Body Composition and Characterization of Skinfold Thicknesses from Polycystic Ovary Syndrome Phenotypes. A Preliminar Case-Control Study
Abstract
1. Introduction
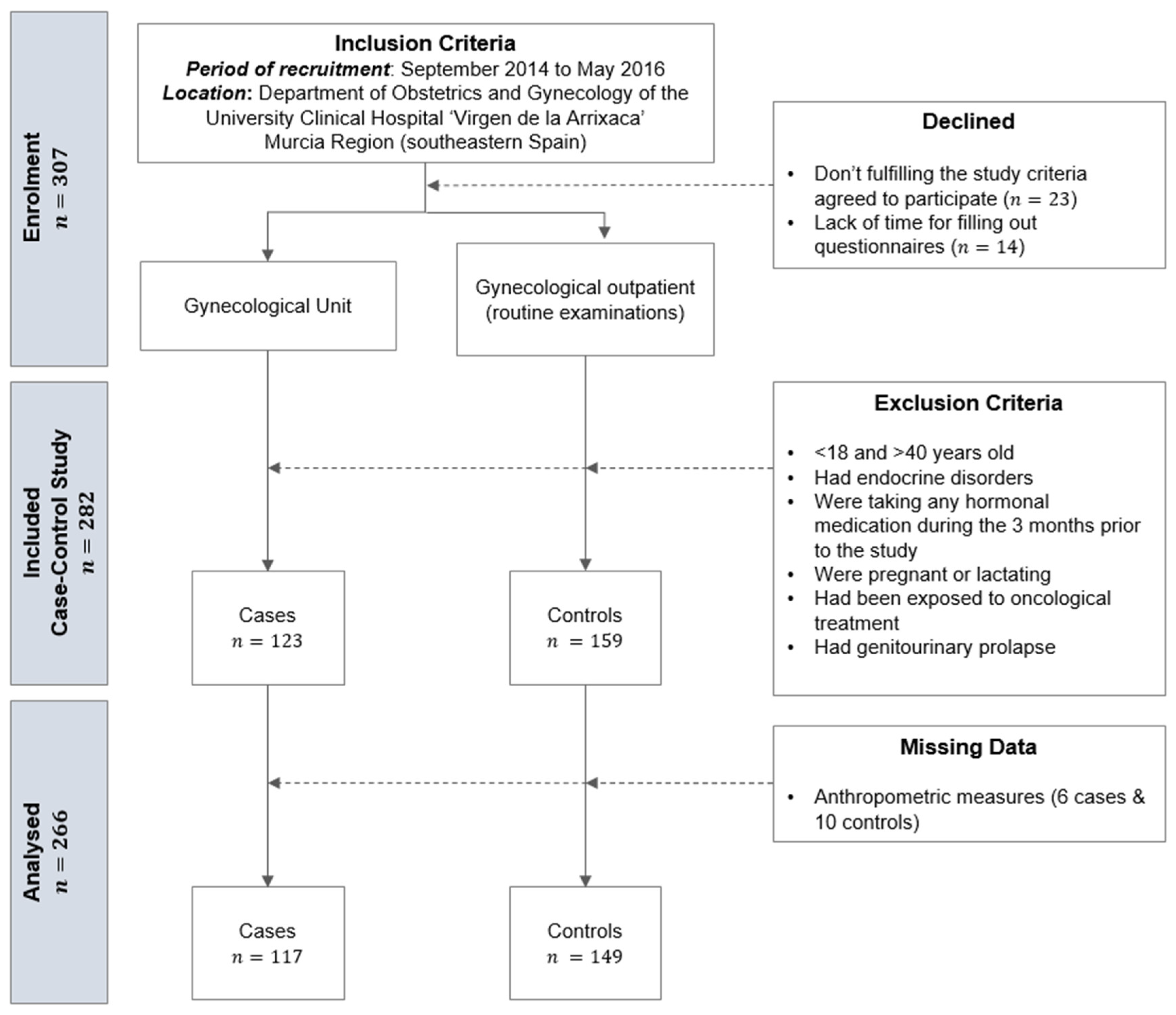
2. Materials and Methods
2.1. Physical and Anthropometric Measurements
2.2. Statistical Analyses
3. Results
3.1. Body Composition
3.1.1. Skinfolds
- PCOS had significantly higher ∑7 SKF (p = 0.013), ∑appendicular SKF (p = 0.017) and ∑arm SKF (p = 0.019) than controls (Table 2);
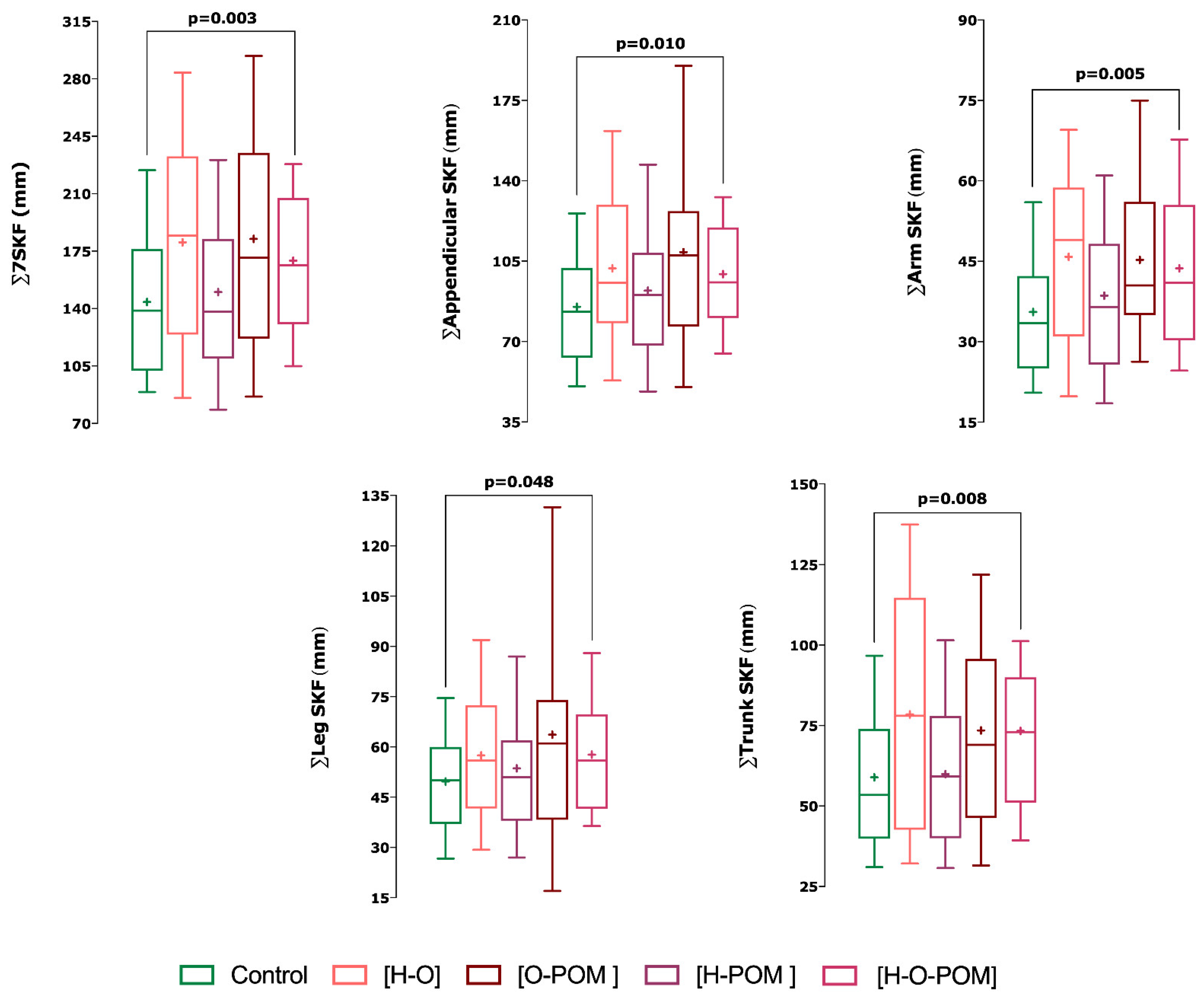
- With regards the phenotypes of PCOS, we only found significant differences in skinfolds with phenotype H-O-POM and controls (Figure 2);
- This phenotype H-O-POM had higher 7∑ SKF (p = 0.003), ∑appendicular SKF (0.01), ∑arm SKF (0.005), ∑leg SKF, and ∑trunk SKF (p = 0.008) (Figure 2);
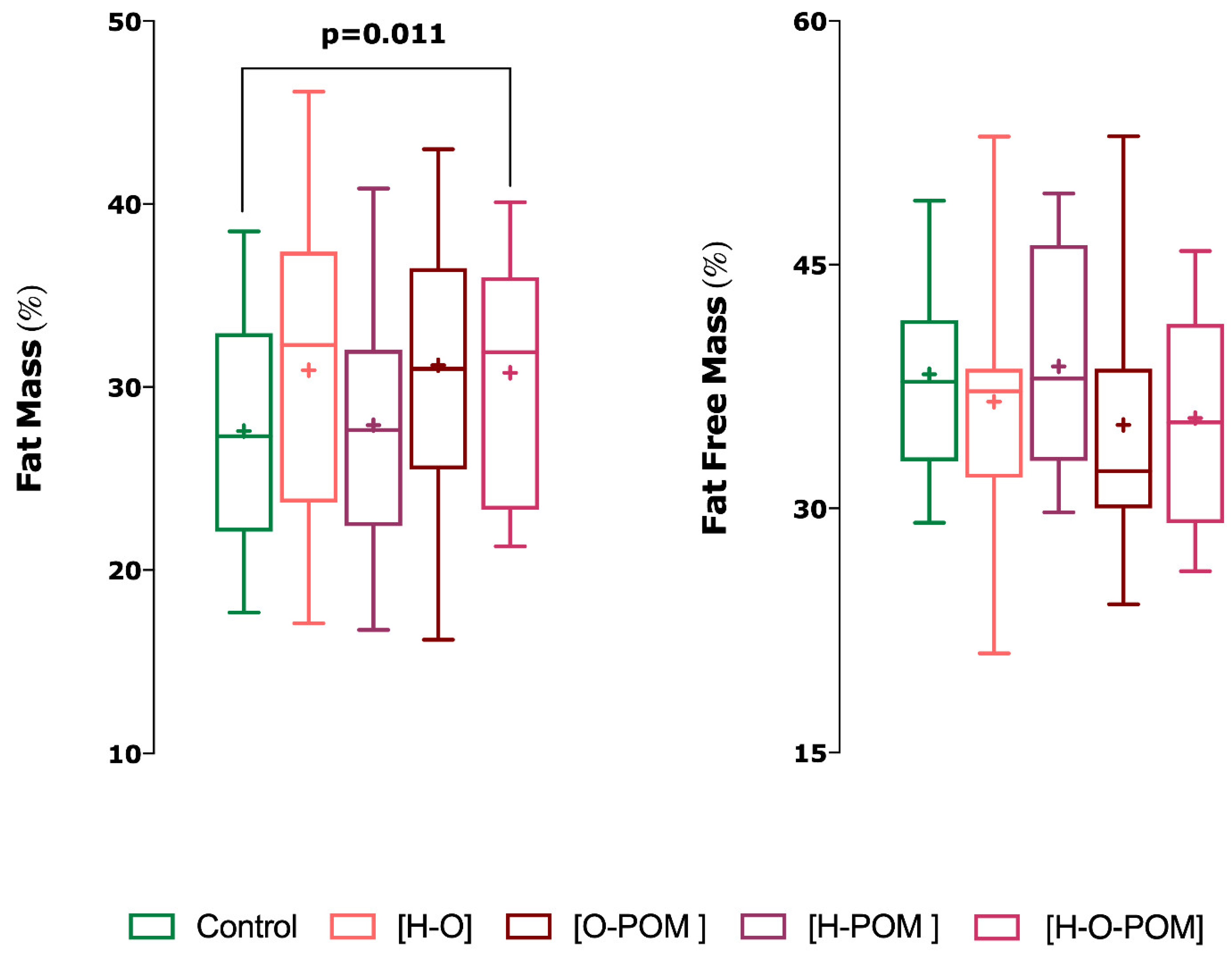
- H-O-POM phenotype had also significantly higher fast mass percentage than controls (p = 0.011) (Figure 3);
3.1.2. Somatotype
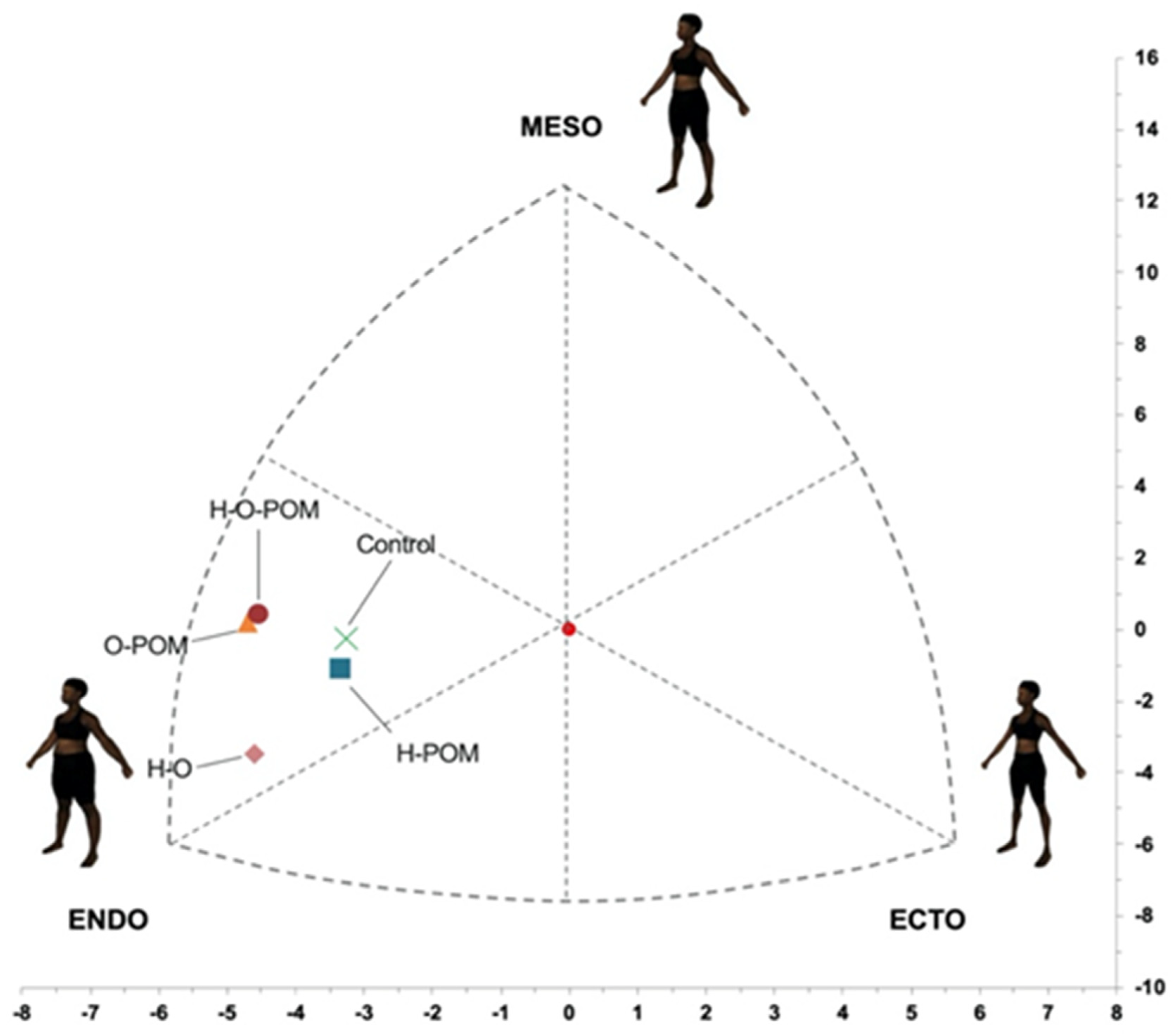
- There were significant differences in the representation on the somatochart in the different phenotypes of PCOS and control women (Figure 4);
- Although the predominant somatotype in both PCOS and controls was the endo-mesomorphic one, controls had the most central position at the somatochart and were closer to the ovulatory phenotype (H-POM) (Figure 4);
- We found that all anovulatories phenotypes of PCOS were located further from the central axis;
