Does Physical Activity Modify the Association between Air Pollution and Recurrence of Cardiovascular Disease?
Abstract
1. Introduction
2. Materials and Methods
2.1. Cohort Description
2.2. Physical Activity
2.3. Air Pollution Assessment
2.4. Health Outcomes
2.5. Statistical Methods
3. Results
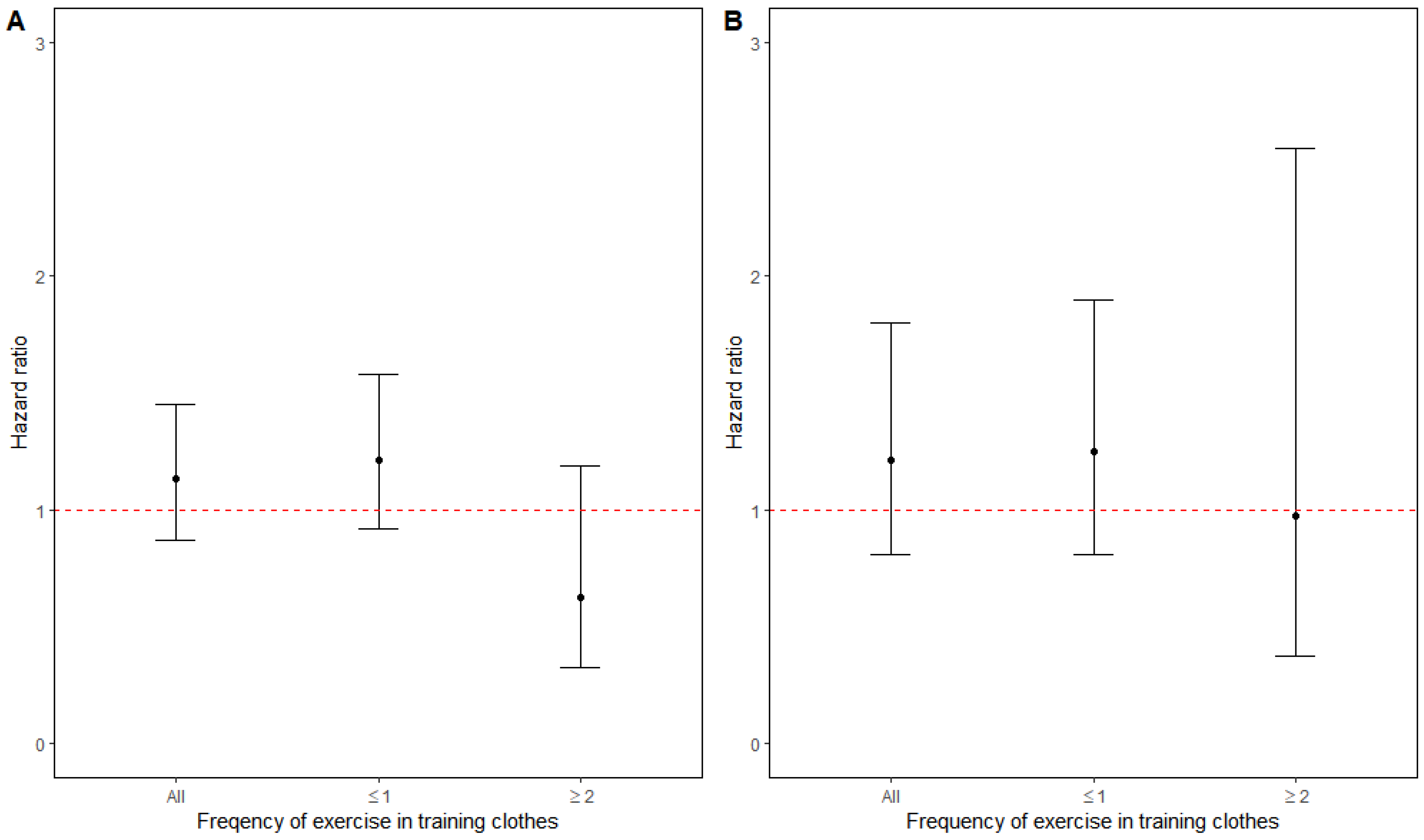
3.1. Association with Air Pollution at Different Levels of Exercise
3.2. Association with Physical Activity at Different Levels of PM2.5
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- GBD 2017 Risk Factors Collaborators. Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks for 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1923–1994. [Google Scholar] [CrossRef]
- Riediker, M.; Zink, D.; Kreyling, W.; Oberdörster, G.; Elder, A.; Graham, U.; Lynch, I.; Duschl, A.; Ichihara, G.; Ichihara, S.; et al. Parti-cle toxicology and health—Where are we? Part. Fibre Toxicol. 2019, 16, 1–33. [Google Scholar]
- Martins, L.C.; Pereira, L.A.A.; Lin, C.A.; Santos, U.P.; Prioli, G.; Luiz, O.D.C.; Saldiva, P.H.N.; Braga, A.L.F. The effects of air pollution on cardiovascular diseases: Lag structures. Revista Saúde Pública 2006, 40, 677–683. [Google Scholar] [CrossRef]
- Townsend, N.; Wilson, L.; Bhatnagar, P.; Wickramasinghe, K.; Rayner, M.; Nichols, M. Cardiovascular disease in Europe: Epidemiolog-ical update 2016. Eur. Heart J. 2016, 42, 3232–3245. [Google Scholar] [CrossRef]
- Miller, M.R.; Shaw, C.A.; Langrish, J.P. From particles to patients: Oxidative stress and the cardiovascular effects of air pollution. Futur. Cardiol. 2012, 8, 577–602. [Google Scholar] [CrossRef] [PubMed]
- Jokhadar, M.; Jacobsen, S.J.; Reeder, G.S.; Weston, S.A.; Roger, V.L. Sudden Death and Recurrent Ischemic Events after Myocardial Infarction in the Community. Am. J. Epidemiol. 2004, 159, 1040–1046. [Google Scholar] [CrossRef] [PubMed]
- Guthold, R.; Stevens, G.A.; Riley, L.M.; Bull, F.C. Worldwide trends in insufficient physical activity from 2001 to 2016: A pooled analysis of 358 population-based surveys with 1·9 million participants. Lancet Glob. Health 2018, 6, e1077–e1086. [Google Scholar] [CrossRef]
- Lee, I.-M.; Shiroma, E.J.; Lobelo, F.; Puska, P.; Blair, S.N.; Katzmarzyk, P.T. Impact of Physical Inactivity on the World’s Major Non-Communicable Diseases. Lancet 2012, 380, 219–229. [Google Scholar] [CrossRef]
- Hallal, P.C.; Andersen, L.B.; Bull, F.C.; Guthold, R.; Haskell, W.L.; Ekelund, U.; Lancet Physical Activity Series Working Group. Global physical activity levels: Surveillance progress, pitfalls, and prospects. Lancet 2012, 380, 247–257. [Google Scholar] [CrossRef]
