Psycho-Electrophysiological Benefits of Forest Therapies Focused on Qigong and Walking with Elderly Individuals
Abstract
1. Introduction
2. Materials and Methods
2.1. Forest Therapy Program
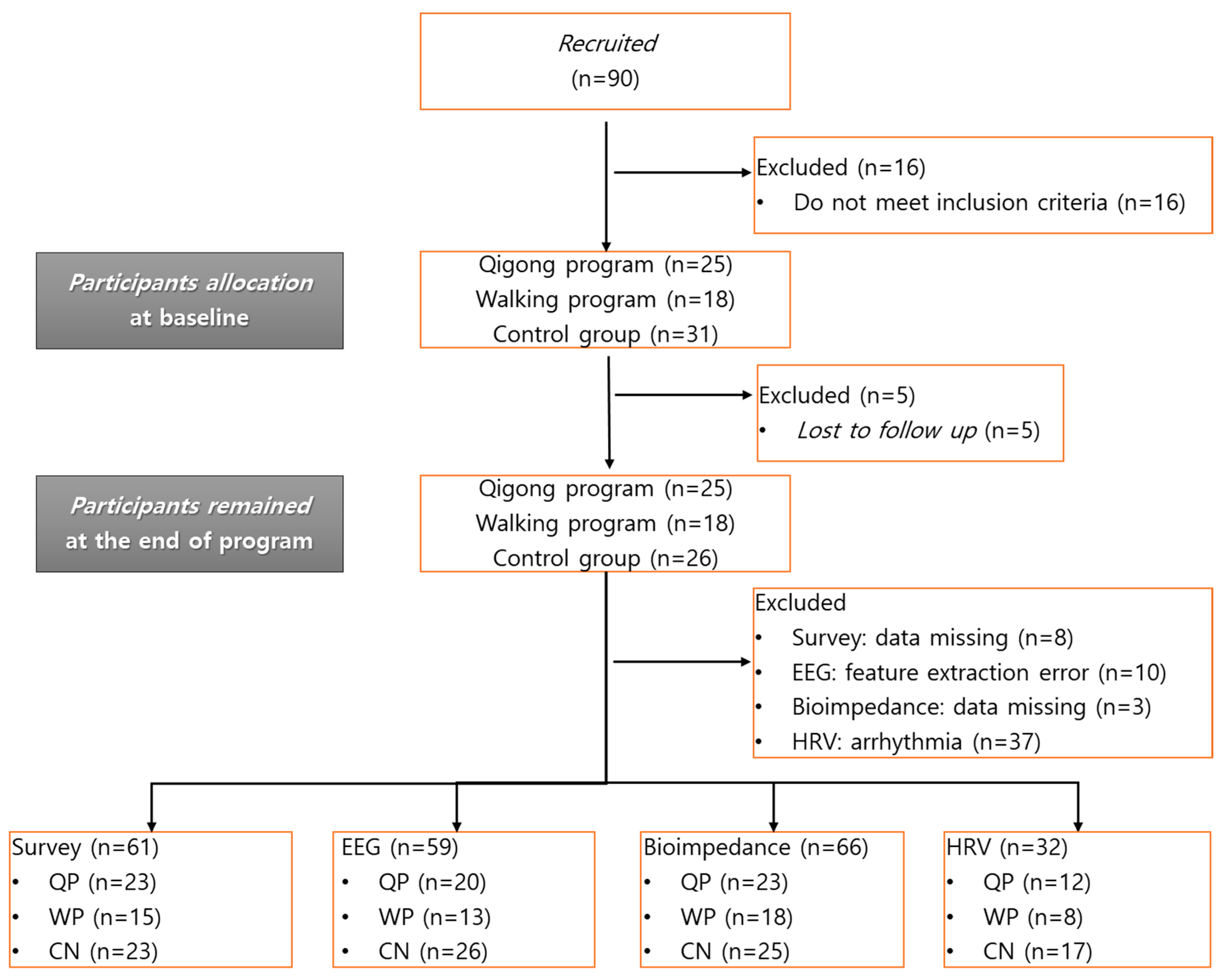
2.2. Subject and Study Protocol
- -
- Aged 65 years or older;
- -
- Not diagnosed with dementia;
- -
- No restrictions on outdoor activity for more than 3 h;
- -
- Able to communicate and complete the self-report questionnaires;
- -
- Able to understand the purpose of the study and voluntarily sign the consent form.
