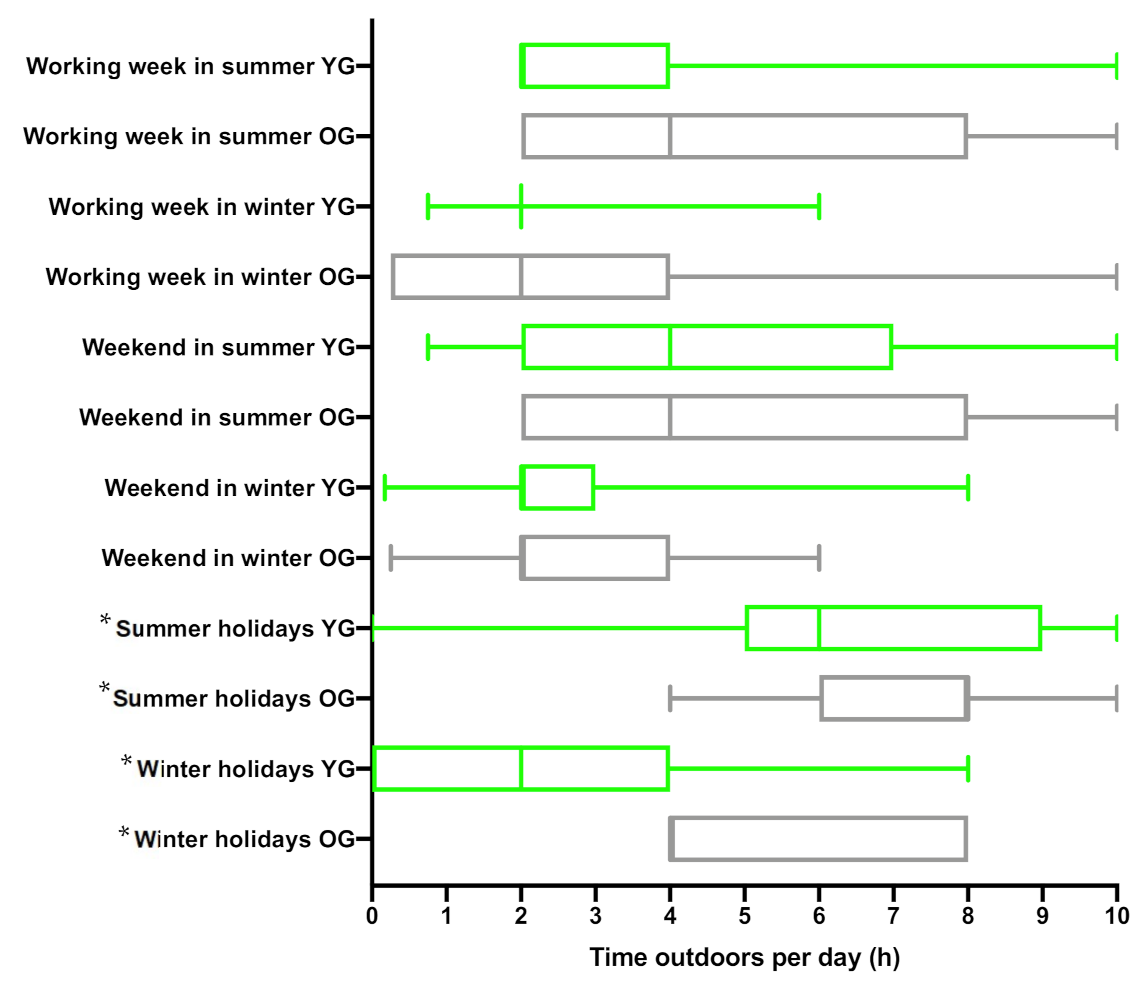
3.3. Time Spent Outdoors in the UK
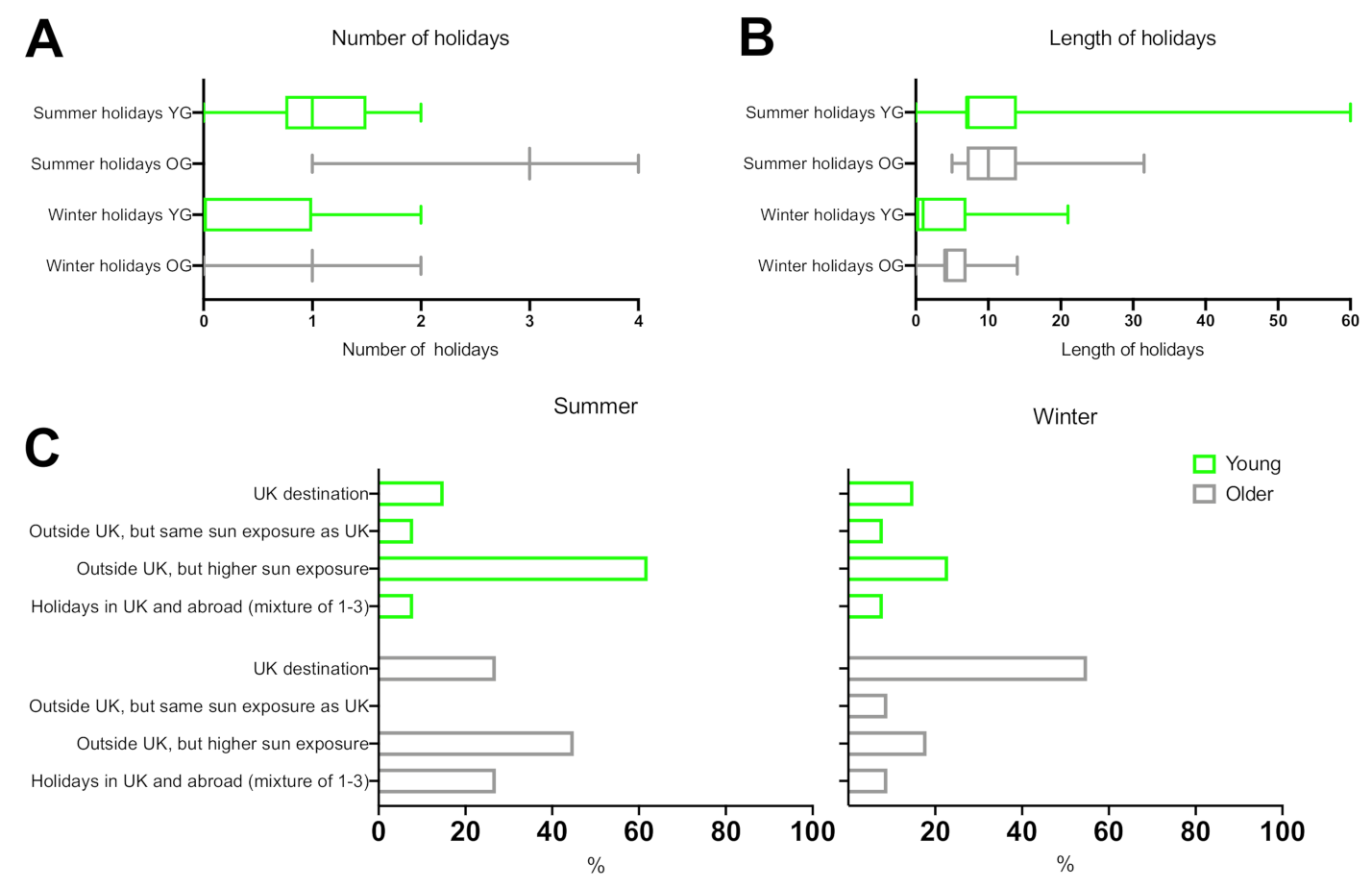
3.4. Holiday Behaviour
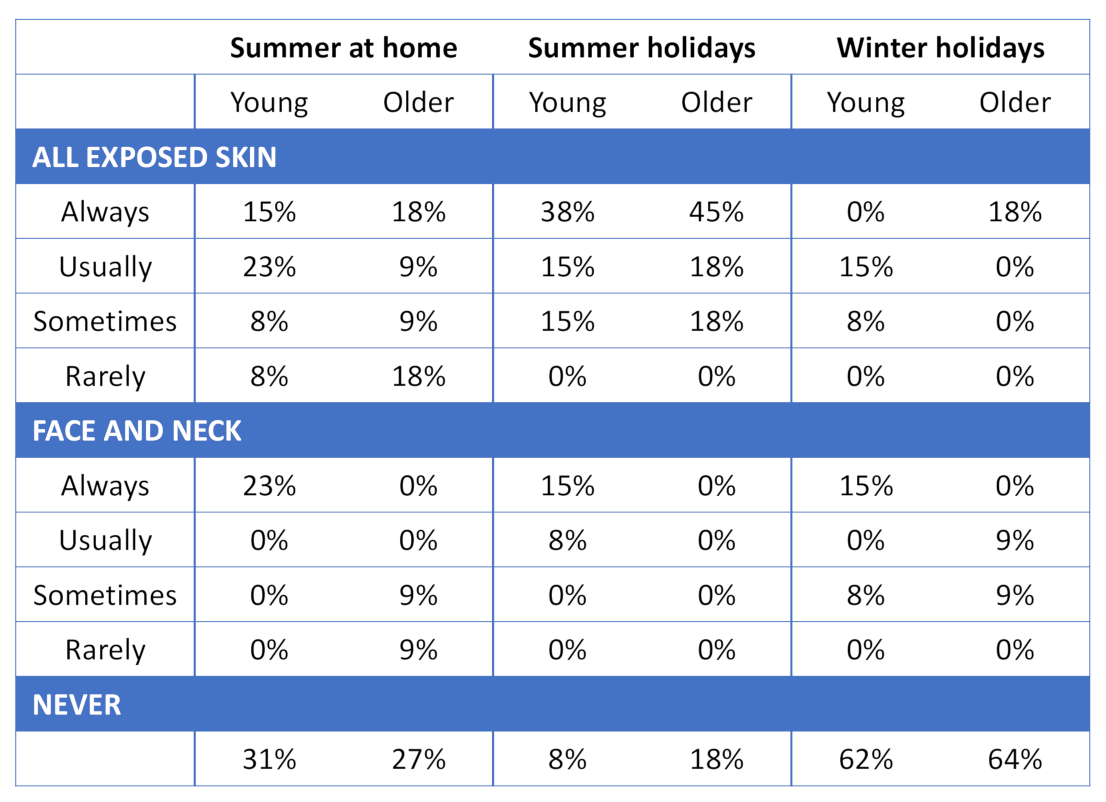
3.5. Holiday Clothing Choices
3.6. Sunscreen Use
3.7. Sunbed Use
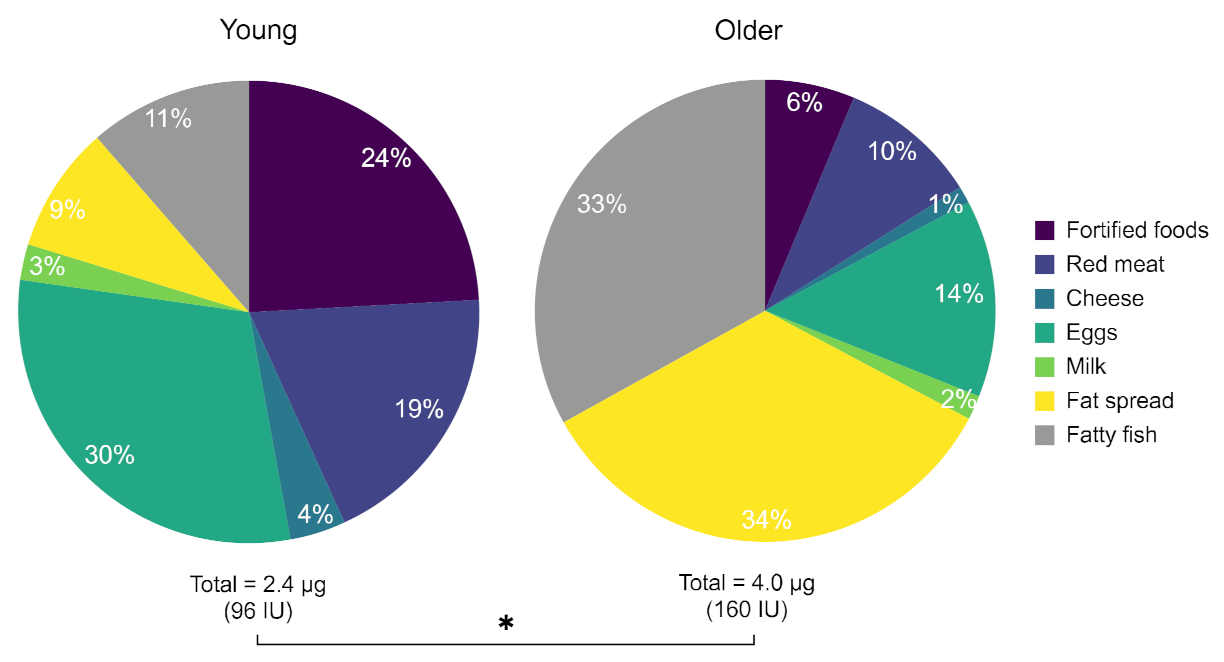
3.8. Dietary Vitamin D Intake
3.9. Vitamin D Supplements
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zerwekh, J.E. Blood biomarkers of vitamin D status. Am. J. Clin. Nutr. 2008, 87, 1087S–1091S. [Google Scholar] [CrossRef] [PubMed]
- SACN Vitamin D and Health. Available online: https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/537616/SACN_Vitamin_D_and_Health_report.pdf (accessed on 28 January 2020).
- Department of Health. Report of the Expert Working Groups of the Committee on Medical Aspects of Food Policy (COMA) on Dietary Reference Values; Stationery Office: London, UK, 1991.
- Society, B.G.N. New Reference Values for Vitamin D. Ann. Nutr. Metab. 2012, 60, 241–246. [Google Scholar] [CrossRef]
- Pilz, S.; Zittermann, A.; Trummer, C.; Theiler-Schwetz, V.; Lerchbaum, E.; Keppel, M.H.; Grübler, M.R.; März, W.; Pandis, M. Vitamin D testing and treatment: A narrative review of current evidence. Endocr. Connect. 2019, 8, R27–R43. [Google Scholar] [CrossRef]
- Ashwell, M.; Stone, E.M.; Stolte, H.; Cashman, K.D.; Macdonald, H.; Lanham-New, S.; Hiom, S.; Webb, A.; Fraser, D. UK Food Standards Agency Workshop Report: An investigation of the relative contributions of diet and sunlight to vitamin D status. Br. J. Nutr. 2010, 104, 603–611. [Google Scholar] [CrossRef]
