Impact of Ginger Root Powder Dietary Supplement on Productive Performance, Egg Quality, Antioxidant Status and Blood Parameters in Laying Japanese Quails
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Birds and Management
2.2. Preparation of Ginger Powder Samples
2.3. Measurement of Productive Performance and Egg Quality
2.4. Blood Biochemical Parameters Analysis
2.5. Antioxidant Status
2.6. Statistical Analysis
3. Results
3.1. Productive Performance
3.2. Quality Traits of Eggs
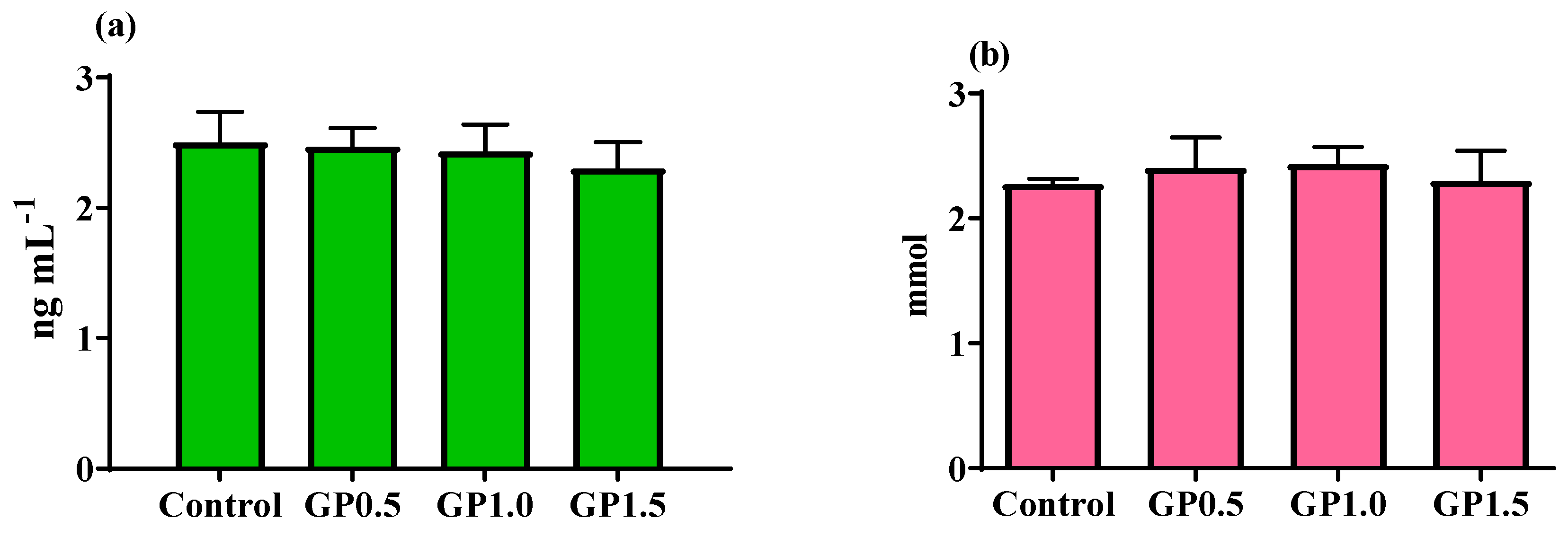
3.3. Blood Parameters and Egg Yolk Phenolic Compounds
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Castanon, J. History of the Use of Antibiotic as Growth Promoters in European Poultry Feeds. Poult. Sci. 2007, 86, 2466–2471. [Google Scholar] [CrossRef]
- Nemati, Z.; Mohammadi, R. The effects of different levels of dietary garlic powder on productive performance, egg quality traits and blood parameters of laying hens. J. Anim. Prod. 2017, 19, 657–670. [Google Scholar]
- Zeng, Z.; Zhang, S.; Wang, H.; Piao, X. Essential oil and aromatic plants as feed additives in non-ruminant nutrition: A review. J. Anim. Sci. Biotechnol. 2015, 6, 7–17. [Google Scholar] [CrossRef]
- Ravindran, P.; Nirmal, B. Ginger: The genus Zingiber. In Medicinal and Aromatic Plant–Industrial Profile; CRC Press: Boca Raton, FL, USA, 2005. [Google Scholar]
- Food and Agriculture Organization of the United Nations, Statistics Division. Ginger production in 2016, Crops/ Regions/ World/ Production/ Quantity (from pick lists). 2017. Available online: http://www.fao.org/faostat/en/#data/QC/visualize (accessed on 2 March 2021).
