Exposure to Perfluoroalkyl Substances and Mortality for COVID-19: A Spatial Ecological Analysis in the Veneto Region (Italy)
Abstract
1. Introduction
2. Materials and Methods
2.1. Data
2.2. Model for Baseline Mortality
2.3. Model for COVID-19 Mortality
2.4. Sensitivity Analysis
3. Results
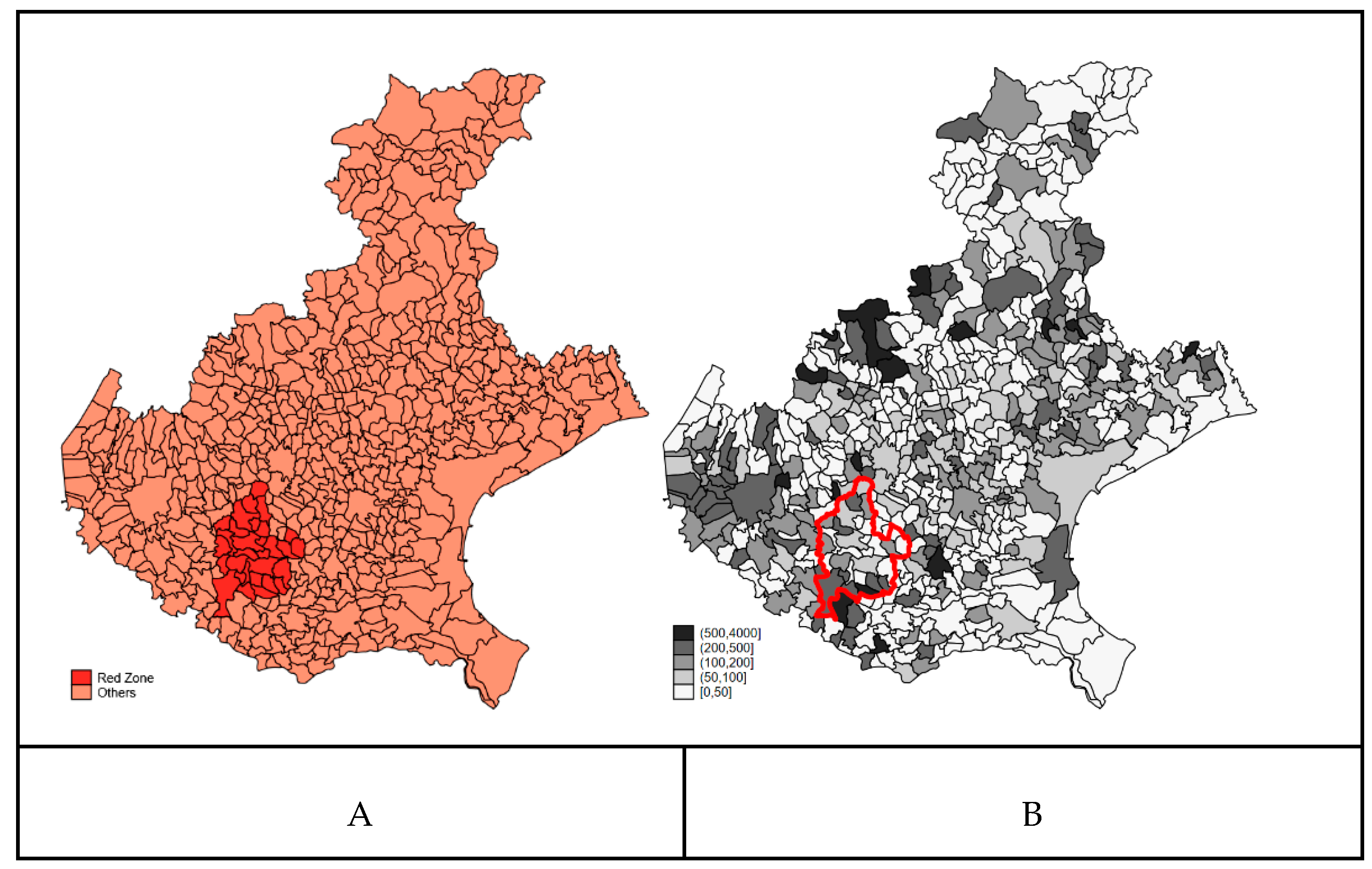
3.1. Descriptive Analysis
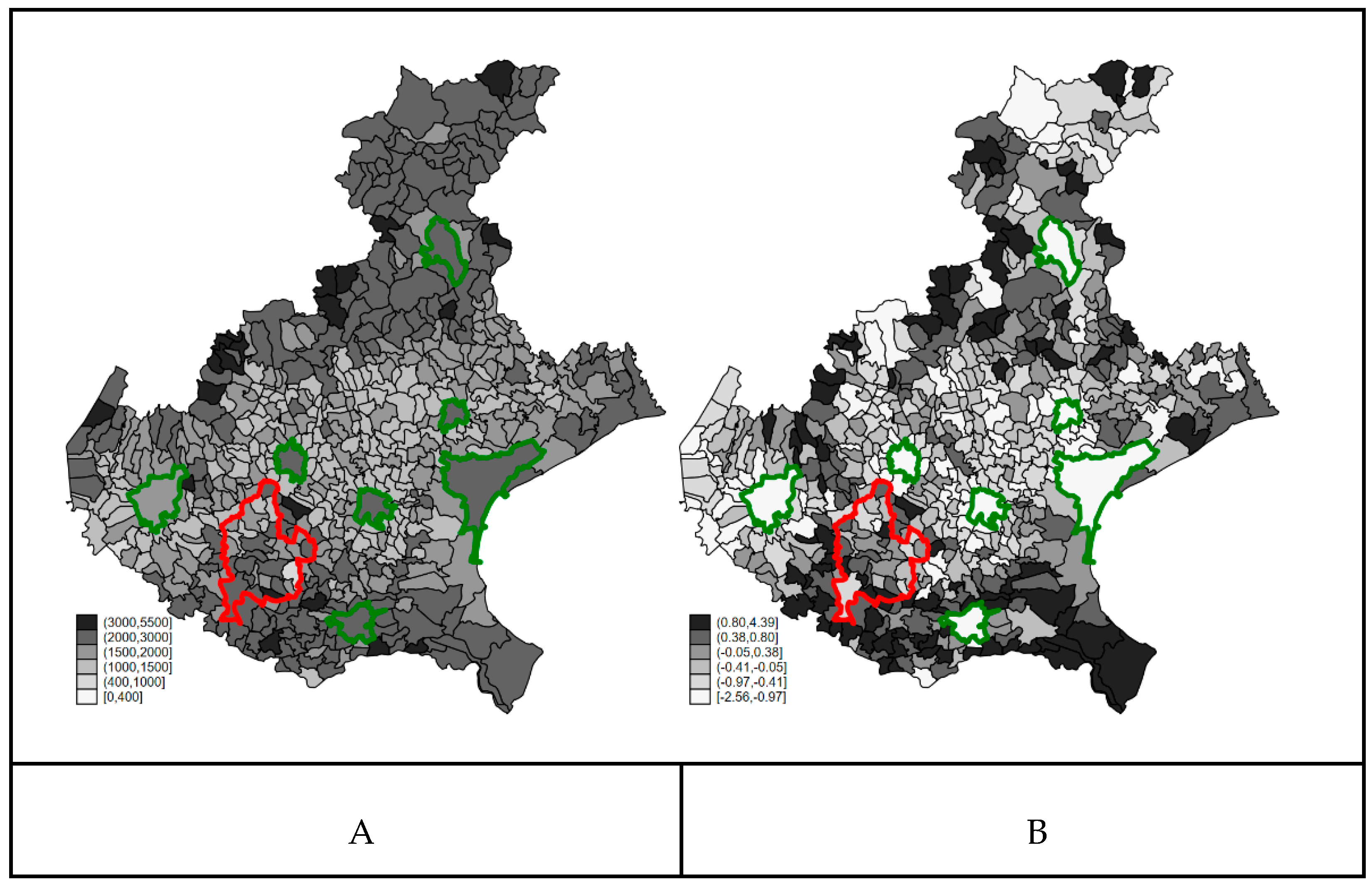
3.2. Confounders’ Spatial Distribution
3.3. Crude Analysis of COVID-19 Mortality in the Red Zone