- Chomistek, A.K.; Chiuve, S.E.; Jensen, M.K.; Cook, N.R.; Rimm, E.B. Vigorous Physical Activity, Mediating Biomarkers, and Risk of Myocardial Infarction. Med. Sci. Sports Exerc. 2011, 43, 1884–1890. [Google Scholar] [CrossRef]
- Reddigan, J.I.; Riddell, M.C.; Kuk, J.L. The joint association of physical activity and glycaemic control in predicting cardiovascular death and all-cause mortality in the US population. Diabetologia 2012, 55, 632–635. [Google Scholar] [CrossRef]
- Raza, W.; Krachler, B.; Forsberg, B.; Sommar, J.N. Health benefits of leisure time and commuting physical activity: A meta-analysis of effects on morbidity. J. Transp. Health 2020, 18, 100873. [Google Scholar] [CrossRef]
- Cepeda, M.M.; Schoufour, J.; Freak-Poli, R.; Koolhaas, M.C.M.; Dhana, K.; Bramer, W.M.; Franco, O.H. Levels of ambient air pollution according to mode of transport: A systematic review. Lancet Public Health 2017, 2, e23–e34. [Google Scholar] [CrossRef]
- Kubesch, N.J.; Jørgensen, J.T.; Hoffmann, B.; Loft, S.; Nieuwenhuijsen, M.J.; Raaschou-Nielsen, O.; Pedersen, M.; Hertel, O.; Overvad, K.; Tjønneland, A.; et al. Effects of Leisure-Time and Transport-Related Physical Activities on the Risk of Incident and Recurrent Myocardial Infarction and Interaction With Traffic-Related Air Pollution: A Cohort Study. J. Am. Hear. Assoc. 2018, 7, e009554. [Google Scholar] [CrossRef]
- Norberg, M.; Wall, S.; Boman, K.; Weinehall, L. The Västerbotten Intervention Programme: Background, design and implications. Glob. Health Action 2010, 3. [Google Scholar] [CrossRef]
- Segersson, D.; Eneroth, K.; Gidhagen, L.; Johansson, C.; Omstedt, G.; Nylén, A.E.; Forsberg, B. Health Impact of PM10, PM2.5 and Black Carbon Exposure Due to Different Source Sectors in Stockholm, Gothenburg and Umea, Sweden. Int. J. Environ. Res. Public Health 2017, 14, 742. [Google Scholar] [CrossRef] [PubMed]
- Hausberger, S.; Rexeis, M.; Zallinger, M.; Luz, R. Emission Factors from the Model PHEM for the HBEFA Version 3. Graz Univ. Technol. 2009, 1, 9–73. [Google Scholar]
- Omstedt, G.; Bringfelt, B.; Johansson, C. A model for vehicle-induced non-tailpipe emissions of particles along Swedish roads. Atmos. Environ. 2005, 39, 6088–6097. [Google Scholar] [CrossRef]
- Denby, B.; Sundvor, I.; Johansson, C.; Pirjola, L.; Ketzel, M.; Norman, M.; Kupiainen, K.; Gustafsson, M.; Blomqvist, G.; Kauhaniemi, M.; et al. A coupled road dust and surface moisture model to predict non-exhaust road traffic induced particle emissions (NORTRIP). Part 2: Surface moisture and salt impact modelling. Atmos. Environ. 2013, 81, 485–503. [Google Scholar] [CrossRef]