- Policegoudra, R.; Aradhya, S. Biochemical changes and antioxidant activity of mango ginger (Curcuma amada Roxb.) rhizomes during postharvest storage at different temperatures. Postharvest Biol. Technol. 2007, 46, 189–194. [Google Scholar] [CrossRef]
- Dieumou, F.; Teguia, A.; Kuiate, J.; Tamokou, J.; Fonge, N.; Dongmo, M. Effects of ginger (Zingiber officinale) and garlic (Allium sativum) essential oils on growth performance and gut microbial population of broiler chickens. Livest. Res. Rural Dev. 2009, 21, 23–32. [Google Scholar]
- Arablou, T.; Aryaeian, N.; Valizadeh, M.; Sharifi, F.; Hosseini, A.; Djalali, M. The effect of ginger consumption on glycemic status, lipid profile and some inflammatory markers in patients with type 2 diabetes mellitus. Int. J. Food Sci. Nutr. 2013, 65, 515–520. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, D.; Mukherjee, A.; Sikdar, S.; Paul, A.; Ghosh, S.; Khuda-Bukhsh, A.R. [6]-Gingerol isolated from ginger attenuates sodium arsneenite induced oxidative stress and plays a corrective role in improving insulin signaling in mice. Toxicol. Lett. 2012, 210, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Vishwakarma, S.L.; Pal, S.C.; Kasture, V.S.; Kasture, S.B. Anxiolytic and antiemetic activity of Zingiber officinale. Phytother. Res. 2002, 16, 621–626. [Google Scholar] [CrossRef] [PubMed]
- Ernst, E.; Pittler, M.H. Efficacy of ginger for nausea and vomiting: A systematic review of randomized clinical trials. Br. J. Anaesth. 2000, 84, 367–371. [Google Scholar] [CrossRef]
- Grzanna, R.; Lindmark, L.; Frondoza, C.G. Ginger—An Herbal Medicinal Product with Broad Anti-Inflammatory Actions. J. Med. Food 2005, 8, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Al-Amin, Z.M.; Thomson, M.; Al-Qattan, K.K.; Peltonen-Shalaby, R.; Ali, M. Anti-diabetic and hypolipidaemic properties of ginger (Zingiber officinale) in streptozotocin-induced diabetic rats. Br. J. Nutr. 2006, 96, 660–666. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.K.; Singh, M.; Jain, P.; Bordia, A. Protective effect of ginger, Zingiber officinale Rosc on experimental atherosclerosis in rabbits. Indian J. Exp. Biol. 2004, 42, 736–738. [Google Scholar]
- Bosisio, E. Effect of the flavanolignans of Silybum marianum L. On lipid peroxidation in rat liver microsomes and freshly isolated hepatocytes. Pharmacol. Res. 1992, 25, 147–165. [Google Scholar] [CrossRef]
- Vipin, A.V.; Raksha Rao, K.; Kurrey, N.K.; Anu Appaiah, K.A.; Venkateswaran, G. Protective effects of phenolics rich extract of ginger against Aflatoxin B1-induced oxidative stress and hepatotoxicity. Biomed. Pharmacother. 2017, 91, 415–424. [Google Scholar]
- Wang, W.; Wang, Z. Studies of commonly used traditional medicine-ginger. Zhongguo Zhong Yao Za Zhi China J. Chin. Mater. Med. 2005, 30, 1569–1573. [Google Scholar]
- Gao, Y.; Ozel, M.Z.; Dugmore, T.; Sulaeman, A.; Matharu, A.S. A biorefinery strategy for spent industrial ginger waste. J. Hazard. Mater. 2021, 401, 123400. [Google Scholar] [CrossRef]
