Activities and Perceived Risk of Transmission and Spread of SARS-CoV-2 among Specialists and Residents in a Third Level University Hospital in Spain
Abstract
1. Introduction
2. Materials and Methods
2.1. Survey Instrument, Administration and Sample Selection
2.2. Data Management and Analysis
2.3. Dissemination of Results
2.4. Transparency Declaration
2.5. Ethics Committee Approval
3. Results
3.1. Usual and Risky Clinical Practice
3.2. Perceived Risk
3.3. Availability and Use of PPE. Hand Hygiene
3.4. Other Protective Measures
3.5. Activities in the Institution
3.6. Time Working with Symptoms
3.7. Close Contact with Suspected/Confirmed Cases
3.8. Public Transport
3.9. Protective and Risk Factors
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lu, R.; Zhao, X.; Li, J.; Niu, P.; Yang, B.; Wu, H.; Wang, W.; Song, H.; Huang, B.; Zhu, N.; et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: Implications for virus origins and receptor binding. Lancet 2020, 395, 565–574. [Google Scholar] [CrossRef]
- Riou, J.; Althaus, C.L. Pattern of early human-to-human transmission of Wuhan 2019 novel coronavirus (2019-nCoV), December 2019 to January 2020. Eurosurveillance 2020, 25, 2000058. [Google Scholar] [CrossRef]
- Endo, A.; Centre for the Mathematical Modelling of Infectious Diseases COVID-19 Working Group; Abbott, S.; Kucharski, A.J.; Funk, S. Estimating the overdispersion in COVID-19 transmission using outbreak sizes outside China. Wellcome Open Res. 2020, 5, 67. [Google Scholar] [CrossRef] [PubMed]
- Trilla, A. One world, one health: The novel coronavirus COVID-19 epidemic. Med. Clin. 2020, 154, 175–177. [Google Scholar] [CrossRef] [PubMed]
- Lauer, S.A.; Grantz, K.H.; Bi, Q.; Jones, F.K.; Zheng, Q.; Meredith, H.R.; Azman, A.S.; Reich, N.G.; Lessler, J. The Incubation Period of Coronavirus Disease 2019 (COVID-19) From Publicly Reported Confirmed Cases: Estimation and Application. Ann. Intern. Med. 2020, 172, 577–582. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, N.M.; Laydon, D.; Nedjati-Gilani, G.; Imai, N.; Ainslie, K.; Baguelin, M.; Bhatia, S.; Boonyasiri, A.; Cucunubá, Z.; Cuomo-Dannenburg, G.; et al. Impact of Non-Pharmaceutical Interventions (NPIS) to Reduce Covid-19 Mortality and Healthcare Demand. Available online: https://www.imperial.ac.uk/media/imperial-college/medicine/mrc-gida/2020-03-16-COVID19-Report-9.pdf (accessed on 25 May 2020).
- Liu, Y.; Yan, L.-M.; Wan, L.; Xiang, T.-X.; Le, A.; Liu, J.-M.; Peiris, M.; Poon, L.L.M.; Zhang, W. Viral dynamics in mild and severe cases of COVID-19. Lancet Infect. Dis. 2020, 20, 656–657. [Google Scholar] [CrossRef]
- Pan, Y.; Zhang, D.; Yang, P.; Poon, L.L.M.; Wang, Q. Viral load of SARS-CoV-2 in clinical samples. Lancet Infect. Dis. 2020, 20, 411–412. [Google Scholar] [CrossRef]
- Zou, L.; Ruan, F.; Huang, M.; Liang, L.; Huang, H.; Hong, Z.; Yu, J.; Kang, M.; Song, Y.; Xia, J.; et al. SARS-CoV-2 Viral Load in Upper Respiratory Specimens of Infected Patients. N. Engl. J. Med. 2020, 382, 1177–1179. [Google Scholar] [CrossRef]
- Ong, S.W.X.; Tan, Y.K.; Chia, P.Y.; Lee, T.H.; Ng, O.T.; Wong, M.S.; Marimuthu, K. Air, Surface Environmental, and Personal Protective Equipment Contamination by Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) From a Symptomatic Patient. JAMA 2020, 323, 1610–1612. [Google Scholar] [CrossRef]
- Modes of Transmission of Virus Causing Covid-19: Implications for IPC Precaution Recommendations. Available online: https://www.who.int/publications-detail/modes-of-transmission-of-virus-causing-covid-19-implications-for-ipc-precaution-recommendations (accessed on 22 May 2020).