3.4. Bayesian Ecological Regression Analysis
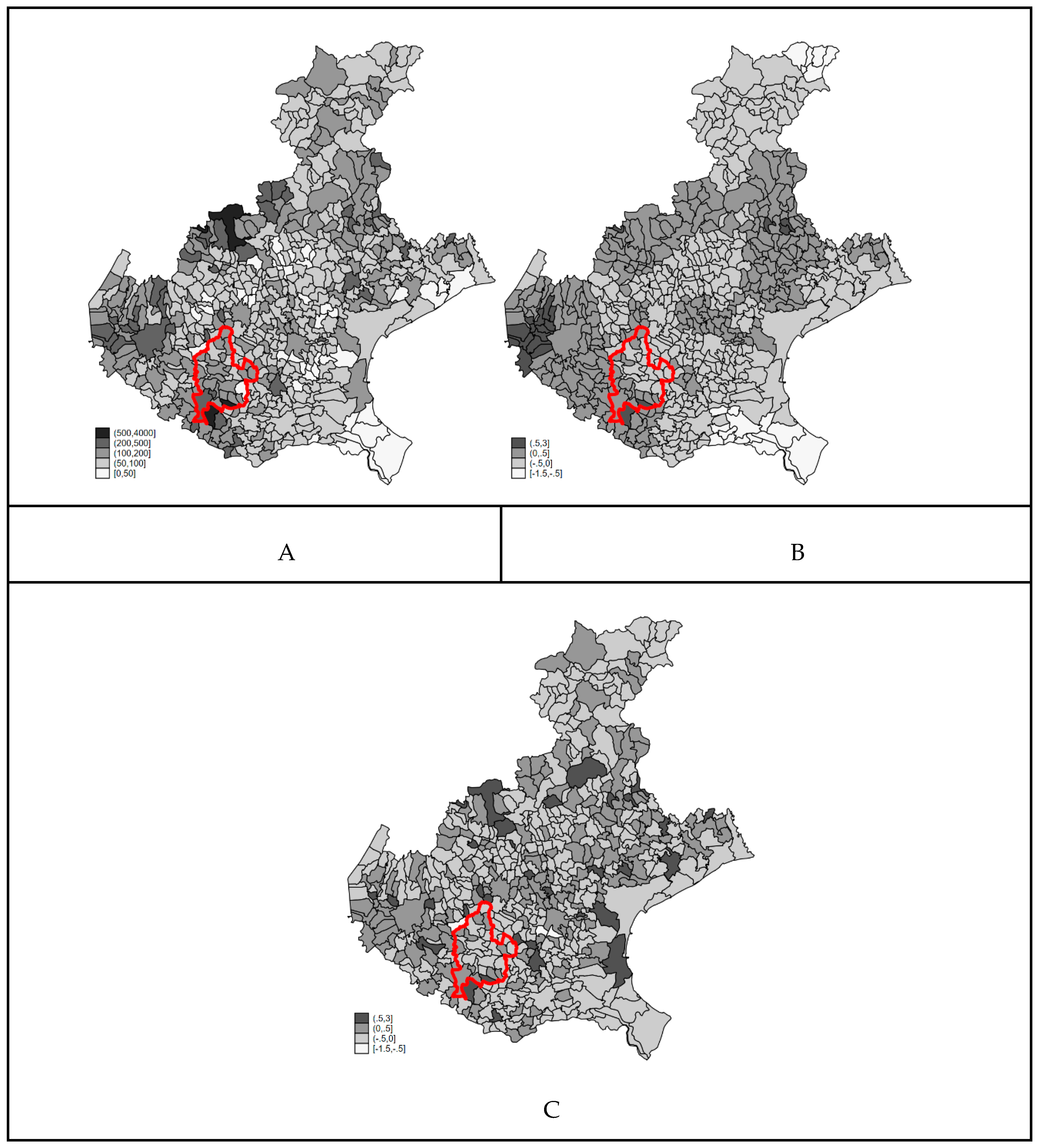
3.5. Sensitivity Aanalysis
4. Discussion
4.1. Consistence of the Definition of Resident Population of the Red Zone as PFAS Highly Exposed Population
4.2. Consistence of the Definition of the Observable Confounders—NH, Baseline Mortality, and Education
4.3. Appropriateness of the Interpretation of the Random Effects as Hidden Confounders in Ecological Analysis
4.4. Interpretation of the Ecological Association between PFAS Exposure and COVID-19 Mortality
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- OECD. Toward a New Comprehensive Global Database of per- and Polyfluoroalkyl Substances (PFASs): Summary Report on Updating the OECD 2007 List of per and Polyfluoroalkyl Substances (PFASs). 2018. Available online: https://www.oecd.org/officialdocuments/publicdisplaydocumentpdf/?cote=ENV-JM-MONO(2018)7&doclanguage=en (accessed on 30 December 2020).