2.3. Test Instrument and Measurement Protocol
2.4. Statistical Analysis
3. Results
3.1. Demographics
3.2. Effects of Forest Therapy Programs
3.2.1. Cognition, Depression, and Quality of Life
3.2.2. Resting-State EEG
3.2.3. Bioimpedance
3.2.4. Heart Rate Variability
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Dataset | Variable | Definition |
|---|---|---|
| EEG | MEF [Hz] | Median frequency: The median frequency used in the dominant intrinsic oscillatory frequency band of 4–13 Hz of the power spectrum |
| Pα [μV2] | Alpha band power: The spectral power integrated over the frequency range of 8–13 Hz (presented in the natural logarithmic scale) | |
| Pβ [μV2] | Beta band power: The spectral power integrated over the frequency range of 13–30 Hz (presented in the natural logarithmic scale) | |
| ATR | Alpha/theta ratio: the power ratio of alpha rhythms (8–13 Hz) to theta rhythms (4–8 Hz) | |
| Bioimpedance | FFM [kg] | Fat-free mass |
| BFM [kg] | Body fat mass | |
| %BF [%] | Percent body fat (body fat/whole body mass) | |
| Imp_arm [Ω] | Impedance averaged over both arms | |
| Imp_leg [Ω] | Impedance averaged over both legs | |
| Reactance_arm [Ω] | Reactance averaged over both arms | |
| Reactance_leg [Ω] | Reactance averaged over both legs | |
| PhA_body | Phase angle of the whole body = (reactance of the whole body)/(impedance of the whole body) | |
| PhA_arm | Phase angle of both arms | |
| PhA_leg | Phase angle of both legs | |
| HRV | HF [m s2] | Spectral power in the high frequency (HF) range of HRV (0.15–0.4 Hz) |
| LF [m s2] | Spectral power in the low frequency (LF) range of HRV (0.040.15 Hz) | |
| %LF | LF power/(LF + HF power) | |
| TP [m s2] | Total power (HF + LF power) | |
| HR [bpm] | Heart rate |
References
- Department of Economic and Social Affairs. World Population Ageing 2019; United Nations: New York, NY, USA, 2019. [Google Scholar]
- Marengoni, A.; Angleman, S.; Melis, R.; Mangialasche, F.; Karp, A.; Garmen, A.; Meinow, B.; Fratiglioni, L. Aging with multimorbidity: A systematic review of the literature. Ageing Res. Rev. 2011, 10, 430–439. [Google Scholar] [CrossRef]
- Nilsson, K.; Sangster, M.; Gallis, C.; Hartig, T.; de Vries, S.; Seeland, K.; Schipperijn, J. Forests, Trees and Human Health; Springer: New York, NY, USA, 2011. [Google Scholar]
- Lee, I.; Choi, H.; Bang, K.S.; Kim, S.; Song, M.; Lee, B. Effects of Forest Therapy on Depressive Symptoms among Adults: A Systematic Review. Int. J. Environ. Res. Public Health 2017, 14, 321. [Google Scholar] [CrossRef]
- Han, J.W.; Choi, H.; Jeon, Y.H.; Yoon, C.H.; Woo, J.M.; Kim, W. The Effects of Forest Therapy on Coping with Chronic Widespread Pain: Physiological and Psychological Differences between Participants in a Forest Therapy Program and a Control Group. Int. J. Environ. Res. Public Health 2016, 13, 255. [Google Scholar] [CrossRef] [PubMed]
- Chun, M.H.; Chang, M.C.; Lee, S.J. The effects of forest therapy on depression and anxiety in patients with chronic stroke. Int. J. Neurosci. 2017, 127, 199–203. [Google Scholar] [CrossRef]
- Kotte, D.; Li, Q.; Shin, W.S.; Michalsen, A. International Handbook of Forest Therapy; Cambridge Scholars Publishing: Newcastle upon Tyne, UK, 2019. [Google Scholar]
- Rajoo, K.S.; Karam, D.S.; Abdul Aziz, N.A. Developing an effective forest therapy program to manage academic stress in conservative societies: A multi-disciplinary approach. Urban For. Urban Green. 2019, 43, 126353. [Google Scholar] [CrossRef]
- Korea Legislation Research Institute. Forestry Culture and Recreation Act. Available online: https://elaw.klri.re.kr/eng_service/lawView.do?hseq=37292&lang=ENG (accessed on 18 January 2021).
- Rajoo, K.S.; Karam, D.S.; Abdullah, M.Z. The physiological and psychosocial effects of forest therapy: A systematic review. Urban For. Urban Green. 2020, 54, 126744. [Google Scholar] [CrossRef]
- Doimo, I.; Masiero, M.; Gatto, P. Forest and Wellbeing: Bridging Medical and Forest Research for Effective Forest-Based Initiatives. Forests 2020, 11, 791. [Google Scholar] [CrossRef]