- Zhang, R.; Li, Y.; Zhang, A.L.; Wang, Y.; Molina, M.J. Identifying airborne transmission as the dominant route for the spread of COVID-19. Proc. Natl. Acad. Sci. USA 2020, 117, 14857–14863. [Google Scholar] [CrossRef]
- Morawska, L.; Cao, J. Airborne transmission of SARS-CoV-2: The world should face the reality. Environ. Int. 2020, 139, 105730. [Google Scholar] [CrossRef] [PubMed]
- Kwok, Y.L.A.; Gralton, J.; McLaws, M.-L. Face touching: A frequent habit that has implications for hand hygiene. Am. J. Infect. Control. 2015, 43, 112–114. [Google Scholar] [CrossRef]
- Kampf, G.; Todt, D.; Pfaender, S.; Steinmann, E. Persistence of coronaviruses on inanimate surfaces and their inactivation with biocidal agents. J. Hosp. Infect. 2020, 104, 246–251. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention (CDC). Coronavirus Disease 2019 (COVID-19)—Prevention & Treatment. Available online: https://www.cdc.gov/coronavirus/2019-ncov/prevent-getting-sick/prevention.html (accessed on 29 May 2020).
- Wikiwand. COVID-19 Pandemic in Japan. Available online: https://www.acpjournals.org/doi/pdf/10.7326/M20-0504 (accessed on 25 May 2020).
- Chen, J.; Lu, H.; Melino, G.; Boccia, S.; Piacentini, M.; Ricciardi, W.; Wang, Y.; Shi, Y.; Zhu, T. COVID-19 infection: The China and Italy perspectives. Cell Death Dis. 2020, 11, 1–17. [Google Scholar] [CrossRef]
- Report on the Situation of COVID-19 in Health Workers in Spain on 21 May 2020. Available online: https://www.isciii.es/QueHacemos/Servicios/VigilanciaSaludPublicaRENAVE/EnfermedadesTransmisibles/Documents/INFORMES/Informes%20COVID-19/COVID-19%20en%20Espa%C3%B1a.%20Situaci%C3%B3n%20en%20Sanitarios%20a%2021%20de%20mayo%20de%202020.pdf (accessed on 2 June 2020).
- Folgueira, M.D.; Munoz-Ruiperez, C.; Alonso-Lopez, M.A.; Delgado, R. SARS-CoV-2 infection in Health Care Workers in a large public hospital in Madrid, Spain, during March 2020. medRxiv 2020. [Google Scholar] [CrossRef]
- Ministry of Health. Action Procedure for Occupational Risk Prevention Services in Relation to Exposure to SARS-Cov-2. 22 May 2020. Available online: https://www.mscbs.gob.es/profesionales/saludPublica/ccayes/alertasActual/nCov-China/documentos/PrevencionRRLL_COVID-19.pdf (accessed on 27 May 2020).
- U.S. Food and Drug Administration. Food and Agriculture: Considerations for Prioritization of PPE, Cloth Face Coverings, Disinfectants, and Sanitation Supplies during the COVID-19 Pandemic. 2020. Available online: https://www.fda.gov/food/food-safety-during-emergencies/food-and-agriculture-considerations-prioritization-ppe-cloth-face-coverings-disinfectants-and (accessed on 27 May 2020).
- Considerations for Acute Personal Protective Equipment (PPE) Shortages. Available online: https://www.gov.uk/government/publications/wuhan-novel-coronavirus-infection-prevention-and-control/managing-shortages-in-personal-protective-equipment-ppe (accessed on 27 May 2020).