- Andersson, S.; Arvelius, J.; Gerner, A.; Danielsson, H.; Ortiz, C.; Svanström, S. Description of Methods and Quality of Spatially Distributed Emissions to Air during 2015; Swedish EPA, Contract No. 309 1235; Swedish EPA: Stockholm, Sweden, 2015.
- World Health Organization International Statistical Classification of Diseases and Related Health Problems: [9th] Ninth Revision, Basic Tabulation List with Alphabetic Index. Available online: https://apps.who.int/iris/handle/10665/39473 (accessed on 27 October 2020).
- World Health Organization International Statistical Classification of Diseases and Related Health Problems, 10th Revision (ICD-10), Fifth Version. Available online: https://www.who.int/classifications/classification-of-diseases (accessed on 27 October 2020).
- Ludvigsson, J.F.; Andersson, E.; Ekbom, A.; Feychting, M.; Kim, J.-L.; Reuterwall, C.; Heurgren, M.; Olausson, P.O. External review and validation of the Swedish national inpatient register. BMC Public Health 2011, 11, 450. [Google Scholar] [CrossRef]
- R Core Team. A Language and Environment for Statistical Computing, R Foundation for Statistical Computing. 2020. Available online: https://www.R-project.org/ (accessed on 8 December 2019).
- Mora, S.; Cook, N.; Buring, J.E.; Ridker, P.M.; Lee, I.-M. Physical Activity and Reduced Risk of Cardiovascular Events. Circulation 2007, 116, 2110–2118. [Google Scholar] [CrossRef] [PubMed]
- Hvidtfeldt, U.A.; Sørensen, M.; Geels, C.; Ketzel, M.; Khan, J.; Tjønneland, A.; Overvad, K.; Brandt, J.; Raaschou-Nielsen, O. Long-term residential exposure to PM2.5, PM10, black carbon, NO2, and ozone and mortality in a Danish cohort. Environ. Int. 2019, 123, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Hållmarker, U.; Åsberg, S.; Michaëlsson, K.; Ärnlöv, J.; Hellberg, D.; Lindbäck, J.; Wester, P.; James, S. Risk of Recurrent Stroke and Death After First Stroke in Long-Distance Ski Race Participants. J. Am. Hear. Assoc. 2015, 4, e002469. [Google Scholar] [CrossRef] [PubMed]
- Hållmarker, U.; Michaëlsson, K.; Ärnlöv, J.; Hellberg, D.; Lagerqvist, B.; Lindbäck, J.; James, S. Risk of recurrent ischaemic events after myocardial infarction in long-distance ski race participants. Eur. J. Prev. Cardiol. 2016, 23, 282–290. [Google Scholar] [CrossRef] [PubMed]
- Svensson, M.; Brundin, L.; Erhardt, S.; Madaj, Z.; Hållmarker, U.; James, S.; Deierborg, T. Long distance ski racing is associated with lower long-term incidence of depression in a population based, large-scale study. Psychiatry Res. 2019, 281, 112546. [Google Scholar] [CrossRef] [PubMed]

| Variables | At Most Once a Week | Twice per Week or More | p-Value | At Most Once a Week | Twice per Week or More | p-Value |
|---|---|---|---|---|---|---|
| IHD | Stroke | |||||