- Burchell, K.; Rhodes, L.E.; Webb, A.R. Public Awareness and Behaviour in Great Britain in the Context of Sunlight Exposure and Vitamin D: Results from the First Large-Scale and Representative Survey. Int. J. Environ. Res. Public Health 2020, 17, 6924. [Google Scholar] [CrossRef] [PubMed]
- Boucher, B.J. The Problems of Vitamin D Insufficiency in Older People. Aging Dis. 2012, 3, 313–329. [Google Scholar]
- MacLaughlin, J.; Holick, M.F. Aging decreases the capacity of human skin to produce vitamin D3. J. Clin. Investig. 1985, 76, 1536–1538. [Google Scholar] [CrossRef]
- Corless, D.; Boucher, B.; Beer, M.; Gupta, S.; Cohen, R. Vitamin-D status in long-stay geriatric patients. Lancet 1975, 305, 1404–1406. [Google Scholar] [CrossRef]
- Lester, E.; Skinner, R.; Wills, M. Seasonal variation in serum-25-hydroxyvitamin-d in the elderly in britain. Lancet 1977, 309, 979–980. [Google Scholar] [CrossRef]
- Hirani, V.; Primatesta, P. Vitamin D concentrations among people aged 65 years and over living in private households and institutions in England: Population survey. Age Ageing 2005, 34, 485–491. [Google Scholar] [CrossRef] [PubMed]
- NDNS. National Diet and Nutrition Survey: People Aged 65 Years and Over. Nutr. Food Sci. 1998, 3, 133–137. [Google Scholar]
- Christensen, K.; Doblhammer, G.; Rau, R.; Vaupel, J.W. Ageing populations: The challenges ahead. Lancet 2009, 374, 1196–1208. [Google Scholar] [CrossRef]
- Nicholson, P.J.; Mayho, G.V. Ageing and employment: Are patients ever too old to work? Br. J. Gen. Pract. 2016, 67, 6–7. [Google Scholar] [CrossRef]