- Of these anovulatories phenotypes, H-O-POM and O-POM had a very close representation in the somatochart, being nearer to the mesomorph axis. However, the HO phenotype was far from them and nearer to the endomorph axis (Figure 4).
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Carmina, E.; Rosato, F.; Jannì, A.; Rizzo, M.; Longo, R.A. Relative Prevalence of Different Androgen Excess Disorders in 950 Women Referred because of Clinical Hyperandrogenism. J. Clin. Endocrinol. Metab. 2006, 91, 2–6. [Google Scholar] [CrossRef]
- Azziz, R.; Woods, K.S.; Reyna, R.; Key, T.J.; Knochenhauer, E.S.; Yildiz, B.O. The Prevalence and Features of the Polycystic Ovary Syndrome in an Unselected Population. J. Clin. Endocrinol. Metab. 2004, 89, 2745–2749. [Google Scholar] [CrossRef]
- Lizneva, D.; Kirubakaran, R.; Mykhalchenko, K.; Suturina, L.; Chernukha, G.; Diamond, M.P.; Azziz, R. Phenotypes and body mass in women with polycystic ovary syndrome identified in referral versus unselected populations: Systematic review and meta-analysis. Fertil. Steril. 2016, 106, 1510–1520.e2. [Google Scholar] [CrossRef]
- Ehrmann, D.A. Polycystic ovary syndrome. N. Engl. J. Med. 2005, 352, 1223–1236. [Google Scholar] [CrossRef]
- Esmaeilzadeh, S.; Andarieh, M.G.; Ghadimi, R.; Delavar, M.A. Body Mass Index and Gonadotropin Hormones (LH & FSH) Associate with Clinical Symptoms among Women with Polycystic Ovary Syndrome. Glob. J. Health Sci. 2014, 7, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Sahin, S.B.; Durakoglugil, T.; Ayaz, T.; Sahin, O.Z.; Durakoglugil, E.; Sumer, F.; Aktas, E.; Alyildiz, N. Evaluation of Para- and Perirenal Fat Thickness and Its Association with Metabolic Disorders in Polycystic Ovary Syndrome. Endocr. Pract. 2015, 21, 878–886. [Google Scholar] [CrossRef] [PubMed]