- Wiastuti, T.; Khasanah, L.U.; Kawiji, W.A.; Manuhara, G.J.; Utami, R. Characterization of active paper packaging incorporated with ginger pulp oleoresin. In Proceedings of the IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2016; Volume 107, p. 012057. [Google Scholar]
- Al-Shuwaili, M.A.; Ibrahim, E.; Naqi Al-Bayati, M. Effect of dietary herbal plants supplement in turkey diet on performance and some blood biochemical parameters. Glob. J. Biosci. Biotechnol. 2015, 4, 153–157. [Google Scholar]
- Yusuf, M.; Hasan, M.; Elnabtiti, A.; Cui, H. Single dose of ginger powder, supported with organic acid or probiotic, maximizes the laying, egg quality hatchability and immune performances of laying Japanese quails. Int. J. Recent Sci. Res. 2015, 6, 6707–6711. [Google Scholar]
- Zhang, G.F.; Yang, Z.B.; Wang, Y.; Yang, W.R.; Jiang, S.Z.; Gai, G.S. Effects of ginger root (Zingiber officinale) processed to different particle sizes on growth performance, antioxidant status, and serum metabolites of broiler chickens. Poult. Sci. 2009, 88, 2159–2166. [Google Scholar] [CrossRef]
- Incharoen, T.; Yamauchi, K. Production Performance, Egg Quality and Intestinal Histology in Laying Hens Fed Dietary Dried Fermented Ginger. Int. J. Poult. Sci. 2009, 8, 1078–1085. [Google Scholar] [CrossRef]
- Najafi, S.; Taherpour, K. Effects of dietary ginger (Zingiber Ofjicinale), cinnamon (Cinnamomum), synbiotic and antibiotic supplementation on performance of broilers. J. Anim. Sci. Adv. 2014, 4, 658–667. [Google Scholar]
- Abd El-Galil, K.; Mahmoud, H.A. Effect of ginger roots meal as feed additives in laying Japanese quail diets. J. Am. Sci. 2015, 2, 233–234. [Google Scholar]
- Nasiroleslami, M.; Torki, M. Including essential oils of fennel (Foeniculum vulgare) and ginger (Zingiber officinale) to diet and evaluating performance of laying hens, white blood cell count and egg quality characteristics. Adv. Environ. Biol. 2010, 4, 341–346. [Google Scholar]
- Ibtisham, F.; Nawab, A.; Niu, Y.; Wang, Z.; Wu, J.; Xiao, M.; An, L. The effect of ginger powder and Chinese herbal medicine on production performance, serum metabolites and antioxidant status of laying hens under heat-stress condition. J. Therm. Biol. 2019, 81, 20–24. [Google Scholar] [CrossRef]
- Habibi, R.; Sadeghi, G.; Karimi, A. Effect of different concentrations of ginger root powder and its essential oil on growth performance, serum metabolites and antioxidant status in broiler chicks under heat stress. Br. Poult. Sci. 2014, 55, 228–237. [Google Scholar] [CrossRef]
- Damaziak, K.; Gozdowski, D.; Niemiec, J.; Riedel, J.; Róg, D.; Siennicka, A. Effects of ginger or ginger and thyme extract in laying hens feeding on productive results and eggs quality. Ann. Wars. Univ. Life Sci. SGGW Anim. Sci. 2018, 57, 5–18. [Google Scholar] [CrossRef]
- Wang, X.; Wu, S.; Zhang, H.; Yue, H.; Qi, G.; Li, J. Effect of dietary protein sources and storage temperatures on egg internal quality of stored shell eggs. Anim. Nutr. 2015, 1, 299–304. [Google Scholar] [CrossRef]
- NRC. Nutrient Requirements of Poultry; National Academy Press: Washington, DC, USA, 1994. [Google Scholar]