- Sewdas, R.; De Wind, A.; Van Der Zwaan, L.G.; Van Der Borg, W.E.; Steenbeek, R.; Van Der Beek, A.J.; Boot, C.R. Why older workers work beyond the retirement age: A qualitative study. BMC Public Health 2017, 17, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Szanton, S.L.; Walker, R.K.; Roberts, L.; Thorpe, R.J.; Wolff, J.; Agree, E.; Roth, D.L.; Gitlin, L.N.; Seplaki, C. Older adults′ favorite activities are resoundingly active: Findings from the NHATS study. Geriatr. Nurs. 2015, 36, 131–135. [Google Scholar] [CrossRef]
- NDNS National Diet and Nutrition Survey (NDNS) Rolling Programme for 2008 and 2009 to 2011 and 2012. Available online: https://www.gov.uk/government/statistics/national-diet-and-nutrition-survey-results-from-years-1-to-4-combined-of-the-rolling-programme-for-2008-and-2009-to-2011-and-2012 (accessed on 28 January 2021).
- Calame, W.; Street, L.; Hulshof, T. Vitamin D Serum Levels in the UK Population, including a Mathematical Approach to Evaluate the Impact of Vitamin D Fortified Ready-to-Eat Breakfast Cereals: Application of the NDNS Database. Nutrients 2020, 12, 1868. [Google Scholar] [CrossRef]
- Diffey, B.L. Human exposure to solar ultraviolet radiation. J. Cosmet. Dermatol. 2002, 1, 124–130. [Google Scholar] [CrossRef] [PubMed]
- O’Mahony, L.; Stępień, M.; Gibney, M.J.; Nugent, A.P.; Brennan, L. The Potential Role of Vitamin D Enhanced Foods in Improving Vitamin D Status. Nutrients 2011, 3, 1023–1041. [Google Scholar] [CrossRef] [PubMed]