- Fauser, B.C.J.M.; Bouchard, P. Uncertainty remains in women with PCOS regarding the increased incidence of cardiovascular disease later in life, despite the indisputable presence of multiple cardiovascular risk factors at a young age. J. Clin. Endocrinol. Metab. 2011, 96, 3675–3677. [Google Scholar] [CrossRef] [PubMed]
- Barry, J.A.; Azizia, M.M.; Hardiman, P.J. Risk of endometrial, ovarian and breast cancer in women with polycystic ovary syndrome: A systematic review and meta-analysis. Hum. Reprod. Update 2014, 20, 748–758. [Google Scholar] [CrossRef]
- Zaadstra, B.M.; Seidell, J.C.; Van Noord, P.A.; te Velde, E.R.; Habbema, J.D.; Vrieswijk, B.; Karbaat, J. Fat and female fecundity: Prospective study of effect of body fat distribution on conception rates. BMJ 1993, 306, 484–487. [Google Scholar] [CrossRef] [PubMed]
- Fauser, B.C.J.M.; Cao, Y.; Li, Q.; Zhou, C.; Li, F.; Li, S.; Zhou, Y.; Calcagno, C.; Lobatto, M.E.; Robson, P.M.; et al. HHS Public Access. Fertil. Steril. 2015, 81, 729–735. [Google Scholar] [CrossRef]
- Srikanthan, P.; Karlamangla, A.S. Relative muscle mass is inversely associated with insulin resistance and prediabetes. Findings from the third National Health and Nutrition Examination Survey. J. Clin. Endocrinol. Metab. 2011, 96, 2898–2903. [Google Scholar] [CrossRef]