- Puci, M.V.; Nosari, G.; Loi, F.; Puci, G.V.; Montomoli, C.; Ferraro, O.E. Risk Perception and Worries among Health Care Workers in the COVID-19 Pandemic: Findings from an Italian Survey. Healthcare 2020, 8, 535. [Google Scholar] [CrossRef]
- Upadhyaya, D.P.; Paudel, R.; Bromberg, D.J.; Acharya, D.; Khoshnood, K.; Lee, K.; Park, J.-H.; Yoo, S.-J.; Shrestha, A.; Bc, B.; et al. Frontline Healthcare Workers’ Knowledge and Perception of COVID-19, and Willingness to Work during the Pandemic in Nepal. Healthcare 2020, 8, 554. [Google Scholar] [CrossRef] [PubMed]
- Peres, D.; Monteiro, J.; Almeida, M.; Ladeira, R. Risk perception of COVID-19 among Portuguese healthcare professionals and the general population. J. Hosp. Infect. 2020, 105, 434–437. [Google Scholar] [CrossRef] [PubMed]
- Health Reputation Monitor 2019. Corporate Business Reputation Monitor. Available online: https://www.imperial.ac.uk/media/imperial-college/medicine/mrc-gida/2020-03-16-COVID19-Report-9.pdf (accessed on 27 May 2020).
- Newsweek. The World’s Best Hospitals. 2020. Available online: https://www.newsweek.com/best-hospitals-2020 (accessed on 27 May 2020).
- Corman, V.M.; Landt, O.; Kaiser, M.; Molenkamp, R.; Meijer, A.; Chu, D.K.; Bleicker, T.; Brünink, S.; Schneider, J.; Schmidt, M.L.; et al. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Eurosurveillance 2020, 25, 2000045. [Google Scholar] [CrossRef]
- StataCorp. Stata Statistical Software: Release 16; StataCorp LLC: College Station, TX, USA, 2019. [Google Scholar]
- Arevalo-Rodriguez, I.; Buitrago-Garcia, D.; Simancas-Racines, D.; Zambrano-Achig, P.; del Campo, R.; Ciapponi, A.; Sued, O.; Martínez-García, L.; Rutjes, A.; Low, N.; et al. False-Negative Results of Initial RT-PCR Assays for COVID-19: A Systematic Review, Infectious Diseases (Except HIV/AIDS). April 2020. Available online: http://medrxiv.org/lookup/doi/10.1101/2020.04.16.20066787 (accessed on 27 May 2020).
- Watson, J.; Whiting, P.F.; Brush, J. Interpreting a covid-19 test result. BMJ 2020, 369. [Google Scholar] [CrossRef]
- COVID-19 Data Community of Madrid. Available online: https://www.comunidad.madrid/sites/default/files/doc/sanidad/200527_cam_covid19.pdf (accessed on 27 May 2020).
- Dryhurst, S.; Schneider, C.R.; Kerr, J.; Freeman, A.L.J.; Recchia, G.; Van Der Bles, A.M.; Spiegelhalter, D.; Van Der Linden, S. Risk perceptions of COVID-19 around the world. J. Risk Res. 2020, 23, 994–1006. [Google Scholar] [CrossRef]
- Wise, T.; Zbozinek, T.D.; Michelini, G.; Hagan, C.C.; Mobbs, D. Changes in risk perception and self-reported protective behaviour during the first week of the COVID-19 pandemic in the United States. R. Soc. Open Sci. 2020, 7, 200742. [Google Scholar] [CrossRef]
- Preparing for a Second Wave of COVID-19. Available online: https://www.bmj.com/content/369/bmj.m2514/rr-1 (accessed on 28 July 2020).