- Black, L.J.; Seamans, K.M.; Cashman, K.D.; Kiely, M. An Updated Systematic Review and Meta-Analysis of the Efficacy of Vitamin D Food Fortification. J. Nutr. 2012, 142, 1102–1108. [Google Scholar] [CrossRef]
- Lamberg-Allardt, C. Vitamin D in foods and as supplements. Prog. Biophys. Mol. Biol. 2006, 92, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Pilz, S.; März, W.; Cashman, K.D.; Kiely, M.E.; Whiting, S.J.; Holick, M.F.; Grant, W.B.; Pludowski, P.; Hiligsmann, M.; Trummer, C.; et al. Rationale and Plan for Vitamin D Food Fortification: A Review and Guidance Paper. Front. Endocrinol. 2018, 9, 373. [Google Scholar] [CrossRef]
- Fitzpatrick, T.B. The Validity and Practicality of Sun-Reactive Skin Types I Through VI. Arch. Dermatol. 1988, 124, 869–871. [Google Scholar] [CrossRef]
- Shih, B.B.; Farrar, M.D.; Cooke, M.S.; Osman, J.; Langton, A.K.; Kift, R.; Webb, A.R.; Berry, J.L.; Watson, R.E.; Vail, A.; et al. Fractional Sunburn Threshold UVR Doses Generate Equivalent Vitamin D and DNA Damage in Skin Types I–VI but with Epidermal DNA Damage Gradient Correlated to Skin Darkness. J. Investig. Dermatol. 2018, 138, 2244–2252. [Google Scholar] [CrossRef] [PubMed]
- Gould, M.; Farrar, M.; Kift, R.; Berry, J.; Mughal, M.; Bundy, C.; Vail, A.; Webb, A.; Rhodes, L. Sunlight exposure and photoprotection behaviour of white Caucasian adolescents in the UK. J. Eur. Acad. Dermatol. Venereol. 2014, 29, 732–737. [Google Scholar] [CrossRef]
- Stafford, R.; Farrar, M.D.; Kift, R.; Durkin, M.; Berry, J.L.; Webb, A.R.; Rhodes, L.E. The impact of photosensitivity disorders on aspects of lifestyle. Br. J. Dermatol. 2010, 163, 817–822. [Google Scholar] [CrossRef] [PubMed]