- Echiburú, B.; Pérez-Bravo, F.; Galgani, J.E.; Sandoval, D.; Saldías, C.; Crisosto, N.; Maliqueo, M.; Sir-Petermann, T. Enlarged adipocytes in subcutaneous adipose tissue associated to hyperandrogenism and visceral adipose tissue volume in women with polycystic ovary syndrome. Steroids 2018, 130, 15–21. [Google Scholar] [CrossRef]
- Elffers, T.W.; de Mutsert, R.; Lamb, H.J.; de Roos, A.; van Dijk, K.W.; Rosendaal, F.R.; Jukema, J.W.; Trompet, S. Body fat distribution, in particular visceral fat, is associated with cardiometabolic risk factors in obese women. PLoS ONE 2017, 12, e0185403. [Google Scholar] [CrossRef] [PubMed]
- Heath, B.H.; Carter, J.E. A comparison of somatotype methods. Am. J. Phys. Anthropol. 1966, 24, 87–99. [Google Scholar] [CrossRef] [PubMed]
- Heath, B.H.; Carter, J.E. A modified somatotype method. Am. J. Phys. Anthropol. 1967, 27, 57–74. [Google Scholar] [CrossRef] [PubMed]
- Amaral, T.F.; Restivo, M.T.; Guerra, R.S.; Marques, E.; Chousal, M.F.; Mota, J. Accuracy of a digital skinfold system for measuring skinfold thickness and estimating body fat. Br. J. Nutr. 2011, 105, 478–484. [Google Scholar] [CrossRef] [PubMed]
- Kaur, R.; Kaur, M.; Suri, V. Somatotype Profile of Obese and Lean Women with Polycystic Ovary Syndrome: A Population Based Cohort Study. Anthropologie 2020, 58, 93–102. [Google Scholar] [CrossRef]
- Vidal Pérez, D.; Martínez-Sanz, J.M.; Ferriz-Valero, A.; Gómez-Vicente, V.; Ausó, E. Relationship of limb lengths and body composition to lifting in weightlifting. Int. J. Environ. Res. Public Health 2021, 18, 756. [Google Scholar] [CrossRef]
- Yeung, E.H.; Hu, F.B.; Solomon, C.G.; Chen, L.; Louis, G.M.; Schisterman, E.; Willett, W.C.; Zhang, C. Life-course weight characteristics and the risk of gestational diabetes. Diabetologia 2010, 53, 668–678. [Google Scholar] [CrossRef]