- Wise, J. COVID-19: Risk of second wave is very real, say researchers. BMJ 2020, 369, m2294. [Google Scholar] [CrossRef] [PubMed]
- Siegel, J.D.; Rhinehart, E.; Jackson, M.; Chiarello, L. 2007 Guideline for Isolation Precautions: Preventing Transmission of Infec-tious Agents in Health Care Settings. Am. J. Infect. Control 2007, 35, S65–S164. [Google Scholar] [CrossRef]
- Jiang, F.-C.; Jiang, X.-L.; Wang, Z.-G.; Meng, Z.-H.; Shao, S.-F.; Anderson, B.D.; Ma, M.-J. Early Release—Detection of Severe Acute Respiratory Syndrome Coronavirus 2 RNA on Surfaces in Quarantine Rooms—Volume 26, Number 9—September 2020—Emerging Infectious Diseases Journal—CDC. Available online: https://wwwnc.cdc.gov/eid/article/26/9/20-1435_article (accessed on 22 May 2020).
- Sendén, M.G.; Schenck-Gustafsson, K.; Fridner, A. Gender differences in Reasons for Sickness Presenteeism—A study among GPs in a Swedish health care organization. Ann. Occup. Environ. Med. 2016, 28, 50. [Google Scholar] [CrossRef]
- Chong, M.-Y.; Wang, W.-C.; Hsieh, W.-C.; Lee, C.-Y.; Chiu, N.-M.; Yeh, W.-C.; Huang, O.-L.; Wen, J.-K.; Chen, C.-L. Psychological impact of severe acute respiratory syndrome on health workers in a tertiary hospital. Br. J. Psychiatry 2004, 185, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Chan, A.O.M. Psychological impact of the 2003 severe acute respiratory syndrome outbreak on health care workers in a medium size regional general hospital in Singapore. Occup. Med. 2004, 54, 190–196. [Google Scholar] [CrossRef] [PubMed]
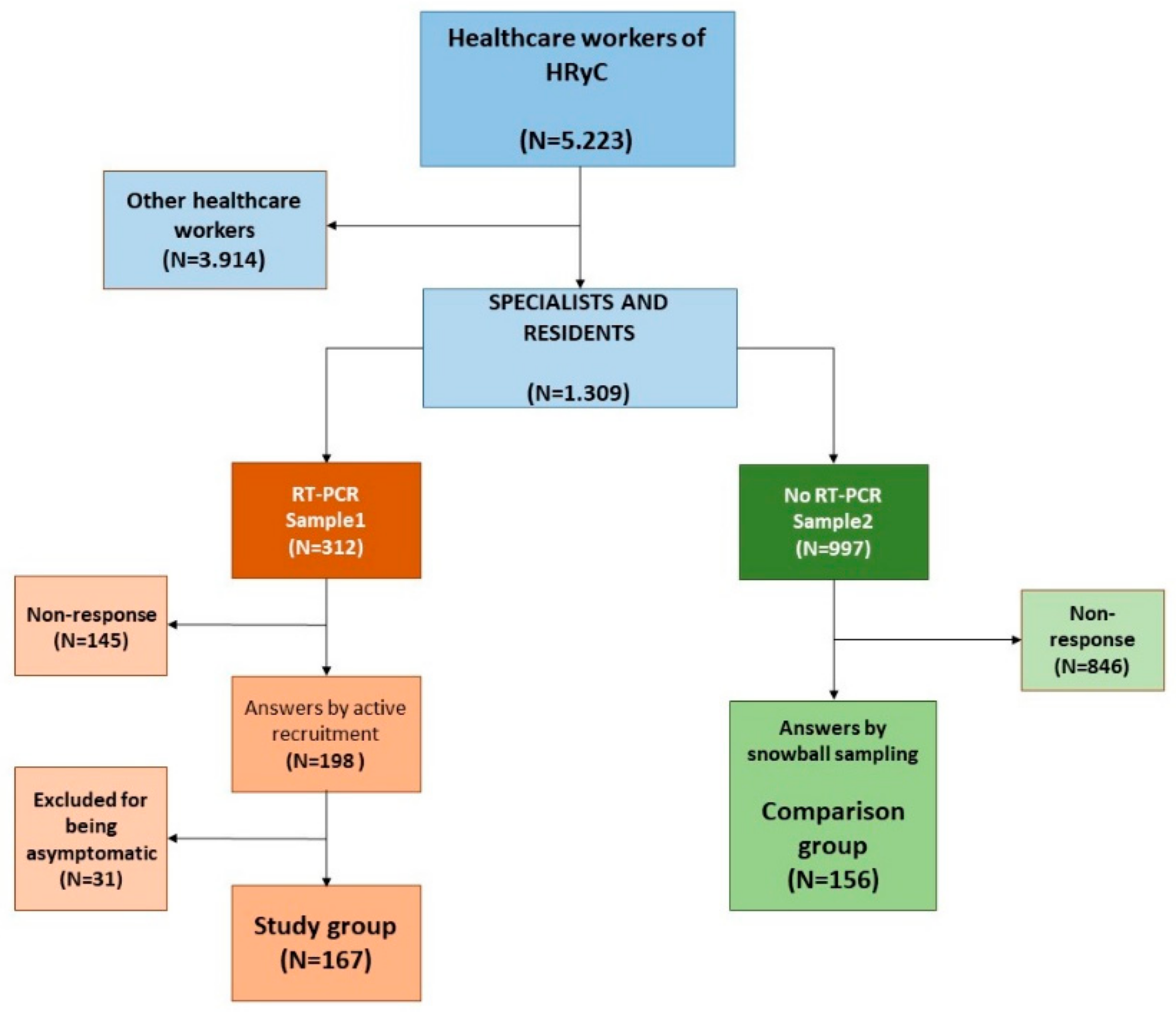

| Study Group | Comparison Group | p-Value | |
|---|---|---|---|
| Median years of experience (interquartile range) | 12 years (4, 22) | 14 years (5, 26) | |
| Sex | 0.3 | ||
| Men N(%) | 79 (47.3) | 65 (41.7) | |
| Women N(%) | 88 (52.7) | 91 (58.3) | |
| Working group | 0.07 | ||
| Resident Intern N(%) | 52 (31.1) | 35 (22.4%) | |
| Specialist physician N(%) | 115 (68.9) | 121 (77.6%) | |
| High-risk specialists 1 N(%) | 20 (12) | 7 (4.5) | 0.01 |
| Study Group N(%) | Comparison Group N(%) | OR (IC95; p-Value) | |
|---|---|---|---|
| Yes, I already knew and used protective measures | 40 (30.3) | 64 (51.2) | 0.41 (0.24–0.71; 0.0006) |