- PHE Public Health England. McCance & Widdowson′s Composition of Foods Integrated Dataset. Available online: https://www.gov.uk/government/publications/composition-of-foods-integrated-dataset-cofid (accessed on 23 January 2021).
- Meehan, M.; Penckofer, S. The Role of Vitamin D in the Aging Adult. J. Aging Gerontol. 2014, 2, 60–71. [Google Scholar] [CrossRef]
- Bunker, J.P. The role of medical care in contributing to health improvements within societies. Int. J. Epidemiol. 2001, 30, 1260–1263. [Google Scholar] [CrossRef]
- Warburton, D.E.; Nicol, C.W.; Bredin, S.S. Health benefits of physical activity: The evidence. Can. Med. Assoc. J. 2006, 174, 801–809. [Google Scholar] [CrossRef]
- Williamson, J. Evidence Regarding the Benefits of Physical Exercise. Arch. Intern. Med. 2010, 170, 124–125. [Google Scholar] [CrossRef] [PubMed]
- Reimers, C.D.; Knapp, G.; Reimers, A.K. Does Physical Activity Increase Life Expectancy? A Review of the Literature. J. Aging Res. 2012, 2012, 1–9. [Google Scholar] [CrossRef]
- Foster, C.; Hillsdon, M.; Thorogood, M.; Kaur, A.; Wedatilake, T. Interventions for promoting physical activity. Cochrane Database Syst. Rev. 2005, 1, CD003180. [Google Scholar] [CrossRef]
- Leavy, J.E.; Bull, F.C.; Rosenberg, M.; Bauman, A. Physical activity mass media campaigns and their evaluation: A systematic review of the literature 2003–2010. Health Educ. Res. 2011, 26, 1060–1085. [Google Scholar] [CrossRef] [PubMed]
- Erzurum, S.C. Aging and scientific medicine: 60 is the new 40. J. Clin. Investig. 2018, 128, 4204–4207. [Google Scholar] [CrossRef] [PubMed]