- Nourbakhsh Amiri, Z.; Najafpour, G.; Mohammadi, M.; Moghadamnia, A. Subcritical water extraction of bioactive compounds from ginger (Zingiber officinale Roscoe). Int. J. Eng. 2018, 31, 1991–2000. [Google Scholar]
- AOAC. Official Methods of Analysis; Association of Official Analytical Chemists: Washington, DC, USA, 1990. [Google Scholar]
- Nemati, Z.; Alirezalu, K.; Besharati, M.; Holman, B.; Hajipour, M.; Bohrer, B. The Effect of Dietary Supplementation with Inorganic or Organic Selenium on the Nutritional Quality and Shelf Life of Goose Meat and Liver. Animals 2021, 11, 261. [Google Scholar] [CrossRef] [PubMed]
- Nemati, Z.; Ahmadian, H.; Besharati, M.; Lesson, S.; Alirezalu, K.; Domínguez, R.; Lorenzo, J.M. Assessment of Dietary Selenium and Vitamin E on Laying Performance and Quality Parameters of Fresh and Stored Eggs in Japanese Quails. Foods 2020, 9, 1324. [Google Scholar] [CrossRef]
- Ahmadian, H.; Nemati, Z.; Karimi, A.; Safari, R. Effect of different dietary selenium sources and storage temperature on enhancing the shelf life of quail eggs. Anim. Prod. Res. 2019, 8, 23–33. (In Persian) [Google Scholar]
- Nemati, Z.; Alirezalu, K.; Besharati, M.; Amirdahri, S.; Franco, D.; Lorenzo, J.M. Improving the Quality Characteristics and Shelf Life of Meat and Growth Performance in Goose Fed Diets Supplemented with Vitamin E. Foods 2020, 9, 798. [Google Scholar] [CrossRef]
- Mohamed, A.B.; Al-Rubaee, M.A.; Jalil, A.Q. Effect of Ginger (Zingiber officinale) on Performance and Blood Serum Parameters of Broiler. Int. J. Poult. Sci. 2012, 11, 143–146. [Google Scholar] [CrossRef]
- Karangiya, V.K.; Savsani, H.H.; Patil, S.S.; Garg, D.D.; Murthy, K.S.; Ribadiya, N.K.; Vekariya, S.J. Effect of dietary supplementation of garlic, ginger and their combination on feed intake, growth performance and economics in commercial broilers. Vet. World 2016, 9, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Oso, A.O.; Awe, A.W.; Awosoga, F.G.; Bello, F.A.; Akinfenwa, T.A.; Ogunremi, E.B. Effect of ginger (Zingiber officinale Roscoe) on growth performance, nutrient digestibility, serum metabolites, gut morphology, and microflora of growing guinea fowl. Trop. Anim. Health Prod. 2013, 45, 1763–1769. [Google Scholar] [CrossRef] [PubMed]
- Thayalini, K.; Shanmugavelu, S.; Saminathan, P.; SitiMasidayu, M.; Noridayusni, Y.; Zainmuddin, H.; Nurul Akmai, C.; Wong, H. Effects of Cymbopogon citratus leaf and Zingiber officinale rhizome supplementation on growth performance, ileal morphology and lactic acid concentration in broilers. Malays. J. Anim. Sci. 2011, 14, 43–49. [Google Scholar]
- Wen, C.; Gu, Y.; Tao, Z.; Cheng, Z.; Wang, T.; Zhou, Y. Effects of Ginger Extract on Laying Performance, Egg Quality, and Antioxidant Status of Laying Hens. Animals 2019, 9, 857. [Google Scholar] [CrossRef] [PubMed]
- Akbarian, A.; Golian, A.; Ahmadi, A.S.; Moravej, H. Effects of ginger root (Zingiber officinale) on egg yolk cholesterol, antioxidant status and performance of laying hens. J. Appl. Anim. Res. 2011, 39, 19–21. [Google Scholar] [CrossRef]
- PR, S.A.; Prakash, J. Chemical composition and antioxidant properties of ginger root (Zingiber officinale). J. Med. Plants Res. 2010, 4, 2674–2679. [Google Scholar]