| Number of incident cases | 1261 | 142 | 731 | 87 | ||
| Number of recurrent cases | 382 | 46 | 136 | 20 | ||
| Time to disease recurrence (days) (mean (SD)) | 161.04 (107.20) | 142.32 (107.67) | 0.05 | 168.43 (107.80) | 156.92 (112.17) | 0.35 |
| PM2.5 (mean (SD)) | 5.57 (0.74) | 5.60 (0.80) | 0.39 | 5.80 (0.89) | 5.62 (0.83) | 0.02 |
| Age, years (mean (SD)) | 52.11 (7.80) | 51.20 (8.29) | 0.19 | 53.31 (7.74) | 51.72 (9.18) | 0.08 |
| Frequency of active commuting (%) | 0.05 | 0.96 | ||||
| At most two seasons of four | 920 (73.0) | 92 (64.8) | 489 (66.9) | 59 (67.8) | ||
| More than two seasons of four | 341 (27.0) | 50 (35.2) | 242 (33.1) | 28 (32.2) | ||
| Gender (% women) | 347 (27.5) | 31 (21.8) | 0.18 | 293 (39.9) | 32 (36.8) | 0.65 |
| Alcohol intake (%) | 0.60 | 291 (39.8) | 32 (36.8) | 0.94 | ||
| Never | 5 (0.4) | 1 (0.7) | 2 (0.3) | 0 (0.0) | ||
| Once/month or sometimes | 373 (29.6) | 41 (28.9) | 223 (30.5) | 25 (28.7) | ||
| 2–4 times/month | 222 (17.6) | 17 (12.0) | 102 (14.0) | 12 (13.8) | ||
| 2–3 times/week | 6 (0.5) | 1 (0.7) | 4 (0.5) | 1 (1.1) | ||
| ≥4 times/week | 14 (1.1) | 2 (1.4) | 0 | 0 | ||
| Missing | 641 (50.8) | 80 (56.3) | 400 (54.7) | 49 (56.3) | ||
| Smoking (%) | 0.03 | 0.11 | ||||
| Never smoker | 451 (35.8) | 54 (38.0) | 303 (41.5) | 41 (47.1) | ||
| Previous non-regular smoker | 93 (7.4) | 14 (9.9) | 53 (7.3) | 8 (9.2) | ||
| Non-regular smoker | 55 (4.4) | 11 (7.7) | 27 (3.7) | 3 (3.4) | ||
| Previous regular smoker | 306 (24.3) | 39 (27.5) | 185 (25.3) | 25 (28.7) | ||
| Cigarette smoker | 299 (23.7) | 16 (11.3) | 145 (19.8) | 6 (6.9) | ||
| Cigar or pipe smoker | 26 (2.1) | 3 (2.1) | 12 (1.6) | 2 (2.3) | ||
| Missing | 31 (2.5) | 5 (3.5) | 6 (0.8) | 2 (2.3) | ||
| Highest education level (%) | 0.06 | 0.07 | ||||
| Compulsory | 715 (56.7) | 70 (49.3) | 438 (59.9) | 40 (46.0) | ||
| High | 216 (17.1) | 21 (14.8) | 119 (16.3) | 21 (24.1) | ||
| University | 305 (24.2) | 49 (34.5) | 1721(23.4) | 26 (29.9) | ||
| Missing | 25 (2.0) | 2 (1.4) | 3 (0.4) | 0 (0.0) | ||
| Occupation (%) | 0.92 | 0.23 | ||||
| Gainfully employed | 1075 (85.2) | 124 (87.3) | 662 (90.6) | 73 (83.9) | ||
| Unemployed | 37 (2.9) | 3 (2.1) | 9 (1.2) | 1 (1.1) | ||
| Not gainfully employed | 14 (1.1) | 1 (0.7) | 4 (0.5) | 1 (1.1) | ||
| Retired | 38 (3.0) | 3 (2.1) | 20 (2.7) | 6 (6.9) | ||
| Missing | 97 (7.7) | 11 (7.7) | 36 (4.9) | 6 (6.9) | ||
| Mean income for the neighborhood (SEK) (mean (SD)) | 130,609 (21,727) | 133,386 (20,993) | 0.15 | 129,489 (21,238) | 132,431 (20,219) | 0.22 |
| Outcome/Exposure | Overall Model Hazard Ratios with no Interaction Effects | a Hazard Ratios in Different Exercise Categories | a Interaction Hazard Ratios | |