- Galić, B.S.; Pavlica, T.; Udicki, M.; Stokić, E.; Mikalački, M.; Korovljev, D.; Čokorilo, N.; Drvendžija, Z.; Adamović, D. Somatotype characteristics of normal-weight and obese women among different metabolic subtypes. Arch. Endocrinol. Metab. 2016, 60, 60–65. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bertrand, K.A.; Giovannucci, E.; Zhang, S.M.; Laden, F.; Rosner, B.; Birmann, B.M. A prospective analysis of body size during childhood, adolescence, and adulthood and risk of non-hodgkin lymphoma. Cancer Prev. Res. 2013, 6, 864–873. [Google Scholar] [CrossRef][Green Version]
- Hidayat, K.; Li, H.J.; Shi, B.M. Anthropometric factors and non-Hodgkin’s lymphoma risk: Systematic review and meta-analysis of prospective studies. Crit. Rev. Oncol. Hematol. 2018, 129, 113–123. [Google Scholar] [CrossRef]
- Doh, E.; Mbanya, A.; Kemfang-Ngowa, J.D.; Dohbit, S.; Tchana-Sinou, M.; Foumane, P.; Donfack, O.T.; Doh, A.S.; Mbanya, J.C.; Sobngwi, E. The Relationship between Adiposity and Insulin Sensitivity in African Women Living with the Polycystic Ovarian Syndrome: A Clamp Study. Int. J. Endocrinol. 2016, 2016, 9201701. [Google Scholar] [CrossRef]
- Rodríguez, A.L.M.; Cervante, T.J.M.; Cortés, L.M.; Eduardo, A. Somatotype of Women with Polycystic Ovary Syndrome. 2012. Available online: https://www.revistafml.es/upload/ficheros/noticias/201208/1604_ai_somatotipo_de_la_mujer_con_sndrome_de_ovario_poliqustico.pdf (accessed on 13 March 2021).