- Hashimoto, K.; Satoh, K.; Murata, P.; Makino, B.; Sakakibara, I.; Kase, Y.; Ishige, A.; Higuchi, M.; Sasaki, H. Component of Zingiber officinale that Improves the Enhancement of Small Intestinal Transport. Planta Med. 2002, 68, 936–939. [Google Scholar] [CrossRef]
- Platel, K.; Srinivasan, K. Influence of dietary spices and their active principles on pancreatic digestive enzymes in albino rats. Food Nahrung 2000, 44, 42–46. [Google Scholar] [CrossRef]
- Herve, T.; Raphaël, K.J.; Ferdinand, N.; Victor Herman, N.; Willy Marvel, N.M.; Cyril D’Alex, T.; Laurine Vitrice, F.T. Effects of ginger (Zingiber officinale, Roscoe) essential oil on growth and laying performances, serum metabolites, and egg yolk antioxidant and cholesterol status in laying Japanese quail. J. Vet. Med. 2019, 2019. [Google Scholar] [CrossRef]
- Zhao, X.; Yang, Z.B.; Yang, W.R.; Wang, Y.; Jiang, S.Z.; Zhang, G.G. Effects of ginger root (Zingiber officinale) on laying performance and antioxidant status of laying hens and on dietary oxidation stability. Poult. Sci. 2011, 90, 1720–1727. [Google Scholar] [CrossRef]
- An, S.; Liu, G.; Guo, X.; An, Y.; Wang, R. Ginger extract enhances antioxidant ability and immunity of layers. Anim. Nutr. 2019, 5, 407–409. [Google Scholar] [CrossRef]
- Kiyama, R. Nutritional implications of ginger: Chemistry, biological activities and signaling pathways. J. Nutr. Biochem. 2020, 86, 108486. [Google Scholar] [CrossRef]
- Saeid, J.M.; Mohamed, A.B.; Al-Baddy, M.A. Effect of Aqueous Extract of Ginger (Zingiber officinale) on Blood Biochemistry Parameters of Broiler. Int. J. Poult. Sci. 2010, 9, 944–947. [Google Scholar] [CrossRef]
- Akhani, S.P.; Vishwakarma, S.L.; Goyal, R.K. Anti-diabetic activity of Zingiber officinale in streptozotocin-induced type I diabetic rats. J. Pharm. Pharmacol. 2004, 56, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Kar, A.; Choudhary, B.K.; Bandyopadhyay, N.G. Preliminary studies on the inorganic constituents of some indigenous hypoglycaemic herbs on oral glucose tolerance test. J. Ethnopharmacol. 1999, 64, 179–184. [Google Scholar] [CrossRef]
- Jang, I.; Ko, Y.; Kang, S.; Lee, C. Effect of a commercial essential oil on growth performance, digestive enzyme activity and intestinal microflora population in broiler chickens. Anim. Feed Sci. Technol. 2007, 134, 304–315. [Google Scholar] [CrossRef]
- Manju, V.; Viswanathan, P.; Nalini, N. Hypolipidemic Effect of Ginger in 1,2-Dimethyl Hydrazine-Induced Experimental Colon Carcinogenesis. Toxicol. Mech. Methods 2006, 16, 461–472. [Google Scholar] [CrossRef] [PubMed]
- Austin, G.E.; Maznicki, E.; Sgoutas, D. Comparison of phosphotungstate and dextran sulfate-Mg2+ precipitation procedures for determination of high density lipoprotein cholesterol. Clin. Biochem. 1984, 17, 166–169. [Google Scholar] [CrossRef]
- Pietta, P.-G. Flavonoids as Antioxidants. J. Nat. Prod. 2000, 63, 1035–1042. [Google Scholar] [CrossRef]
- Khalifa, M.I.; Noseer, E.A. Cholesterol quality of edible eggs produced by quail fed diets containing probiotic and/or ginger (Zingiber officinale). Livest. Res. Rural Dev. 2019, 31, 165–175. [Google Scholar]