| Yes, I already knew but I did not use protective measures | 1 (0.8) | 2 (1.6) | 0.47 (0.00–9.14; 0.6135) 1 |
| Yes, but I did not know, and I did not use protective measures | 37 (28) | 30 (24) | 1.23 (0.68–2.25; 0.4620) |
| Yes, but I did not know, and, despite everything, I used protective measures | 14 (10.6) | 16 (12.8) | 0.81 (0.35–1.86; 0.5841) |
| I have not cared for any confirmed or suspected patients | 40 (30.3) | 13 (10.4) | 3.75 (1.82–8.07; 0.0001) |
| Total | 132 (100) | 125 (100) |
| Study Group N (%) | Comparison Group N (%) | p-Value | Work Setting | ||||
|---|---|---|---|---|---|---|---|
| Study Group N (%) | Comparison Group N (%) | p-Value | |||||
| Work setting | 130 (77.8) | 139 (89.1%) | 0.1089 | Colleagues | 35 (26.9) | 19 (13.7) | 0.0067 |
| Unknown setting | 14 (8.4) | 9 (5.8%) | 0.4331 | Patients | 27 (20.8) | 34 (24.5) | 0.2599 |
| Personal environment | 11 (6.6) | Mixed | 15 (11.5) | 29 (20.9) | 0.0388 | ||
| Mixed area | 11 (6.6) | 3 (1.9%) | 0.0541 1 | PPE | 19 (14.6) | 35 (25.2) | 0.1202 |
| Public transport | 1 (0.6) | 1 (0.6%) | Common spaces | 1 (0.8) | 13 (9.3) | 0.0015 1 | |
| Lack of testing | 4 (2.6%) | Not specified | 33 (25.4) | 9 (6.4) | <0.0001 | ||
| Study Group N (%) | Comparison Group N (%) | |||||
|---|---|---|---|---|---|---|
| Optimal (≥7) | Suboptimal (<7) | Optimal (≥7) | Suboptimal (<7) | p-Value | ||
| Mask | Availability | 38 (28.8%) | 94 (71.2%) | 35 (28%) | 90 (72%) | 0.8887 |
| Correct use | 74 (56.1%) | 58 (43.9%) | 70 (56%) | 55 (44%) | 0.9922 | |
| Goggles | Availability | 37 (28%) | 95 (72%) | 42 (33.6%) | 83 (66.4%) | 0.3334 |
| Correct use | 46 (34.9%) | 86 (65.1%) | 58 (46.4%) | 67 (53.6%) | 0.0593 | |
| Gloves | Availability | 98 (74.2%) | 34 (25.8%) | 97 (77.6%) | 28 (22.4%) | 0.5292 |
| Correct use | 103 (78%) | 28 (22%) | 103 (82.4%) | 22 (17.6%) | 0.3792 | |
| Gowns | Availability | 28 (21.2%) | 104 (78.8%) | 22 (17.6%) | 103 (82.4%) | 0.4641 |
| Correct use | 51 (38.7%) | 81 (61.3%) | 59 (47.2%) | 66 (52.8%) | 0.9052 | |
| Hand sanitiser | Availability | 77 (58.3%) | 55 (41.7%) | 72 (57.6%) | 53 (42.4%) | 0.9052 |
| Correct use | 102 (77.3%) | 30 (22.7%) | 95 (76%) | 30 (24%) | 0.8095 | |