- Geronikolou, S.A.; Bacopoulou, F.; Cokkinos, D. Bioimpedance Measurements in Adolescents with Polycystic Ovary Syndrome: A Pilot Study. Adv. Exp. Med. Biol. 2017, 987, 291–299. [Google Scholar] [CrossRef]
- Sánchez-Ferrer, M.L.; Mendiola, J.; Hernández-Peñalver, A.I.; Corbalán-Biyang, S.; Carmona-Barnosi, A.; Prieto-Sánchez, M.T.; Nieto, A.; Torres-Cantero, A.M. Presence of polycystic ovary syndrome is associated with longer anogenital distance in adult Mediterranean women. Hum. Reprod. 2017, 32, 2315–2323. [Google Scholar] [CrossRef]
- Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Hum. Reprod. 2004, 19, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Ferriman, D.; Gallwey, J.D. Clinical assessment of body hair growth in women. J. Clin. Endocrinol. Metab. 1961, 21, 1440–1447. [Google Scholar] [CrossRef]
- Afifi, L.; Saeed, L.; Pasch, L.A.; Huddleston, H.G.; Cedars, M.I.; Zane, L.T.; Shinkai, K. Association of ethnicity, Fitzpatrick skin type, and hirsutism: A retrospective cross-sectional study of women with polycystic ovarian syndrome. Int. J. Women’s Dermatol. 2017, 3, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Conway, G.; Dewailly, D.; Diamanti-Kandarakis, E.; Escobar-Morreale, H.F.; Franks, S.; Gambineri, A.; Kelestimur, F.; Macut, D.; Micic, D.; Pasquali, R.; et al. The polycystic ovary syndrome: A position statement from the European Society of Endocrinology. Eur. J. Endocrinol. 2014, 171, P1–P29. [Google Scholar] [CrossRef] [PubMed]
- National Institutes of Health 2012. Evidence-Based Methodology Workshop on Polycystic Ovary Syndrome 3–5 December 2012. Available online: https://prevention.nih.gov/docs/programs/pcos/FinalReport.pdf (accessed on 20 May 2017).
- Santos, D.A.; Dawson, J.A.; Matias, C.N.; Rocha, P.M.; Minderico, C.S.; Allison, D.B.; Sardinha, L.B.; Silva, A.M. Reference values for body composition and anthropometric measurements in athletes. PLoS ONE 2014, 9, e97846. [Google Scholar] [CrossRef]
- Jackson, A.S.; Pollock, M.L.; Ward, A. Generalized equations for predicting body density of women. Med. Sci. Sports Exerc. 1980, 12, 175–181. [Google Scholar] [CrossRef]
- Carter, J.E.L.; Honeyman Heath, B. Somatotyping: Development and Applications; Cambridge University Press: New York, NY, USA, 1990. [Google Scholar]
- Cabañas Armesilla, M.D.; Esparza Ros, F. Compendio de Cineantropometría; CTO Editorial: Madrid, Spain, 2009; ISBN 9788492523726. [Google Scholar]
- Alvero Cruz, A.; Cabañas, M.D.; Herrero De Lucas, A.; Martinez Riaza, L.; Moreno Pascual, C.; Porta Manzañido, J. Protocolo de Valoración de la Composición Corporal para el Reconocimiento Médico-Deportivo. Documento de Consenso del Grupo Español de Cineantropometría de la Federación Española de Medicina del Deporte. Archivos de Medicina del Deporte 2009, 131, 166–179. [Google Scholar]
- Zabuliene, L.; Urboniene, J.; Tutkuviene, J. Body composition of lean women with polycystic ovary syndrome. Anthropol. Rev. 2013, 76, 183–198. [Google Scholar] [CrossRef]
- Sánchez-Ferrer, M.L.; Prieto-Sánchez, M.T.; Corbalán-Biyang, S.; Mendiola, J.; Adoamnei, E.; Hernández-Peñalver, A.I.; Carmona-Barnosi, A.; Salido-Fiérrez, E.J.; Torres-Cantero, A.M. Are there differences in basal thrombophilias and C-reactive protein between women with or without PCOS? Reprod. Biomed. Online 2019, 38, 1018–1026. [Google Scholar] [CrossRef] [PubMed]
- Hudson, G.M.; Lu, Y.; Zhang, X.; Hahn, J.; Zabal, J.E.; Latif, F.; Philbeck, J. The Development of a BMI-Guided Shape Morphing Technique and the Effects of an Individualized Figure Rating Scale on Self-Perception of Body Size. Eur. J. Investig. Health Psychol. Educ. 2020, 10, 579–594. [Google Scholar] [CrossRef]
- Koleva, M.; Nacheva, A.; Boev, M. Somatotype and disease prevalence in adults. Rev. Environ. Health 2002, 17, 65–84. [Google Scholar] [CrossRef] [PubMed]