- Herve, T.; Raphaël, K.J.; Ferdinand, N.; Vitrice, F.T.L.; Gaye, A.; Outman, M.M.; Marvel, N.M.W. Growth Performance, Serum Biochemical Profile, Oxidative Status, and Fertility Traits in Male Japanese Quail Fed on Ginger (Zingiber officinale, Roscoe) Essential Oil. Vet. Med. Int. 2018, 2018, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, M.; Liang, W.; Geng, Z.; Chen, X. Green tea powder supplementation increased viscosity and decreased lysozyme activity of egg white during storage of eggs from Huainan partridge chicken. Ital. J. Anim. Sci. 2020, 19, 586–592. [Google Scholar] [CrossRef]
- Hasan, H.A.; Raauf, A.M.R.; Razik, B.M.A.; Hassan, B.A.R. Chemical Composition and Antimicrobial Activity of the Crude Extracts Isolated from Zingiber Officinale by Different Solvents. Pharm. Anal. Acta 2012, 3, 1–5. [Google Scholar] [CrossRef]
- Spasevski, N.; Puvača, N.; Pezo, L.; Tasić, T.; Vukmirović, Ð.; Banjac, V.; Čolović, R.; Rakita, S.; Kokić, B.; Džinić, N. Optimisation of egg yolk colour using natural colourants. Eur. Poult. Sci. 2018, 82, 2018–2035. [Google Scholar]
- Ajileye, B.A.; Iteire, A.K.; Arigi, Q.B. Zingiber officinale (ginger) extract as a histological dye for muscle fibers and cytoplasm. Int. J. Med. Sci. 2015, 4, 1445–1448. [Google Scholar] [CrossRef]
| Item | Value (%) | Diet Composition | Value |
|---|---|---|---|
| Corn | 45.38 | ME (kcal/kg) | 2900 |
| Soybean meal | 32.50 | Crude protein (%) | 20 |
| Wheat bran | 10.51 | Ca (%) | 2.5 |
| Soybean oil | 3.2 | Available P (%) | 0.39 |
| Di calcium phosphate | 1.8 | Na | 0.15 |
| Calcium carbonate | 5.3 | Methionine (%) | 0.7 |
| Methionine | 0.39 | Lysine (%) | 1.12 |
| Sodium chloride | 0.32 | Sys (%) | 0.34 |
| Bicarbonate | 0.1 | ||
| Vitamin 1 | 0.3 | ||
| Trace mineral 2 | 0.3 |
| Item | Level (%) |
|---|---|
| Dry matter, DM | 88.5 |
| Phenol, component (g/100 gr) | 0.703 |
| Crude protein | 5.5 |
| Ash | 11.75 |
| Organic matter, OM | 88.25 |
| Ether extract, EE | 1.35 |
| Neutral detergent fiber, NDF | 7.5 |
| Acid detergent fiber, ADF | 4.5 |
| Natural detergent soluble fiber, NDS | 92.5 |
| Acid Detergent soluble fiber, ADS | 95.5 |
| Item | Feed Intake (g/day) | Feed Conversion Ratio (g:g) | Egg Production Rate (%) | Egg Weight (g) | Egg Mass (%) | |
|---|---|---|---|---|---|---|
| Treat | ||||||
| Control | 33.87 a | 3.03 | 92.99 | 12.01 | 11.16 | |
| GP0.5 | 32.93 b | 2.88 | 94.10 | 12.13 | 11.41 | |
| GP1.0 | 32.19 c | 2.77 | 94.42 | 12.31 | 11.62 | |
| GP1.5 | 31.33 d | 2.72 | 94.77 | 12.15 | 11.51 | |
| SEM | 0.17 | 0.02 | 1.19 | 0.13 | 0.13 | |
| Week 0 | 34.02 | 2.99 | 93.87 | 12.12 | 11.37 | |
| Time, w | ||||||
| Week 2 | 34.21 a | 2.95 | 94.51 | 12.25 a | 11.58 a | |
| Week 4 | 32.13 b | 2.78 | 94.91 | 12.17 a | 11.54 a | |
| Week 6 | 32.06 b | 2.85 | 93.84 | 11.99 b | 11.25 b | |
| Week 8 | 31.93 b | 2.82 | 93.03 | 12.18 a | 11.33 b | |
| SEM | 0.21 | 0.01 | 0.82 | 0.08 | 0.08 | |
| treat × Time | ||||||
| Control | Week 2 | 34.71 | 3.02 a,b | 93.92 | 12.22 | 11.48 |
| Control | Week 4 | 34.45 | 3.01 a,b,c | 94.10 | 12.13 | 11.41 |