- Sanderson, W.C.; Scherbov, S. Faster Increases in Human Life Expectancy Could Lead to Slower Population Aging. PLoS ONE 2015, 10, e0121922. [Google Scholar] [CrossRef]
- Sanderson, W.; Scherbov, S. NEW WAYS TO REDEFINE OLD AGE. Innov. Aging 2017, 1, 1370–1371. [Google Scholar] [CrossRef]
- Miles, A.; Waller, J.; Hiom, S.; Swanston, D. SunSmart? Skin cancer knowledge and preventive behaviour in a British population representative sample. Health Educ. Res. 2005, 20, 579–585. [Google Scholar] [CrossRef] [PubMed]
- Iannacone, M.R.; Green, A.C. Towards skin cancer prevention and early detection: Evolution of skin cancer awareness campaigns in Australia. Melanoma Manag. 2014, 1, 75–84. [Google Scholar] [CrossRef] [PubMed]

| Food | Vitamin D (μg/100 g) |
|---|---|
| Eel | 25.6 |
| Herring | 15.4 |
| Salmon | 12.4 |
| Egg yolk (egg) | 7.8 (2.8) |
| Tuna | 7.2 |
| Cod | 7.0 |
| Milk, fortified | 1.3–2.0 |
| Beef liver | 0.8 |
| Cheddar cheese | 0.3–0.6 |
| Butter | 0.3–1.5 |
| Milk, whole, unfortified | 0.1 |
| Hours Outdoors—Available Answers | Score |
|---|---|
| 30 min or less | 1 |
| >30 min to <1 h | 2 |
| 1 h to <3 h | 3 |
| 3 h to <5 h | 4 |
| 5 h to <7 h | 5 |
| 7 h to <9 h | 6 |
| 9 h or more | 7 |
| Group | Young (18–40 Years Old) | Older (65–89 Years Old) |
|---|---|---|
| Participants, n | 13 | 11 |
| Gender: male, n (%) | 7 (54) | 6 (55) |
| Gender: female, n (%) | 6 (46) | 5 (45) |
| Mean 25(OH)D [nmol/L] (±SD) | 43.2 (21.1) | 56.9 (24.9) |
| Mean BMI [kg/m2] (±SD) | 27.1 (4.6) | 27.1 (5.4) |
| Skin type I, II, III | 3, 3, 7 | 2, 2, 7 |