- Glüge, J.; Scheringer, M.; Cousins, I.T.; DeWitt, J.C.; Goldenman, G.; Herzke, D.; Lohmann, R.; Ng, C.A.; Trier, X.; Wang, Z. An overview of the uses of per- and polyfluoroalkyl substances (PFAS). Environ. Sci. Process. Impacts 2020, 22, 2345–2373. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Fletcher, T.; Mucs, D.; Scott, K.; Lindh, C.H.; Tallving, P.; Jakobsson, K. Half-lives of PFOS, PFHxS and PFOA after end of exposure to contaminated drinking water. Occup. Environ. Med. 2018, 75, 46–51. [Google Scholar] [CrossRef]
- Perfluorooctanoic Acid (PFOA), Its Salts and PFOA-Related Compounds. Available online: http://chm.pops.int/Implementation/Alternatives/AlternativestoPOPs/ChemicalslistedinAnnexA/PFOA/tabid/8292/Default.aspx (accessed on 30 December 2020).
- EFSA. Risk to Human Health Related to the Presence of Perfluorooctane Sulfonic Acid and Perfluorooctanoic Acid in Food. EFSA J. 2018, 16, 5194. [Google Scholar]
- Abraham, K.; Mielke, H.; Fromme, H.; Völkel, W.; Menzel, J.; Peiser, M.; Zepp, F.; Willich, S.N.; Weikert, C. Internal exposure to perfluoroalkyl substances (PFASs) and biological markers in 101 healthy 1-year-old children: Associations between levels of perfluorooctanoic acid (PFOA) and vaccine response. Arch. Toxicol. 2020, 94, 2131–2147. [Google Scholar] [CrossRef]
- Kvalem, H.; Nygaard, U.; Carlsen, K.L.; Haug, L.; Granum, B. Perfluoroalkyl substances, airways infections, allergy and asthma related health outcomes—implications of gender, exposure period and study design. Environ. Int. 2020, 134, 105259. [Google Scholar] [CrossRef] [PubMed]
- Looker, C.; Luster, M.I.; Calafat, A.M.; Johnson, V.J.; Burleson, G.R.; Burleson, F.G.; Fletcher, T. Influenza Vaccine Response in Adults Exposed to Perfluorooctanoate and Perfluorooctanesulfonate. Toxicol. Sci. 2014, 138, 76–88. [Google Scholar] [CrossRef] [PubMed]
- National Toxicology Program (NTP). Monograph on Immunotoxicity Associated with Exposure to Perfluorooctanoic Acid or Perfluorooctane Sulfonate; National Toxicology Program: Research Triangle Park, NC, USA, 2016. [Google Scholar]
- DeWitt, J.C.; Blossom, S.J.; Schaider, L.A. Exposure to per-fluoroalkyl and polyfluoroalkyl substances leads to immunotoxicity: Epidemiological and toxicological evidence. J. Expo. Sci. Environ. Epidemiol. 2019, 29, 148–156. [Google Scholar] [CrossRef]
- Chang, E.T.; Adami, H.-O.; Boffetta, P.; Wedner, H.J.; Mandel, J.S. A critical review of perfluorooctanoate and perfluorooctanesulfonate exposure and immunological health conditions in humans. Crit. Rev. Toxicol. 2016, 46, 279–331. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, M.A.; Hossain, N.; Kashem, M.A.; Shahid, A.; Alam, A. Immune response in COVID-19: A review. J. Infect. Public Health 2020, 13, 1619–1629. [Google Scholar] [CrossRef]
- Quinete, N.; Hauser-Davis, R.A. Drinking water pollutants may affect the immune system: Concerns regarding COVID-19 health effects. Environ. Sci. Pollut. Res. 2021, 28, 1235–1246. [Google Scholar] [CrossRef] [PubMed]
- Grandjean, P.; Timmermann, C.A.G.; Kruse, M.; Nielsen, F.; Vinholt, P.J.; Boding, L.; Heilmann, C.; Molbak, K. Severity of COVID-19 at Elevated Exposure to Perfluorinated Alkylates. PLoS ONE 2020, 15, e0244815. [Google Scholar] [CrossRef]