|---|---|---|---|---|
| Exercise in training clothes | ||||
| IHD | ≤once/week | ≥twice/week | ||
| Low PM2.5 b | 1 | 1 | 1 | |
| High PM2.5 b | 1.13 (0.87–1.45) | 1.21 (0.92–1.58) | 0.62 (0.32–1.19) | 0.51 (0.26–1.02) |
| Stroke | ||||
| Low PM2.5 b | 1 | 1 | 1 | |
| High PM2.5 b | 1.21 (0.81–1.80) | 1.25 (0.81–1.9) | 0.97 (0.37–2.55) | 0.78 (0.28–2.19) |
| Active commuting | ||||
| IHD | ≤two seasons of four | >two seasons of four | ||
| Low PM2.5 b | 1 | 1 | 1 | |
| High PM2.5 b | 1.13 (0.87–1.45) | 1.23 (0.92–1.64) | 0.89 (0.58–1.35) | 0.72 (0.45–1.16) |
| Stroke | ||||
| Low PM2.5 b | 1 | |||
| High PM2.5 b | 1.21 (0.81–1.80) | 1.1 (0.68–1.76) | 1.46 (0.77–2.74) | 1.33 (0.64–2.77) |
| Exercise in Training Clothes | Overall Model Hazard Ratios with no Interaction Effects | a Hazard Ratios in Categories of High and Low Particle Exposure | |
|---|---|---|---|
| IHD | Low PM2.5 b | High PM2.5 b | |
| ≤once/week | 1 | 1 | 1 |
| ≥twice/week | 1.35 (0.92–1.98) | 1.96 (1.23–3.15) | 1.01 (0.59–1.72) |
| Stroke | |||
| ≤once/week | 1 | 1 | 1 |
| ≥twice/week | 1.75 (0.97–3.19) | 2.07 (0.90–4.76) | 1.61 (0.77–3.36) |
| Active commuting per season | Low PM2.5 b | High PM2.5 b | |
| IHD | |||
| ≤two seasons of four | 1 | 1 | 1 |
| >two seasons of four | 0.96 (0.75–1.25) | 1.18 (0.82–1.71) | 0.86 (0.62–1.19) |
| Stroke | |||
| ≤two seasons of four | 1 | 1 | 1 |
| >two seasons of four | 1.11 (0.75–1.65) | 0.93 (0.51–1.70) | 1.23 (0.76–2.01) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raza, W.; Krachler, B.; Forsberg, B.; Sommar, J.N. Does Physical Activity Modify the Association between Air Pollution and Recurrence of Cardiovascular Disease? Int. J. Environ. Res. Public Health 2021, 18, 2631. https://doi.org/10.3390/ijerph18052631
Raza W, Krachler B, Forsberg B, Sommar JN. Does Physical Activity Modify the Association between Air Pollution and Recurrence of Cardiovascular Disease? International Journal of Environmental Research and Public Health. 2021; 18(5):2631. https://doi.org/10.3390/ijerph18052631
Chicago/Turabian StyleRaza, Wasif, Benno Krachler, Bertil Forsberg, and Johan Nilsson Sommar. 2021. "Does Physical Activity Modify the Association between Air Pollution and Recurrence of Cardiovascular Disease?" International Journal of Environmental Research and Public Health 18, no. 5: 2631. https://doi.org/10.3390/ijerph18052631
APA StyleRaza, W., Krachler, B., Forsberg, B., & Sommar, J. N. (2021). Does Physical Activity Modify the Association between Air Pollution and Recurrence of Cardiovascular Disease? International Journal of Environmental Research and Public Health, 18(5), 2631. https://doi.org/10.3390/ijerph18052631