- Lee, J.; Park, B.J.; Tsunetsugu, Y.; Ohira, T.; Kagawa, T.; Miyazaki, Y. Effect of forest bathing on physiological and psychological responses in young Japanese male subjects. Public Health 2011, 125, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Morimoto, K.; Nakadai, A.; Inagaki, H.; Katsumata, M.; Shimizu, T.; Hirata, Y.; Hirata, K.; Suzuki, H.; Miyazaki, Y.; et al. Forest bathing enhances human natural killer activity and expression of anti-cancer proteins. Int. J. Immunopathol. Pharm. 2007, 20 (Suppl. S2), 3–8. [Google Scholar] [CrossRef]
- Lee, J.; Tsunetsugu, Y.; Takayama, N.; Park, B.J.; Li, Q.; Song, C.; Komatsu, M.; Ikei, H.; Tyrvainen, L.; Kagawa, T.; et al. Influence of forest therapy on cardiovascular relaxation in young adults. Evid. Based Complementary Altern. Med. Ecam 2014, 2014, 834360. [Google Scholar] [CrossRef]
- Park, B.J.; Tsunetsugu, Y.; Kasetani, T.; Kagawa, T.; Miyazaki, Y. The physiological effects of Shinrin-yoku (taking in the forest atmosphere or forest bathing): Evidence from field experiments in 24 forests across Japan. Environ. Health Prev. Med. 2010, 15, 18–26. [Google Scholar] [CrossRef]
- Park, B.J.; Shin, C.S.; Shin, W.S.; Chung, C.Y.; Lee, S.H.; Kim, D.J.; Kim, Y.H.; Park, C.E. Effects of Forest Therapy on Health Promotion among Middle-Aged Women: Focusing on Physiological Indicators. Int. J. Environ. Res. Public Health 2020, 17, 4348. [Google Scholar] [CrossRef]
- Shin, W.S.; Shin, C.S.; Yeoun, P.S. The influence of forest therapy camp on depression in alcoholics. Environ. Health Prev. Med. 2012, 17, 73–76. [Google Scholar] [CrossRef] [PubMed]
- Shin, W.S.; Shin, C.S.; Yeoun, P.S.; Kim, J.J. The Influence of interaction with forest on cognitive function. Scand. J. For. Res. 2011, 26, 595–598. [Google Scholar] [CrossRef]
- Jung, W.H.; Woo, J.-M.; Ryu, J.S. Effect of a forest therapy program and the forest environment on female workers’ stress. Urban For. Urban Green. 2015, 14, 274–281. [Google Scholar] [CrossRef]
- Kim, J.G.; Khil, T.G.; Lim, Y.; Park, K.; Shin, M.; Shin, W.S. The Psychological Effects of a Campus Forest Therapy Program. Int. J. Environ. Res. Public Health 2020, 17, 3409. [Google Scholar] [CrossRef]
- World Health Organization. Meeting on the Implementation of the Global Action Plan on the Public Health Response to Dementia; World Health Organization: Geneva, Swizerland, 2018. [Google Scholar]
- Livingston, G.; Sommerlad, A.; Orgeta, V.; Costafreda, S.G.; Huntley, J.; Ames, D.; Ballard, C.; Banerjee, S.; Burns, A.; Cohen-Mansfield, J.; et al. The Lancet International Commission on Dementia Prevention and Care. Lancet Comm. 2017, 390, 2673–2734. [Google Scholar] [CrossRef]
- Intzandt, B.; Black, S.E.; Lanctot, K.L.; Herrmann, N.; Oh, P.; Middleton, L.E. Is Cardiac Rehabilitation Exercise Feasible for People with Mild Cognitive Impairment? Can. Geriatr. J. Cgj 2015, 18, 65–72. [Google Scholar] [CrossRef][Green Version]
- Yi, J.; Ku, B.; Kim, S.G.; Khil, T.; Lim, Y.; Shin, M.; Jeon, S.; Kim, J.; Kang, B.; Shin, J.; et al. Traditional Korean Medicine-Based Forest Therapy Programs Providing Electrophysiological Benefits for Elderly Individuals. Int. J. Environ. Res. Public Health 2019, 16, 4325. [Google Scholar] [CrossRef]
- Sung, J.; Woo, J.M.; Kim, W.; Lim, S.K.; Chung, E.J. The effect of cognitive behavior therapy-based “forest therapy” program on blood pressure, salivary cortisol level, and quality of life in elderly hypertensive patients. Clin. Exp. Hypertens. 2012, 34, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Shin, W.S.; Lee, J. Forest Therapy. In Outdoor Therapies: An Introduction to Practices, Possibilities, and Critical Perspectives; Harper, N.J., Dobud, W.W., Eds.; Taylor & Francis Group: Abingdon, UK, 2020. [Google Scholar]
- Mao, G.; Cao, Y.; Wang, B.; Wang, S.; Chen, Z.; Wang, J.; Xing, W.; Ren, X.; Lv, X.; Dong, J.; et al. The Salutary Influence of Forest Bathing on Elderly Patients with Chronic Heart Failure. Int. J. Environ. Res. Public Health 2017, 14, 368. [Google Scholar] [CrossRef]