- Pérez, F.; Nadal, M.; Navarro-Ortega, A.; Fàbrega, F.; Domingo, J.L.; Barceló, D.; Farré, M. Accumulation of perfluoroalkyl substances in human tissues. Environ. Int. 2013, 59, 354–362. [Google Scholar] [CrossRef]
- Pitter, G.; Da Re, F.; Canova, C.; Barbieri, G.; Jeddi, M.Z.; Daprà, F.; Manea, F.; Zolin, R.; Bettega, A.M.; Stopazzolo, G.; et al. Serum Levels of Perfluoroalkyl Substances (PFAS) in Adolescents and Young Adults Exposed to Contaminated Drinking Water in the Veneto Region, Italy: A Cross-Sectional Study Based on a Health Surveillance Program. Environ. Health Perspect. 2020, 128, 027007. [Google Scholar] [CrossRef] [PubMed]
- Ingelido, A.M.; Abballe, A.; Gemma, S.; Dellatte, E.; Iacovella, N.; De Angelis, G.; Zampaglioni, F.; Marra, V.; Miniero, R.; Valentini, S.; et al. Biomonitoring of perfluorinated compounds in adults exposed to contaminated drinking water in the Veneto Region, Italy. Environ. Int. 2018, 110, 149–159. [Google Scholar] [CrossRef] [PubMed]
- Available online: http://demo.istat.it (accessed on 30 December 2020).
- Rosano, A.; Pacelli, B.; Zengarini, N.; Costa, G.; Cislaghi, C.; Caranci, N. Aggiornamento e revisione dell’indice di deprivazione italiano 2011 a livello di sezione di censimento. Epidemiol. Prev. 2020, 44, 162–170. [Google Scholar] [CrossRef]
- Available online: http://extraospedaliero.regione.veneto.it/area-anziani/anagrafica-udo (accessed on 30 December 2020).
- Besag, J.; York, J. Bayesian image restoration, with two applications in spatial statistics. Ann. Inst. Stat. Math. 1991, 43, 1–20. [Google Scholar] [CrossRef]
- Kelsall, J.E.; Wakefield, J.C. Bayesian Models for Spatially Correlated Disease and Exposure Data. In Bayesian Statistics 6; Oxford University Press: Oxford, UK, 1999. [Google Scholar]
- Johnson, N.L.; Kemp, A.W.; Kotz, S. Univariate Discrete Distributions: Johnson/Univariate Discrete Distributions; Wiley Series in Probability and Statistics; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2005; ISBN 978-0-471-71581-8. [Google Scholar]
- Clayton, D.G.; Bernardinelli, L.; Montomoli, C. Spatial Correlation in Ecological Analysis. Int. J. Epidemiol. 1993, 22, 1193–1202. [Google Scholar] [CrossRef]
- Lunn, D.J.; Thomas, A.; Best, N.; Spiegelhalter, D. WinBUGS—A Bayesian modelling framework: Concepts, structure, and extensibility. Stat. Comput. 2000, 10, 325–337. [Google Scholar] [CrossRef]
- Makowski, D.; Ben Sachar, M.S.; Chen, S.H.A.; Luedecke, D. Indices of Effect Existence and Significance in the Bayesian Framework. Front. Psychol. 2019, 10, 2767. [Google Scholar] [CrossRef]
- Makowski, D.; Ben-Shachar, M.; Lüdecke, D. bayestestR: Describing Effects and their Uncertainty, Existence and Significance within the Bayesian Framework. J. Open Source Softw. 2019, 4, 1541. [Google Scholar] [CrossRef]
- Rosenbaum, P.R.; Rubin, D.B. Difficulties with regression analyses of age-adjusted rates. Biometrics 1984, 40, 437–443. [Google Scholar] [CrossRef] [PubMed]
- Biggeri, A.; Catelan, D.; Stoppa, G.; Lagazio, C. Joint Analysis of Short and Long-Term Effects of Air Pollution. Book of Short Papers SIS 2020. 2020, pp. 551–556. Available online: https://it.pearson.com/content/dam/region-core/italy/pearson-italy/pdf/Docenti/Universit%C3%A0/Pearson-SIS-2020-atti-convegno.pdf (accessed on 30 December 2020).