| Mean age (±SD) | 30.1 (6.2) | 70.8 (4.6) |
| Employed, n (%) | 11 (85) | 2 (18) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borecka, O.; Farrar, M.D.; Osman, J.E.; Rhodes, L.E.; Webb, A.R. Older Adults Who Spend More Time Outdoors in Summer and Have Higher Dietary Vitamin D Than Younger Adults Can Present at Least as High Vitamin D Status: A Pilot Study. Int. J. Environ. Res. Public Health 2021, 18, 3364. https://doi.org/10.3390/ijerph18073364
Borecka O, Farrar MD, Osman JE, Rhodes LE, Webb AR. Older Adults Who Spend More Time Outdoors in Summer and Have Higher Dietary Vitamin D Than Younger Adults Can Present at Least as High Vitamin D Status: A Pilot Study. International Journal of Environmental Research and Public Health. 2021; 18(7):3364. https://doi.org/10.3390/ijerph18073364
Chicago/Turabian StyleBorecka, Oktawia, Mark D. Farrar, Joanne E. Osman, Lesley E. Rhodes, and Ann R. Webb. 2021. "Older Adults Who Spend More Time Outdoors in Summer and Have Higher Dietary Vitamin D Than Younger Adults Can Present at Least as High Vitamin D Status: A Pilot Study" International Journal of Environmental Research and Public Health 18, no. 7: 3364. https://doi.org/10.3390/ijerph18073364
APA StyleBorecka, O., Farrar, M. D., Osman, J. E., Rhodes, L. E., & Webb, A. R. (2021). Older Adults Who Spend More Time Outdoors in Summer and Have Higher Dietary Vitamin D Than Younger Adults Can Present at Least as High Vitamin D Status: A Pilot Study. International Journal of Environmental Research and Public Health, 18(7), 3364. https://doi.org/10.3390/ijerph18073364