- Lee, J.-Y.; Lee, D.-C. Cardiac and pulmonary benefits of forest walking versus city walking in elderly women: A randomised, controlled, open-label trial. Eur. J. Integr. Med. 2014, 6, 5–11. [Google Scholar] [CrossRef]
- Yu, C.P.; Lin, C.M.; Tsai, M.J.; Tsai, Y.C.; Chen, C.Y. Effects of Short Forest Bathing Program on Autonomic Nervous System Activity and Mood States in Middle-Aged and Elderly Individuals. Int. J. Environ. Res. Public Health 2017, 14, 897. [Google Scholar] [CrossRef]
- Lee, H.J.; Son, S.A. Psychological and Physical Effects of 10 Weeks Urban Forest Therapy Program on Dementia Prevention in Low-Income Elderly Living Alone. J. People Plants Environ. 2018, 21, 557–564. [Google Scholar] [CrossRef]
- Jack, C.R., Jr.; Bennett, D.A.; Blennow, K.; Carrillo, M.C.; Dunn, B.; Haeberlein, S.B.; Holtzman, D.M.; Jagust, W.; Jessen, F.; Karlawish, J.; et al. Contributors, NIA-AA Research Framework: Toward a biological definition of Alzheimer’s disease. Alzheimer’s Dement. J. Alzheimer’s Assoc. 2018, 14, 535–562. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Son, S.A. Qualitative Assessment of Experience on Urban Forest Therapy Program for Preventing Dementia of the Elderly Living Alone in Low-Income Class. J. People Plants Environ. 2018, 21, 565–574. [Google Scholar] [CrossRef]
- Horowitz, S. Evidence-Based Health Benefits of Qigong. Altern. Complementary Ther. 2009, 15, 178–183. [Google Scholar] [CrossRef]
- Oh, B.; Butow, P.; Mullan, B.; Clarke, S. Medical Qigong for Cancer Patients: Pilot Study of Impact on Quality of Life, Side Effects of Treatment and Inflammation. Am. J. Chin. Med. 2008, 36, 459–472. [Google Scholar] [CrossRef]
- Solloway, M.R.; Taylor, S.L.; Shekelle, P.G.; Miake-Lye, I.M.; Beroes, J.M.; Shanman, R.M.; Hempel, S. An evidence map of the effect of Tai Chi on health outcomes. Syst. Rev. 2016, 5, 126. [Google Scholar] [CrossRef]
- Jahnke, R.; Larkey, L.; Rogers, C.; Etnier, J.; Lin, F. A comprehensive review of health benefits of qigong and tai chi. Am. J. Health Promot. Ajhp 2010, 24, e1–e25. [Google Scholar] [CrossRef]
- Zeng, Y.; Luo, T.; Xie, H.; Huang, M.; Cheng, A.S. Health benefits of qigong or tai chi for cancer patients: A systematic review and meta-analyses. Complementary Ther. Med. 2014, 22, 173–186. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Kim, S.Y.; Chae, Y.; Kim, M.Y.; Yin, C.; Jung, W.S.; Cho, K.H.; Kim, S.N.; Park, H.J.; Lee, H. Turo (Qi Dance) Program for Parkinson’s Disease Patients: Randomized, Assessor Blind, Waiting-List Control, Partial Crossover Study. Explore 2018, 14, 216–223. [Google Scholar] [CrossRef] [PubMed]
- Chaochao, Y.; Le, W.; Lihong, K.; Feng, S.; Chaoyang, M.; Yanjun, D.; Hua, Z. Acupoint combinations used for treatment of Alzheimer’s disease: A data mining analysis. J. Tradit. Chin. Med. 2018, 38, 943–952. [Google Scholar] [CrossRef]
- Shen, K.; Cho, Y.; Pascoe, E.M.; Hawley, C.M.; Oliver, V.; Hughes, K.M.; Baer, R.; Frazier, J.; Jarvis, E.; Tan, K.S.; et al. The SIESTA Trial: A Randomized Study Investigating the Efficacy, Safety, and Tolerability of Acupressure versus Sham Therapy for Improving Sleep Quality in Patients with End-Stage Kidney Disease on Hemodialysis. Evid. Based Complementary Altern. Med. 2017, 2017, 7570352. [Google Scholar] [CrossRef]
- Kim, J.-H.; Cho, M.-R.; Park, G.-C.; Lee, J.-S. Effects of different acupuncture treatment methods on mild cognitive impairment: A study protocol for a randomized controlled trial. Trials 2019, 20, 551. [Google Scholar] [CrossRef] [PubMed]
- Borg, G.A. Psychophysical bases of perceived exertion. Med. Sci. Sports Exerc. 1982, 14, 377–381. [Google Scholar] [CrossRef]
- Han, J.W.; Kim, T.H.; Jhoo, J.H.; Park, J.H.; Kim, J.L.; Ryu, S.H.; Moon, S.W.; Choo, I.H.; Lee, D.W.; Yoon, J.C.; et al. A Normative Study of the Mini-Mental State Examination for Dementia Screening (MMSE-DS) and Its Short form(SMMSE-DS) in the Korean Elderly. J. Korean Geriatr. Psychiatry 2010, 14, 27–37. [Google Scholar]