- Jamil, A.S.; Alalaf, S.K.; Al-Tawil, N.G.; Al-Shawaf, T. Comparison of clinical and hormonal characteristics among four phenotypes of polycystic ovary syndrome based on the Rotterdam criteria. Arch. Gynecol. Obstet. 2016, 293, 447–456. [Google Scholar] [CrossRef]
- Pinola, P.; Puukka, K.; Piltonen, T.T.; Puurunen, J.; Vanky, E.; Sundström-Poromaa, I.; Stener-Victorin, E.; Lindén Hirschberg, A.; Ravn, P.; Skovsager Andersen, M.; et al. Normo- and hyperandrogenic women with polycystic ovary syndrome exhibit an adverse metabolic profile through life. Fertil. Steril. 2017, 107, 788–795.e2. [Google Scholar] [CrossRef] [PubMed]
- Condorelli, R.A.; Calogero, A.E.; Di Mauro, M.; Mongioi’, L.M.; Cannarella, R.; Rosta, G.; La Vignera, S. Androgen excess and metabolic disorders in women with PCOS: Beyond the body mass index. J. Endocrinol. Investig. 2018, 41, 383–388. [Google Scholar] [CrossRef] [PubMed]
- Franik, G.; Bizoń, A.; Włoch, S.; Pluta, D.; Blukacz, Ł.; Milnerowicz, H.; Madej, P. The effect of abdominal obesity in patients with polycystic ovary syndrome on metabolic parameters. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 4755–4761. [Google Scholar] [PubMed]
- Dou, P.; Ju, H.; Shang, J.; Li, X.; Xue, Q.; Xu, Y.; Guo, X. Application of receiver operating characteristic curve in the assessment of the value of body mass index, waist circumference and percentage of body fat in the Diagnosis of Polycystic Ovary Syndrome in childbearing women. J. Ovarian Res. 2016, 9, 51. [Google Scholar] [CrossRef] [PubMed]
- Arpaci, D.; Gurkan Tocoglu, A.; Yilmaz, S.; Ergenc, H.; Tamer, A.; Keser, N.; Gunduz, H. The relationship between epicardial fat tissue thickness and visceral adipose tissue in lean patients with polycystic ovary syndrome. J. Ovarian Res. 2015, 8, 71. [Google Scholar] [CrossRef] [PubMed]
- Kirchengast, S.; Huber, J. Body composition characteristics and body fat distribution in lean women with polycystic ovary syndrome. Hum. Reprod. 2001, 16, 1255–1260. [Google Scholar] [CrossRef]
- Ezeh, U.; Pall, M.; Mathur, R.; Azziz, R. Association of fat to lean mass ratio with metabolic dysfunction in women with polycystic ovary syndrome. Hum. Reprod. 2014, 29, 1508–1517. [Google Scholar] [CrossRef]
- Durmus, U.; Duran, C.; Ecirli, S. Visceral adiposity index levels in overweight and/or obese, and non-obese patients with polycystic ovary syndrome and its relationship with metabolic and inflammatory parameters. J. Endocrinol. Investig. 2017, 40, 487–497. [Google Scholar] [CrossRef]
- Baltadjiev, A.G. Somatotype characteristics of female patients with type 2 diabetes mellitus. Folia Med. (Plovdiv) 2013, 55, 64–69. [Google Scholar] [CrossRef]
- Crosignani, P.G.; Colombo, M.; Vegetti, W.; Somigliana, E.; Gessati, A.; Ragni, G. Overweight and obese anovulatory patients with polycystic ovaries: Parallel improvements in anthropometric indices, ovarian physiology and fertility rate induced by diet. Hum. Reprod. 2003, 18, 1928–1932. [Google Scholar] [CrossRef] [PubMed]
- Hill, A.B. The environment and disease: Association or causation? Proc. R. Soc. Med. 1965, 58, 295–300. [Google Scholar] [CrossRef] [PubMed]
- Pasquali, R.; Gambineri, A.; Cavazza, C.; Ibarra Gasparini, D.; Ciampaglia, W.; Cognigni, G.E.; Pagotto, U. Heterogeneity in the responsiveness to long-term lifestyle intervention and predictability in obese women with polycystic ovary syndrome. Eur. J. Endocrinol. 2011, 164, 53–60. [Google Scholar] [CrossRef]
- Pasquali, R.; Antenucci, D.; Casimirri, F.; Venturoli, S.; Paradisi, R.; Fabbri, R.; Balestra, V.; Melchionda, N.; Barbara, L. Clinical and hormonal characteristics of obese amenorrheic hyperandrogenic women before and after weight loss. J. Clin. Endocrinol. Metab. 1989, 68, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Kiddy, D.S.; Hamilton-Fairley, D.; Bush, A.; Short, F.; Anyaoku, V.; Reed, M.J.; Franks, S. Improvement in endocrine and ovarian function during dietary treatment of obese women with polycystic ovary syndrome. Clin. Endocrinol. 1992, 36, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Huber-Buchholz, M.M.; Carey, D.G.; Norman, R.J. Restoration of reproductive potential by lifestyle modification in obese polycystic ovary syndrome: Role of insulin sensitivity and luteinizing hormone. J. Clin. Endocrinol. Metab. 1999, 84, 1470–1474. [Google Scholar] [CrossRef] [PubMed]