| Soap | Availability | 100 (75.8%) | 32 (24.2%) | 94 (75.2%) | 31 (24.8%) | 0.9173 |
| Correct use | 105 (79.6%) | 27 (20.4%) | 92 (73.6%) | 33 (26.4%) | 0.2600 | |
| Before touching the patient | Compliance | 98 (74.2%) | 34 (25.8%) | 92 (73.6%) | 33 (26.4%) | 0.9067 |
| Before the aseptic technique | Compliance | 106 (80.3%) | 26 (19.7%) | 101 (80.8%) | 24 (19.2%) | 0.9199 |
| After exposure to fluids | Compliance | 111 (84.1%) | 21 (15.9%) | 107 (85.6%) | 18 (14.4%) | 0.7360 |
| After contact with the patient | Compliance | 110 (83.3%) | 22 (16.7%) | 103 (82.4%) | 22 (17.6%) | 0.8427 |
| After the environment | Compliance | 94 (71.2%) | 38 (28.8%) | 99 (79.2%) | 26 (20.8%) | 0.1378 |
| Study Group N (%) | Comparison Group N (%) | ||||
|---|---|---|---|---|---|
| Optimal (≥7) | Suboptimal (<7) | Optimal (≥7) | Suboptimal (<7) | p-Value | |
| Hand hygiene before the workday | 127 (76.1%) | 40 (23.9%) | 130 (83.3%) | 26 (16.7%) | 0.1034 |
| Hand hygiene after the workday | 137 (82.1%) | 30 (17.9%) | 132 (84.6%) | 24 (15.4%) | 0.5342 |
| Personal object sanitation before the start of the day | 43 (25.7%) | 124 (74.3%) | 76 (48.7%) | 80 (51.3%) | <0.0001 |
| Personal object sanitation at the end of the work day | 69 (41.3%) | 98 (58.7%) | 99 (63.5%) | 57 (36.5%) | 0.0001 |
| Personal distancing | 77 (46.1%) | 90 (53.9%) | 113 (74.4%) | 43 (27.6%) | <0.0001 |
| Prior disinfection of shared material | 48 (28.7%) | 119 (71.3%) | 97 (62.2%) | 59 (37.8%) | <0.0001 |
| Study Group N (%) | Comparison Group N (%) | p-Value | |
|---|---|---|---|
| 0 days | 66 (39.5%) | 3 (13.1%) | |
| 1–3 days | 68 (40.8%) | 10 (43.4%) | |
| 4 or more days | 33 (19.7%) | 10 (43.4%) | |
| Total | 167 (100%) | 23 (100%) | 0.011 |
| OR (DE) | p-Value | CI95% | |
|---|---|---|---|
| Univariate Regression | |||
| Public transport | 2.79 (0.72) | <0.0001 | 1.68–4.64 |
| Having carried out AGP | 2.45 (1.08) | 0.042 | 1.03–5.84 |
| Risk speciality | 2.9 (1.31) | 0.019 | 1.18–7.05 |
| Personal object sanitation before the workday | 0.37 (0.09) | <0.0001 | 0.23–0.58 |
| Personal object sanitation at the end of the workday | 0.41 (0.09) | <0.0001 | 0.26–0.63 |
| Personal distancing | 0.33 (0.7) | <0.0001 | 0.20–0.51 |