- Chin, J.; Park, J.; Yang, S.J.; Yeom, J.; Ahn, Y.; Baek, M.J.; Ryu, H.J.; Lee, B.H.; Han, N.E.; Ryu, K.H.; et al. Re-standardization of the Korean-Instrumental Activities of Daily Living (K-IADL): Clinical Usefulness for Various Neurodegenerative Diseases. Dement. Neurocognitive Disord. 2018, 17, 11–22. [Google Scholar] [CrossRef]
- Nasreddine, Z.S.; Phillips, N.A.; Bedirian, V.; Charbonneau, S.; Whitehead, V.; Collin, I.; Cummings, J.L.; Chertkow, H. The Montreal Cognitive Assessment, MoCA: A brief screening tool for mild cognitive impairment. J. Am. Geriatr. Soc. 2005, 53, 695–699. [Google Scholar] [CrossRef]
- Lee, J.Y.; Dong Woo, L.; Cho, S.J.; Na, D.L.; Hong Jin, J.; Kim, S.K.; You Ra, L.; Youn, J.H.; Kwon, M.; Lee, J.H.; et al. Brief screening for mild cognitive impairment in elderly outpatient clinic: Validation of the Korean version of the Montreal Cognitive Assessment. J. Geriatr. Psychiatry Neurol. 2008, 21, 104–110. [Google Scholar] [CrossRef]
- Brink, T.L.; Yesavage, J.A.; Lum, O.; Heersema, P.H.; Adey, M.; Rose, T.L. Screening Tests for Geriatric Depression. Clin. Gerontol. 2008, 1, 37–43. [Google Scholar] [CrossRef]
- Jung, I.-K.; Kwak, D.-I.; Shin, D.-K.; Lee, M.-S.; Lee, H.-S.; Kim, J.-Y. A Reliabilty and Validity Study of Geriatric Depression Scale. J. Korean Neuropsychiatr. Assoc. 1997, 36, 103–112. [Google Scholar]
- Devlin, N.J.; Brooks, R. EQ-5D and the EuroQol Group: Past, Present and Future. Appl. Health Econ. Health Policy 2017, 15, 127–137. [Google Scholar] [CrossRef]
- Jelic, V.; Kowalski, J. Evidence-Based Evaluation of Diagnostic Accuracy of Resting EEG in Dementia and Mild Cognitive Impairment. Clin. EEG Neurosci. 2009, 40, 129–142. [Google Scholar] [CrossRef] [PubMed]
- Jackson, C.E.; Snyder, P.J. Electroencephalography and event-related potentials as biomarkers of mild cognitive impairment and mild Alzheimer’s disease. Alzheimers Dement. 2008, 4 (Suppl. S1), S137–S143. [Google Scholar] [CrossRef]
- Kyle, U.G.; Bosaeus, I.; De Lorenzo, A.D.; Deurenberg, P.; Elia, M.; Gomez, J.M.; Heitmann, B.L.; Kent-Smith, L.; Melchior, J.C.; Pirlich, M.; et al. Bioelectrical impedance analysis—Part I: Review of principles and methods. Clin. Nutr. 2004, 23, 1226–1243. [Google Scholar] [CrossRef] [PubMed]
- Task Force of The European Society of Cardiology and The North American Society of Pacing and Electrophysiology, Heart Rate Variability. Standards of measurement, physiological interpretation, and clinical use. Eur. Heart J. 1996, 17, 354–381. [Google Scholar] [CrossRef]
- Rossini, P.M.; Di Iorio, R.; Vecchio, F.; Anfossi, M.; Babiloni, C.; Bozzali, M.; Bruni, A.C.; Cappa, S.F.; Escudero, J.; Fraga, F.J.; et al. Early diagnosis of Alzheimer’s disease: The role of biomarkers including advanced EEG signal analysis. Report from the IFCN-sponsored panel of experts. Clin. Neurophysiol. Off. J. Int. Fed. Clin. Neurophysiol. 2020, 131, 1287–1310. [Google Scholar] [CrossRef] [PubMed]
- Dittmar, M. Reliability and variability of bioimpedance measures in normal adults: Effects of age, gender, and body mass. Am. J. Phys. Anthr. 2003, 122, 361–370. [Google Scholar] [CrossRef]
- Jun, M.H.; Kim, S.; Ku, B.; Cho, J.; Kim, K.; Yoo, H.R.; Kim, J.U. Glucose-independent segmental phase angles from multi-frequency bioimpedance analysis to discriminate diabetes mellitus. Sci. Rep. 2018, 8, 648. [Google Scholar] [CrossRef]
- Grundmann, O.; Yoon, S.L.; Williams, J.J. The value of bioelectrical impedance analysis and phase angle in the evaluation of malnutrition and quality of life in cancer patients—A comprehensive review. Eur. J. Clin. Nutr 2015, 69, 1290–1297. [Google Scholar] [CrossRef]
- Choi, J.; Ku, B.; You, Y.G.; Jo, M.; Kwon, M.; Choi, Y.; Jung, S.; Ryu, S.; Park, E.; Go, H.; et al. Resting-state prefrontal EEG biomarkers in correlation with MMSE scores in elderly individuals. Sci. Rep. 2019, 9, 10468. [Google Scholar] [CrossRef] [PubMed]
- Development R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2011; Volume 1. [Google Scholar]