| Control | Week 6 | 33.66 | 3.1 a | 92.32 | 11.77 | 10.85 |
| Control | Week 8 | 32.68 | 2.99 a,b,c,d | 91.61 | 11.91 | 10.91 |
| GP0.5 | Week 2 | 34.82 | 3.05 a,b | 93.21 | 12.26 | 11.42 |
| GP0.5 | Week 4 | 32.11 | 2.81 d,e,f | 94.46 | 12.08 | 11.39 |
| GP0.5 | Week 6 | 31.94 | 2.85 b,c,d,e,f | 93.03 | 12.02 | 11.18 |
| GP0.5 | Week 8 | 32.84 | 2.82 c,d,e,f | 95.71 | 12.15 | 11.63 |
| GP1.0 | Week 2 | 34.05 | 2.89 b,c,d,e | 95.17 | 12.36 | 11.77 |
| GP1.0 | Week 4 | 31.19 | 2.69 f,g | 94.28 | 12.26 | 11.56 |
| GP1.0 | Week 6 | 32.03 | 2.77 e,f,g | 94.50 | 12.23 | 11.56 |
| GP1.0 | Week 8 | 31.50 | 2.71 e,f,g | 93.75 | 12.37 | 11.59 |
| GP1.5 | Week 2 | 33.25 | 2.85 b,c,d,e,f | 95.71 | 12.15 | 11.65 |
| GP1.5 | Week 4 | 30.80 | 2.61 g | 96.78 | 12.19 | 11.80 |
| GP1.5 | Week 6 | 30.58 | 2.68 f,g | 95.53 | 11.94 | 11.42 |
| GP1.5 | Week 8 | 30.69 | 2.74 e,f,g | 91.07 | 12.31 | 11.19 |
| SEM | 0.42 | 0.03 | 1.64 | 0.16 | 0.17 | |
| Probability | ||||||
| Treat | >0.0001 | >0.0001 | 0.74 | 0.48 | 0.16 | |
| Time | >0.0001 | >0.0001 | 0.32 | 0.001 | 0.007 | |
| Treat × Time | 0.07 | 0.01 | 0.23 | 0.41 | 0.06 | |
| Traits | Experimental Diets | p Value | ||||||
|---|---|---|---|---|---|---|---|---|
| Ginger Powder (g/kg of Diet) | SEM | Treatment | Time | Treatment × Time | ||||
| 0 | 0.5 | 1 | 1.5 | |||||
| Shape index % | 128.97 | 130.25 | 253.90 | 122.66 | 62.09 | 0.43 | 0.45 | 0.43 |
| Albumen index % | 10.36 | 10.74 | 10.89 | 10.79 | 0.23 | 0.46 | 0.01 | 0.009 |
| Yolk index % | 44.77 c | 47.02 a | 46.16 a,b | 45.17 b,c | 0.54 | 0.005 | >0.001 | 0.13 |
| Albumen height (mm) | 3.81 b | 3.99 a | 4.11 a | 4.04 a | 0.04 | 0.004 | 0.21 | 0.02 |
| Haugh unit | 84.88 b | 86.06 a | 86.62 a | 86.27 a | 0.24 | 0.003 | 0.13 | 0.07 |
| Albumen weight | 7.45 | 7.49 | 7.52 | 7.53 | 0.09 | 0.93 | 0.49 | 0.15 |
| Yolk colour | 4.25 b | 4.45 b | 4.70 a | 4.75 a | 0.08 | 0.004 | >0.001 | >0.001 |
| Shell weight (g) | 0.965 | 0.966 | 0.987 | 0.989 | 0.01 | 0.56 | 0.59 | 0.98 |
| Albumen percentage % | 59.92 | 60.80 | 60.14 | 60.41 | 0.36 | 0.39 | 0.11 | 0.29 |
| Yolk percentage % | 32.31 | 31.35 | 31.95 | 31.64 | 0.30 | 0.20 | 0.03 | 0.44 |
| Shell percentage % | 7.76 | 7.84 | 7.90 | 7.93 | 0.11 | 0.73 | 0.59 | 0.29 |
| Albumen to yolk | 0.539 | 0.515 | 0.531 | 0.524 | 0.008 | 0.26 | 0.03 | 0.33 |
| Yolk weight (g) | 4.02 | 3.86 | 3.99 | 3.94 | 0.05 | 0.21 | 0.08 | 0.70 |
| Yolk pH | 5.87 a | 5.84 b | 5.81 b | 5.85 b | 0.01 | 0.02 | >0.001 | 0.02 |
| Albumen pH | 8.91 a | 8.78 b | 8.77 b | 8.83 b | 0.02 | 0.01 | >0.001 | 0.009 |
| Egg specific gravity (g/cm3) | 1.0740 | 1.0745 | 1.0748 | 1.0750 | 0.0006 | 0.72 | 0.60 | 0.97 |
| Shell thickness (mm × 10)2 | 20.61 | 20.67 | 20.29 | 20.76 | 0.35 | 0.80 | 0.005 | 0.51 |
| Shell strength (hg/cm2) | 19.51 | 19.71 | 19.86 | 19.94 | 0.28 | 0.73 | 0.59 | 0.97 |
| Treat | Time (Week) | Albumen Index | Albumen Height (mm) | Yolk Colour | Yolk pH | Albumen pH |
|---|---|---|---|---|---|---|
| Control | 2 | 10.38 a,b | 3.71 b | 3.87 d | 5.91 a,b | 8.99 a,b |
| Control | 4 | 9.75 b | 3.68 b | 4.56 b,c | 5.85 a,b,c,d | 9.10 a |