| Disinfection of shared material | 0.24 (0.05) | <0.0001 | 0.15–0.39 |
| Change of uniform | 0.55 (0.13) | 0.013 | 0.35–0.88 |
| Close contact with a sick SARS-CoV-2 confirmed health worker | 0.61 (0.15) | 0.045 | 0.38–0.99 |
| Multivariate regression | |||
| Risk speciality | 4.45 (2.23) | 0.003 | 1.66–11.91 |
| Public transport | 3.27 (0.94) | <0.0001 | 1.87–5.73 |
| Personal object sanitation before the workday | 0.55 (0.16) | 0.040 | 0.31–0.97 |
| Disinfection of shared material | 0.34 (0.09) | <0.0001 | 0.19–0.58 |
| Change of uniform | 0.53 (0.14) | 0.019 | 0.32–0.90 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aranaz-Andrés, J.M.; McGee-Laso, A.; Galán, J.C.; Cantón, R.; Mira, J.; on behalf of the Team of Work COVID-19. Activities and Perceived Risk of Transmission and Spread of SARS-CoV-2 among Specialists and Residents in a Third Level University Hospital in Spain. Int. J. Environ. Res. Public Health 2021, 18, 2838. https://doi.org/10.3390/ijerph18062838
Aranaz-Andrés JM, McGee-Laso A, Galán JC, Cantón R, Mira J, on behalf of the Team of Work COVID-19. Activities and Perceived Risk of Transmission and Spread of SARS-CoV-2 among Specialists and Residents in a Third Level University Hospital in Spain. International Journal of Environmental Research and Public Health. 2021; 18(6):2838. https://doi.org/10.3390/ijerph18062838
Chicago/Turabian StyleAranaz-Andrés, Jesús María, Amaranta McGee-Laso, Juan Carlos Galán, Rafael Cantón, José Mira, and on behalf of the Team of Work COVID-19. 2021. "Activities and Perceived Risk of Transmission and Spread of SARS-CoV-2 among Specialists and Residents in a Third Level University Hospital in Spain" International Journal of Environmental Research and Public Health 18, no. 6: 2838. https://doi.org/10.3390/ijerph18062838
APA StyleAranaz-Andrés, J. M., McGee-Laso, A., Galán, J. C., Cantón, R., Mira, J., & on behalf of the Team of Work COVID-19. (2021). Activities and Perceived Risk of Transmission and Spread of SARS-CoV-2 among Specialists and Residents in a Third Level University Hospital in Spain. International Journal of Environmental Research and Public Health, 18(6), 2838. https://doi.org/10.3390/ijerph18062838