| Control | PCOS | p-Value * | |
|---|---|---|---|
| Age (years) | 30.68 | 27.38 | 0.000 |
| Weight (Kg) | 63.66 | 68.54 | 0.010 |
| Height (cm) | 164.64 | 164.64 | 0.995 |
| BMI | 23.51 | 25.22 | 0.009 |
| Polycystic Ovary Morphology | 17.4 (11.4–23.5) | 86.4 (80.3–92.5) | <0.001 |
| Hyperandrogenism | 32.7 (26.0–41.0) | 84.1 (79.0–92.0) | <0.001 |
| Oligo/amenorrhoea | 7.6 (3.0–12.0) | 73.0 (65.0–81.0) | <0.001 |
| Hyperandrogenism + Oligo/amenorrhoea | 0 | 14.2 (8.1–20.3) | - |
| Hyperandrogenism + POM | 0 | 27.0 (19.2–34.8) | - |
| Oligo/amenorrhoea + POM | 0 | 15.9 (9.5–22.3) | - |
| Hyperandrogenism + Oligo/amenorrhoea + POM | 0 | 42.9 (34.3–51.5) | - |
| FM (%) | 27.59 | 30.04 | 0.012 |
| BM (%) | 13.26 | 12.52 | 0.139 |
| LM (%) | 38.26 | 36.54 | 0.089 |
| Endomorph | 5.30 | 5.93 | 0.004 |
| Mesomorph | 3.54 | 3.81 | 0.485 |
| Ectomorph | 2.05 | 1.71 | 0.062 |
| Control | PCOS (All) | PCOS (H-O) | PCOS (O-POM) | PCOS (H-O-POM) | |
|---|---|---|---|---|---|
| ∑7SKF (mm) | 143.68 ± 49.96 | 167.57 ± 59.05 † | 180.35 ± 71.83 | 182.44 ± 74.71 | 169.16 ± 47.90 † |
| ∑Appendicular SKF (mm) | 85.09 ± 27.34 | 99.11 ± 34.65 † | 101.88 ± 36.79 | 108.93 ± 46.63 | 99.40 ± 28.15 † |
| ∑Arm SKF (mm) | 35.54 ± 12.95 | 42.83 ± 16.28 † | 45.83 ± 17.25 | 45.27 ± 16.37 | 43.68 ± 16.77 † |
| ∑Leg SKF (mm) | 49.64 ± 17.14 | 57.54 ± 22.59 | 57.46 ± 21.95 | 63.66 ± 35.06 | 57.70 ± 17.58 † |
| ∑Trunk SKF (mm) | 58.60 ± 25.95 | 70.27 ± 30.06 | 78.48 ± 39.08 | 73.51 ± 32.25 | 73.32 ± 28.28 † |
| Fat Mass (%) | 27.59 ± 7.23 | 30.04 ± 8.01 | 30.91 ± 9.50 | 31.19 ± 8.87 | 30.78 ± 7.11 † |
| Fat Free Mass (%) | 38.25 ± 7.55 | 36.54 ± 8.35 | 36.57 ± 9.45 | 35.13 ± 9.61 | 35.56 ± 7.52 † |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez-Ferrer, M.L.; De La Cruz-Sánchez, E.; Arense-Gonzalo, J.J.; Prieto-Sánchez, M.T.; Bernabeu-González, I.; Carmona-Barnosi, A.; Mendiola, J.; Torres-Cantero, A.M. Body Composition and Characterization of Skinfold Thicknesses from Polycystic Ovary Syndrome Phenotypes. A Preliminar Case-Control Study. Int. J. Environ. Res. Public Health 2021, 18, 2977. https://doi.org/10.3390/ijerph18062977
Sánchez-Ferrer ML, De La Cruz-Sánchez E, Arense-Gonzalo JJ, Prieto-Sánchez MT, Bernabeu-González I, Carmona-Barnosi A, Mendiola J, Torres-Cantero AM. Body Composition and Characterization of Skinfold Thicknesses from Polycystic Ovary Syndrome Phenotypes. A Preliminar Case-Control Study. International Journal of Environmental Research and Public Health. 2021; 18(6):2977. https://doi.org/10.3390/ijerph18062977
Chicago/Turabian StyleSánchez-Ferrer, María L., Ernesto De La Cruz-Sánchez, Julián J. Arense-Gonzalo, María T. Prieto-Sánchez, Itziar Bernabeu-González, Ana Carmona-Barnosi, Jaime Mendiola, and Alberto M. Torres-Cantero. 2021. "Body Composition and Characterization of Skinfold Thicknesses from Polycystic Ovary Syndrome Phenotypes. A Preliminar Case-Control Study" International Journal of Environmental Research and Public Health 18, no. 6: 2977. https://doi.org/10.3390/ijerph18062977
APA StyleSánchez-Ferrer, M. L., De La Cruz-Sánchez, E., Arense-Gonzalo, J. J., Prieto-Sánchez, M. T., Bernabeu-González, I., Carmona-Barnosi, A., Mendiola, J., & Torres-Cantero, A. M. (2021). Body Composition and Characterization of Skinfold Thicknesses from Polycystic Ovary Syndrome Phenotypes. A Preliminar Case-Control Study. International Journal of Environmental Research and Public Health, 18(6), 2977. https://doi.org/10.3390/ijerph18062977