| Control | 6 | 10.36 a,b | 3.90 a,b | 4.35 b,c,d | 5.84 a,b,c,d | 8.81 c,d,e,f |
| Control | 8 | 10.95 a,b | 3.94 a,b | 4.20 b,c,d | 5.89 a,b,c | 8.75 c,d,e,f |
| GP0.5 | 2 | 9.98 a,b | 4.01 a,b | 4.20 b,c,d | 5.81 b,c,d | 8.73 e,f,g |
| GP0.5 | 4 | 11.09 a,b | 4.001 a,b | 4.12 c,d | 5.82 a,b,c,d | 8.91 a,b,c,d |
| GP0.5 | 6 | 10.86 a,b | 3.91 a,b | 4.56 b,c,d | 5.85 a,b,c,d | 8.74 e,f,g |
| GP0.5 | 8 | 11.02 a,b | 4.04 a,b | 4.91 a,b | 5.88 a,b,c | 8.72 e,f,g |
| GP1.0 | 2 | 10.99 a,b | 4.26 a | 4.29 b,c,d | 5.76 d | 8.76 c,d,e,f |
| GP1.0 | 4 | 10.33 a,b | 3.98 a,b | 4.41 b,c,d | 5.81 a,b,c,d | 8.90 a,b,c,e |
| GP1.0 | 6 | 11.50 a | 4.25 a | 4.54 b,c,d | 5.81 a,b,c,d | 8.72 d,f,g |
| GP1.0 | 8 | 10.71 a,b | 3.94 a,b | 5.56 a | 5.86 a,b,c,d | 8.70 f |
| GP1.5 | 2 | 10.24 a,b | 4.05 a,b | 4.35 b,c,d | 5.85 a,b,c,d | 8.81 b,c,d,e,f |
| GP1.5 | 4 | 10.45 a,b | 4.04 a,b | 4.50 b,c,d | 5.79 c,d | 8.89 a,b,c,d,e,f |
| GP1.5 | 6 | 11.26 a,b | 4.06 a,b | 4.58 b,c,d | 5.84 a,b,c,d | 8.76 c,d,e,f |
| GP1.5 | 8 | 10.87 a,b | 4.02 a,b | 5.56 a | 5.92 a | 8.86 b,c,d,e,f |
| SEM | 0.34 | 0.08 | 0.13 | 0.02 | 0.04 | |
| p value | 0.009 | 0.02 | 0.001 | 0.02 | 0.009 |
| Treatments | Protein (g dL−1) | Albumin (g dL−1) | Triacylglycerol (mg dL−1) | Cholesterol (mg dL−1) | Glucose (mg dL−1) |
|---|---|---|---|---|---|
| Control | 5.80 | 1.60 | 453.1 a | 243 | 195 |
| Ginger (0.5 g/kg of diet) | 5.70 | 1.43 | 351.8 b | 247 | 242 |
| Ginger (1 g/kg of diet) | 5.73 | 1.56 | 336.8 b | 210 | 173 |
| Ginger (1.5 g/kg of diet) | 6.15 | 1.70 | 342.6 b | 219 | 221 |
| SEM | 0.28 | 0.1 | 23.03 | 22.18 | 21.80 |
| p-value | 0.64 | 0.39 | 0.01 | 0.58 | 0.17 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nemati, Z.; Moradi, Z.; Alirezalu, K.; Besharati, M.; Raposo, A. Impact of Ginger Root Powder Dietary Supplement on Productive Performance, Egg Quality, Antioxidant Status and Blood Parameters in Laying Japanese Quails. Int. J. Environ. Res. Public Health 2021, 18, 2995. https://doi.org/10.3390/ijerph18062995
Nemati Z, Moradi Z, Alirezalu K, Besharati M, Raposo A. Impact of Ginger Root Powder Dietary Supplement on Productive Performance, Egg Quality, Antioxidant Status and Blood Parameters in Laying Japanese Quails. International Journal of Environmental Research and Public Health. 2021; 18(6):2995. https://doi.org/10.3390/ijerph18062995
Chicago/Turabian StyleNemati, Zabihollah, Zahra Moradi, Kazem Alirezalu, Maghsoud Besharati, and António Raposo. 2021. "Impact of Ginger Root Powder Dietary Supplement on Productive Performance, Egg Quality, Antioxidant Status and Blood Parameters in Laying Japanese Quails" International Journal of Environmental Research and Public Health 18, no. 6: 2995. https://doi.org/10.3390/ijerph18062995
APA StyleNemati, Z., Moradi, Z., Alirezalu, K., Besharati, M., & Raposo, A. (2021). Impact of Ginger Root Powder Dietary Supplement on Productive Performance, Egg Quality, Antioxidant Status and Blood Parameters in Laying Japanese Quails. International Journal of Environmental Research and Public Health, 18(6), 2995. https://doi.org/10.3390/ijerph18062995