- Petti, S.; Cowling, B.J. Ecologic association between influenza and COVID-19 mortality rates in European countries. Epidemiol. Infect. 2020, 148, 1–21. [Google Scholar] [CrossRef] [PubMed]
- The Territorial Impact of COVID-19: Managing the Crisis across Levels of Government; OECD Policy Responses to Coronavirus (COVID-19). 2020. Available online: https://www.oecd.org/coronavirus/policy-responses/the-territorial-impact-of-COVID-19-managing-the-crisis-across-levels-of-government-d3e314e1/ (accessed on 30 December 2020).
- Available online: https://www.epicentro.iss.it/en/coronavirus/sars-cov-2-survey-rsa (accessed on 30 December 2020).
- Morgenstern, H. Ecologic Studies. In Modern Epidemiology, 3rd ed.; Lippincott: New York, NY, USA, 2008. [Google Scholar]
- Richardson, S.; Hirsch, J.S.; Narasimhan, M.; Crawford, J.M.; McGinn, T.; Davidson, K.W.; The Northwell COVID-19 Research Consortium. Presenting Characteristics, Comorbidities, and Outcomes among 5700 Patients Hospitalized With COVID-19 in the New York City Area. JAMA 2020, 323, 2052–2059. [Google Scholar] [CrossRef] [PubMed]
- Steenland, K.; Tinker, S.; Frisbee, S.; Ducatman, A.; Vaccarino, V. Association of Perfluorooctanoic Acid and Perfluorooctane Sulfonate with Serum Lipids Among Adults Living Near a Chemical Plant. Am. J. Epidemiol. 2009, 170, 1268–1278. [Google Scholar] [CrossRef]
- Canova, C.; Barbieri, G.; Jeddi, M.Z.; Gion, M.; Fabricio, A.; Daprà, F.; Russo, F.; Fletcher, T.; Pitter, G. Associations between perfluoroalkyl substances and lipid profile in a highly exposed young adult population in the Veneto Region. Environ. Int. 2020, 145, 106117. [Google Scholar] [CrossRef] [PubMed]
| Number of Municipalities | COVID-19 Deaths | Population | Rate (×100,000) | 90% CI | |
|---|---|---|---|---|---|
| Red Zone | 30 | 63 | 187,375 | 33.6 | 27.3; 41.4 |
| Others | 533 | 1032 | 4,750,548 | 21.7 | 26.6; 22.9 |
| RR | 90% CrI | |
|---|---|---|
| General Mortality | 1.04 | 1.03; 1.05 |
| Education Level | 0.92 | 0.83; 0.99 |
| Red Zone | 1.60 | 0.94; 2.51 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Catelan, D.; Biggeri, A.; Russo, F.; Gregori, D.; Pitter, G.; Da Re, F.; Fletcher, T.; Canova, C. Exposure to Perfluoroalkyl Substances and Mortality for COVID-19: A Spatial Ecological Analysis in the Veneto Region (Italy). Int. J. Environ. Res. Public Health 2021, 18, 2734. https://doi.org/10.3390/ijerph18052734
Catelan D, Biggeri A, Russo F, Gregori D, Pitter G, Da Re F, Fletcher T, Canova C. Exposure to Perfluoroalkyl Substances and Mortality for COVID-19: A Spatial Ecological Analysis in the Veneto Region (Italy). International Journal of Environmental Research and Public Health. 2021; 18(5):2734. https://doi.org/10.3390/ijerph18052734
Chicago/Turabian StyleCatelan, Dolores, Annibale Biggeri, Francesca Russo, Dario Gregori, Gisella Pitter, Filippo Da Re, Tony Fletcher, and Cristina Canova. 2021. "Exposure to Perfluoroalkyl Substances and Mortality for COVID-19: A Spatial Ecological Analysis in the Veneto Region (Italy)" International Journal of Environmental Research and Public Health 18, no. 5: 2734. https://doi.org/10.3390/ijerph18052734
APA StyleCatelan, D., Biggeri, A., Russo, F., Gregori, D., Pitter, G., Da Re, F., Fletcher, T., & Canova, C. (2021). Exposure to Perfluoroalkyl Substances and Mortality for COVID-19: A Spatial Ecological Analysis in the Veneto Region (Italy). International Journal of Environmental Research and Public Health, 18(5), 2734. https://doi.org/10.3390/ijerph18052734