- Van Buuren, S.; Groothuis-Oudshoorn, K. Mice: Multivariate Imputation by Chained Equations in R. J. Stat. Softw. 2011, 45, 67. [Google Scholar] [CrossRef]
- Dobson, A.J.; Barnett, A.G. An Introduction to Generalized Linear Models; CRC Press: New York, NY, USA, 2018. [Google Scholar]
- Dunnett, C.W. New tables for multiple comparisons with a control. Biometrics 1964, 20, 482–491. [Google Scholar] [CrossRef]
- Rosnow, R.L.; Rosenthal, R.; Rubin, D.B. Contrasts and Correlations in Effect-Size Estimation. Psychol. Sci. 2016, 11, 446–453. [Google Scholar] [CrossRef] [PubMed]
- Souza, M.F.; Tomeleri, C.M.; Ribeiro, A.S.; Schoenfeld, B.J.; Silva, A.M.; Sardinha, L.B.; Cyrino, E.S. Effect of resistance training on phase angle in older women: A randomized controlled trial. Scand. J. Med. Sci. Sports 2017, 27, 1308–1316. [Google Scholar] [CrossRef]
- Kyle, U.G.; Genton, L.; Pichard, C. Low phase angle determined by bioelectrical impedance analysis is associated with malnutrition and nutritional risk at hospital admission. Clin. Nutr. 2013, 32, 294–299. [Google Scholar] [CrossRef] [PubMed]
- Maddocks, M.; Kon, S.S.; Jones, S.E.; Canavan, J.L.; Nolan, C.M.; Higginson, I.J.; Gao, W.; Polkey, M.I.; Man, W.D. Bioelectrical impedance phase angle relates to function, disease severity and prognosis in stable chronic obstructive pulmonary disease. Clin. Nutr. 2015, 34, 1245–1250. [Google Scholar] [CrossRef]
- Stapel, S.N.; Looijaard, W.G.P.M.; Dekker, I.M.; Girbes, A.R.J.; Weijs, P.J.M.; Straaten, H.M.O.V. Bioelectrical impedance analysis-derived phase angle at admission as a predictor of 90-day mortality in intensive care patients. Eur. J. Clin. Nutr. 2018, 72, 1019–1025. [Google Scholar] [CrossRef]
- Moretti, D. Individual analysis of EEG frequency and band power in mild Alzheimer’s disease. Clin. Neurophysiol. 2004, 115, 299–308. [Google Scholar] [CrossRef]
- Hogan, M.J.; Swanwick, G.R.J.; Kaiser, J.; Rowan, M.; Lawlor, B. Memory-related EEG power and coherence reductions in mild Alzheimer’s disease. Int. J. Psychophysiol. 2003, 49, 147–163. [Google Scholar] [CrossRef]
- Tsang, H.W.; Fung, K.M.; Chan, A.S.; Lee, G.; Chan, F. Effect of a qigong exercise programme on elderly with depression. Int. J. Geriatr. Psychiatry 2006, 21, 890–897. [Google Scholar] [CrossRef]
- Kim, K.B.; Cohen, S.M.; Oh, H.K.; Sok, S.R. The effects of meridian exercise on anxiety, depression, and self-esteem of female college students in Korea. Holist. Nurs. Pract. 2004, 18, 230–234. [Google Scholar] [CrossRef]
- Song, C.; Ikei, H.; Miyazaki, Y. Physiological Effects of Nature Therapy: A Review of the Research in Japan. Int. J. Environ. Res. Public Health 2016, 13, 781. [Google Scholar] [CrossRef]
- Song, C.; Ikei, H.; Kobayashi, M.; Miura, T.; Taue, M.; Kagawa, T.; Li, Q.; Kumeda, S.; Imai, M.; Miyazaki, Y. Effect of forest walking on autonomic nervous system activity in middle-aged hypertensive individuals: A pilot study. Int. J. Environ. Res. Public Health 2015, 12, 2687–2699. [Google Scholar] [CrossRef]
- Aspinall, P.; Mavros, P.; Coyne, R.; Roe, J. The urban brain: Analysing outdoor physical activity with mobile EEG. Br. J. Sports Med. 2015, 49, 272–276. [Google Scholar] [CrossRef] [PubMed]
- Neale, C.; Aspinall, P.; Roe, J.; Tilley, S.; Mavros, P.; Cinderby, S.; Coyne, R.; Thin, N.; Ward Thompson, C. The impact of walking in different urban environments on brain activity in older people. Cities Health 2019, 4, 94–106. [Google Scholar] [CrossRef]
- Shafer, K.J.; Siders, W.A.; Johnson, L.K.; Lukaski, H.C. Validity of segmental multiple-frequency bioelectrical impedance analysis to estimate body composition of adults across a range of body mass indexes. Nutrition 2009, 25, 25–32. [Google Scholar] [CrossRef]
- Raja, M.K.; Raymer, G.H.; Moran, G.R.; Marsh, G.; Thompson, R.T. Changes in tissue water content measured with multiple-frequency bioimpedance and metabolism measured with 31P-MRS during progressive forearm exercise. J. Appl. Physiol. 2006, 101, 1070–1075. [Google Scholar] [CrossRef]
- Gates, N.; Fiatarone Singh, M.A.; Sachdev, P.S.; Valenzuela, M. The effect of exercise training on cognitive function in older adults with mild cognitive impairment: A meta-analysis of randomized controlled trials. Am. J. Geriatr. Psychiatry Off. J. Am. Assoc. Geriatr. Psychiatry 2013, 21, 1086–1097. [Google Scholar] [CrossRef]

| Control | Qigong Program | Walking Program | p-Value | |
|---|---|---|---|---|
| N (%) | 31 (41.9%) | 25 (33.8%) | 18 (24.3%) | |
| Sex | 0.003 | |||
| Male | 21 (67.7%) | 6 (24.0%) | 11 (61.1%) | |
| Female | 10 (32.3%) | 19 (76.0%) | 7 (38.9%) | |
| Age (y) | 75.7 ± 4.2 | 75.1 ± 4.9 | 78.0 ± 5.5 | 0.145 |
| Height (cm) | 160.0 ± 10.1 | 154.2 ± 7.3 | 159.1 ± 8.4 | 0.047 |
| Weight (kg) | 63.6 ± 11.8 | 60.7 ± 7.9 | 62.3 ± 8.3 | 0.558 |
| BMI (kg/m2) | 24.7 ± 3.4 | 25.6 ± 3.5 | 24.5 ± 2.2 | 0.490 |
| MMSE | 25.7 ± 2.4 | 26.8 ± 2.0 | 26.2 ± 2.2 | 0.170 |
| Smoking: Yes | 1 (3.3%) | 2 (9.5%) | 0 (0.0%) | 0.338 |
| Alcohol: Yes | 10 (33.3%) | 8 (40.0%) | 3 (17.6%) | 0.327 |
| Religion: Yes | 22 (73.3%) | 17 (68.0%) | 10 (55.6%) | 0.444 |
| Marital status: Married | 17 (56.7%) | 6 (24.0%) | 8 (44.4%) | 0.050 |
| Education level | 6.2 ± 4.0 | 5.2 ± 4.2 | 8.4 ± 2.5 | 0.023 |
| Medical History | ||||
| Hypertension: Yes | 9 (42.9%) | 13 (59.1%) | 8 (61.5%) | 0.456 |
| Diabetes: Yes | 8 (38.1%) | 6 (27.3%) | 3 (23.1%) | 0.601 |
| Dyslipidemia: Yes | 3 (14.3%) | 12 (54.5%) | 3 (23.1%) | 0.013 |
| Arthritis: Yes | 6 (28.6%) | 9 (40.9%) | 3 (23.1%) | 0.500 |
| Cerebrovascular disease: Yes | 2 (9.5%) | 1 (4.5%) | 1 (7.7%) | 0.815 |
| Depression: Yes | 1 (4.8%) | 0 (0.0%) | 0 (0.0%) | 0.428 |
| Other | 10 (50.0%) | 11 (50.0%) | 5 (38.5%) | 0.767 |
| Daily activity hours (h/day) | 1.0 ± 0.8 | 1.0 ± 0.9 | 1.1 ± 0.9 | 0.880 |
| Control | Qigong Program | Walking Program | QP–CN | WP–CN | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
(95% CI) | (95% CI) | (95% CI) | (95% CI) | (95% CI) | |||||||||
| MoCA | 19.18 | 2.09 ** (0.76, 3.41) | 0.85 | 20.48 | 2.22 *** (1.10, 3.34) | 0.89 | 20.67 | 3.46 *** (2.12, 4.80) | 1.34 | 0.14 (−1.88, 2.15) | 0.05 | 1.37 (−0.82, 3.56) | 0.46 |
| GDS | 12.45 | 0.40 (−2.28, 3.09) | 0.10 | 14.33 | −2.23· (−4.49, 0.04) | 0.52 | 7.07 | −0.03 (−2.73, 2.66) | 0.01 | −2.63 (−6.64, 1.38) | 0.59 | −0.44 (−5.02, 4.14) | 0.09 |
| EQ-5D | 0.84 | 0.01 (−0.05, 0.07) | 0.09 | 0.84 | −0.00 (−0.05, 0.04) | 0.02 | 0.85 | 0.05· (−0.00, 0.11) | 0.50 | −0.01 (−0.10, 0.07) | 0.11 | 0.04 (−0.05, 0.14) | 0.35 |
| Control | Qigong Program | Walking Program | QP−CN | WP−CN | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
(95% CI) | (95% CI) | (95% CI) | (95% CI) | (95% CI) | |||||||||
| MEF (Hz) | 9.06 | −0.13 (−0.31, 0.06) | 0.27 | 9.16 | 0.09 (−0.11, 0.29) | 0.20 | 9.03 | 0.04 (−0.21, 0.29) | 0.10 | 0.22 (−0.11, 0.54) | 0.43 | 0.17 (−0.19, 0.53) | 0.32 |
| 2.44 | 0.01 (−0.24, 0.26) | 0.02 | 2.59 | −0.28 * (−0.55, −0.00) | 0.45 | 2.52 | 0.33· (−0.01, 0.67) | 0.54 | −0.29 (−0.73, 0.15) | 0.42 | 0.32 (−0.17, 0.81) | 0.45 | |
| 2.99 | −0.17 (−0.46, 0.12) | 0.24 | 3.21 | −0.39 * (−0.71, −0.06) | 0.53 | 3.03 | 0.19 (−0.21, 0.58) | 0.26 | −0.21 (−0.73, 0.30) | 0.27 | 0.36 (−0.21, 0.93) | 0.43 | |
| ATR | 1.12 | 0.08 (−0.02, 0.17) | 0.33 | 1.12 | 0.08 (−0.03, 0.18) | 0.34 | 1.15 | 0.04 (−0.09, 0.16) | 0.16 | 0.00 (−0.16, 0.17) | 0.01 | −0.04 (−0.22, 0.14) | 0.15 |
| Control | Qigong Program | Walking Program | BP−CN | WP−CN | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
(95% CI) | (95% CI) | (95% CI) | (95% CI) | (95% CI) | |||||||||
| FFM (kg) | 47.27 | −0.57· (−1.20, 0.06) | 0.36 | 40.80 | −0.68· (−1.37, 0.00) | 0.42 | 44.65 | −1.05 ** (−1.80, −0.29) | 0.66 | −0.12 (−1.22, 0.99) | 0.06 | −0.48 (−1.61, 0.64) | 0.26 |
| BFM (kg) | 16.91 | 0.36 (−0.35, 1.07) | 0.20 | 20.24 | 0.63 (−0.14, 1.40) | 0.34 | 17.62 | 0.47 (−0.37, 1.31) | 0.26 | 0.27 (−0.98, 1.52) | 0.13 | 0.11 (−1.14, 1.37) | 0.05 |
| %BF (%) | 26.33 | 0.58 (−0.31, 1.47) | 0.26 | 32.67 | 1.08 * (0.11, 2.05) | 0.46 | 28.20 | 1.07 * (0.02, 2.13) | 0.48 | 0.50 (−1.08, 2.08) | 0.19 | 0.49 (−1.09, 2.08) | 0.19 |
| Imp_arm (Ω) | 312.72 | −7.13 * (−12.62, −1.63) | 0.52 | 333.27 | 2.08 (−3.92, 8.07) | 0.14 | 325.74 | 0.89 (−5.79, 7.58) | 0.06 | 9.20· (−0.42, 18.82) | 0.58 | 8.02 (−1.94, 17.98) | 0.49 |
| Imp_leg (Ω) | 164.66 | −0.99 (−6.41, 4.44) | 0.07 | 159.62 | 15.63 *** (9.97, 21.29) | 1.15 | 150.42 | 13.06 *** (6.89, 19.22) | 1.00 | 16.62 *** (7.05, 26.18) | 1.05 | 14.04 ** (4.40, 23.69) | 0.89 |
| Reactance_arm (Ω) | 31.67 | −2.68 *** (−3.66, −1.70) | 1.09 | 30.77 | −0.86 (−1.91, 0.20) | 0.34 | 32.09 | −2.09 *** (−3.27, −0.91) | 0.84 | 1.82 * (0.12, 3.53) | 0.65 | 0.59 (−1.18, 2.36) | 0.20 |
| Reactance_leg (Ω) | 14.91 | −0.14 (−0.86, 0.57) | 0.08 | 13.85 | 1.84 *** (1.06, 2.63) | 0.98 | 12.73 | 2.15 *** (1.29, 3.02) | 1.17 | 1.99 ** (0.72, 3.25) | 0.95 | 2.30 *** (1.01, 3.58) | 1.10 |
| PhA_body | 5.62 | −0.22 *** (−0.32, −0.12) | 0.89 | 5.28 | −0.15 ** (−0.25, −0.04) | 0.56 | 5.45 | −0.08 (−0.20, 0.04) | 0.32 | 0.07 (−0.10, 0.25) | 0.26 | 0.14 (−0.03, 0.32) | 0.50 |
| PhA_arm | 5.86 | −0.38 *** (−0.47, −0.28) | 1.58 | 5.33 | −0.23 *** (−0.34, −0.13) | 0.94 | 5.65 | −0.32 *** (−0.43, −0.21) | 1.35 | 0.14 (−0.03, 0.31) | 0.51 | 0.05 (−0.11, 0.22) | 0.20 |
| PhA_leg | 5.23 | 0.02 (−0.13, 0.16) | 0.04 | 4.98 | 0.12 (−0.03, 0.28) | 0.33 | 4.88 | 0.37 *** (0.20, 0.54) | 1.03 | 0.11 (−0.15, 0.37) | 0.26 | 0.35 ** (0.10, 0.61) | 0.86 |
| Control | Qigong Program | Walking Program | BP−CN | WP−CN | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
(95% CI) | (95% CI) | (95% CI) | (95% CI) | (95% CI) | |||||||||
| HF (msec2) | 4.62 | 0.19 (−0.28, 0.66) | 0.16 | 4.85 | −0.11 (−0.61, 0.40) | 0.09 | 4.88 | −0.28 (−0.85, 0.30) | 0.23 | −0.29 (−1.11, 0.52) | 0.21 | −0.46 (−1.32, 0.39) | 0.33 |
| LF (msec2) | 4.48 | 0.40 (−0.16, 0.95) | 0.28 | 4.51 | −0.16 (−0.76, 0.43) | 0.11 | 4.96 | −0.34 (−1.02, 0.34) | 0.24 | −0.56 (−1.52, 0.41) | 0.34 | −0.74 (−1.75, 0.27) | 0.44 |
| %LF | 49.36 | 1.25 (−0.97, 3.47) | 0.22 | 47.99 | −0.50 (−2.86, 1.86) | 0.09 | 50.31 | 0.24 (−2.45, 2.94) | 0.04 | −1.75 (−5.58, 2.07) | 0.27 | −1.00 (−5.02, 3.01) | 0.15 |
| TP (msec2) | 6.36 | 0.07 (−0.48, 0.62) | 0.05 | 6.46 | −0.25 (−0.83, 0.34) | 0.17 | 6.54 | −0.12 (−0.80, 0.55) | 0.09 | −0.32 (−1.27, 0.63) | 0.20 | −0.20 (−1.20, 0.80) | 0.12 |
| HR (bpm) | 67.42 | 2.42 (−0.92, 5.76) | 0.28 | 67.87 | 2.97· (−0.58, 6.51) | 0.34 | 68.65 | −0.44 (−4.49, 3.62) | 0.05 | 0.54 (−5.21, 6.29) | 0.06 | −2.86 (−8.90, 3.18) | 0.29 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yi, J.; Kim, S.G.; Khil, T.; Shin, M.; You, J.-H.; Jeon, S.; Park, G.H.; Jeong, A.Y.; Lim, Y.; Kim, K.; et al. Psycho-Electrophysiological Benefits of Forest Therapies Focused on Qigong and Walking with Elderly Individuals. Int. J. Environ. Res. Public Health 2021, 18, 3004. https://doi.org/10.3390/ijerph18063004
Yi J, Kim SG, Khil T, Shin M, You J-H, Jeon S, Park GH, Jeong AY, Lim Y, Kim K, et al. Psycho-Electrophysiological Benefits of Forest Therapies Focused on Qigong and Walking with Elderly Individuals. International Journal of Environmental Research and Public Health. 2021; 18(6):3004. https://doi.org/10.3390/ijerph18063004
Chicago/Turabian StyleYi, Jiyune, Seul Gee Kim, Taegyu Khil, Minja Shin, Jin-Hee You, Sookja Jeon, Gue Hong Park, Ah Young Jeong, Youngsuwn Lim, Kahye Kim, and et al. 2021. "Psycho-Electrophysiological Benefits of Forest Therapies Focused on Qigong and Walking with Elderly Individuals" International Journal of Environmental Research and Public Health 18, no. 6: 3004. https://doi.org/10.3390/ijerph18063004
APA StyleYi, J., Kim, S. G., Khil, T., Shin, M., You, J.-H., Jeon, S., Park, G. H., Jeong, A. Y., Lim, Y., Kim, K., Kim, J., Kang, B., Lee, J., Park, J. H., Ku, B., Choi, J., Cha, W., Lee, H.-J., Shin, C., ... Kim, J. U. (2021). Psycho-Electrophysiological Benefits of Forest Therapies Focused on Qigong and Walking with Elderly Individuals. International Journal of Environmental Research and Public Health, 18(6), 3004. https://doi.org/10.3390/ijerph18063004








