MAternal Mental Health in the WORKplace (MAMH@WORK): A Protocol for Promoting Perinatal Maternal Mental Health and Wellbeing
Abstract
1. Introduction
1.1. Return to Work after Childbirth
1.2. Aims and Objectives
2. Materials and Methods
2.1. Study Design and Setting
2.2. Sampling Details and Characteristics of the Sample
2.3. Description of the Intervention
2.4. Data Collection and Variables under Study
2.5. Statistical Analyses
2.6. Ethics Approval and Consent to Participate
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Committee on Obstetric Practice. The American College of Obstetricians and Gynecologists Committee Opinion No. 630. Screening for perinatal depression. Obstet. Gynecol. 2015, 125, 1268–1271. [Google Scholar] [CrossRef] [PubMed]
- Salmela-Aro, K.; Nurmi, J.E.; Saisto, T.; Halmesmäki, E. Goal reconstruction and depressive symptoms during the transition to motherhood: Evidence from two cross-lagged longitudinal studies. J. Pers. Soc. Psychol. 2001, 81, 1144–1159. [Google Scholar] [CrossRef]
- Miller, L.J. Postpartum depression. JAMA 2002, 287, 762–765. [Google Scholar] [CrossRef] [PubMed]
- Seyfried, L.S.; Marcus, S.M. Postpartum mood disorders. Int. Rev. Psychiatry 2003, 15, 231–242. [Google Scholar] [CrossRef] [PubMed]
- Skouteris, H. Pregnancy: Physical and Body Image Changes. In Encyclopedia of Body Image and Human Appearance; Cash, T.F., Ed.; Academic Press: Oxford, UK, 2012; Volume 2, pp. 664–668. ISBN 978-0-12-384925-0. [Google Scholar]
- Watson, B.; Fuller-Tyszkiewicz, M.; Broadbent, J.; Skouteris, H. The meaning of body image experiences during the perinatal period: A systematic review of the qualitative literature. Body Image 2015, 14, 102–113. [Google Scholar] [CrossRef] [PubMed]
- Brazelton, T.B.; Cranmer, B.G. The Earliest Relationship; Maddison-Wesley Publishing Company: Reading, MA, USA, 1990. [Google Scholar]
- Stern, D.N. Maternal representations: A clinical and subjective phenomenological view. Infant Ment. Health J. 1991, 12, 174–186. [Google Scholar] [CrossRef]
- Kennerley, H.; Gath, D. Maternity blues. I. Detection and measurement by questionnaire. Br. J. Psychiatry 1989, 155, 356–362. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, M.; Wada, K.; Sakata, Y.; Aratake, Y.; Kato, N.; Ohta, H.; Tanaka, K. Maternity blues as predictor of postpartum depression: A prospective cohort study among Japanese women. J. Psychosom. Obstet. Gynecol. 2008, 29, 206–212. [Google Scholar] [CrossRef]
- Reck, C.; Stehle, E.; Reinig, K.; Mundt, C. Maternity blues as a predictor of DSM-IV depression and anxiety disorders in the first three months postpartum. J. Affect. Disord. 2009, 113, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Wisner, K.L.; Chambers, C.; Sit, D.K.Y. Postpartum depression—A major public health problem. JAMA 2006, 296, 2616–2618. [Google Scholar] [CrossRef] [PubMed]
- O’Hara, M.W.; McCabe, J.E. Postpartum depression: Current status and future directions. Annu. Rev. Clin. Psychol. 2013, 9, 379–407. [Google Scholar] [CrossRef]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: DSM-5, 5th ed.; American Psychiatric Publishing: Washington, DC, USA, 2013. [Google Scholar]
- Breedlove, G.; Fryzelka, D. Depression screening during pregnancy. J. Midwifery Womens Health 2011, 56, 18–25. [Google Scholar] [CrossRef]
- Fuller-Tyszkiewicz, M.; Skouteris, H.; Watson, B.E.; Hill, B. Body dissatisfaction during pregnancy: A systematic review of cross-sectional and prospective correlates. J. Health Psychol. 2013, 18, 1411–1421. [Google Scholar] [CrossRef]
- Paulson, J.F.; Bazemore, S.D. Prenatal and postpartum depression in fathers and its association with maternal depression. JAMA 2010, 303, 1961–1969. [Google Scholar] [CrossRef]
- Letourneau, N.L.; Dennis, C.-L.; Benzies, K.; Duffett-Leger, L.; Stewart, M.; Tryphonopoulos, P.D.; Este, D.; Watson, W. Postpartum depression is a family affair: Addressing the impact on mothers, fathers, and children. Issues Ment. Health Nurs. 2012, 33, 445–457. [Google Scholar] [CrossRef]
- Burke, L. The impact of maternal depression on familial relationships. Int. Rev. Psychiatry 2003, 15, 243–255. [Google Scholar] [CrossRef] [PubMed]
- Feldman, R.; Weller, A.; Zagoory-Sharon, O.; Levine, A. Evidence for a neuroendocrinological foundation of human affiliation: Plasma oxytocin levels across pregnancy and the postpartum period predict mother-infant bonding. Psychol. Sci. 2007, 18, 965–970. [Google Scholar] [CrossRef] [PubMed]
- Galbally, M.; Lewis, A.J.; van Ijzendoorn, M.; Permezel, M. The role of oxytocin in mother-infant relations: A systematic review of human studies. Harv. Rev. Psychiatry 2011, 19, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.C. Anybody’s child: Severe disorders of mother-to- infant bonding. Br. J. Psychiatry 1997, 171, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Taylor, A.; Atkins, R.; Kumar, R.; Adams, D.; Glover, V. A new Mother-to-Infant Bonding Scale: Links with early maternal mood. Arch. Womens Ment. Health 2005, 8, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, K.; Yamashita, H.; Conroy, S.; Marks, M.; Kumar, C. A Japanese version of Mother-to-Infant Bonding Scale: Factor structure, longitudinal changes and links with maternal mood during the early postnatal period in Japanese mothers. Arch. Womens Ment. Health 2012, 15, 343–352. [Google Scholar] [CrossRef]
- Hornstein, C.; Trautmann-Villalba, P.; Hohm, E.; Rave, E.; Wortmann-Fleischer, S.; Schwarz, M. Maternal bond and mother-child interaction in severe postpartum psychiatric disorders: Is there a link? Arch. Womens Ment. Health 2006, 9, 279–284. [Google Scholar] [CrossRef] [PubMed]
- O’Higgins, M.; Roberts, I.S.J.; Glover, V.; Taylor, A. Mother-child bonding at 1 year; Associations with symptoms of postnatal depression and bonding in the first few weeks. Arch. Womens Ment. Health 2013, 16, 381–389. [Google Scholar] [CrossRef]
- Righetti-Veltema, M.; Conne-Perréard, E.; Bousquet, A.; Manzano, J. Postpartum depression and mother-infant relationship at 3 months old. J. Affect. Disord. 2002, 70, 291–306. [Google Scholar] [CrossRef]
- Benoit, D. Infant-parent attachment: Definition, types, antecedents, measurement and outcome. Paediatr. Child Health 2004, 9, 541–545. [Google Scholar] [CrossRef] [PubMed]
- Dias, C.C.; Figueiredo, B. Breastfeeding and depression: A systematic review of the literature. J. Affect. Disord. 2015, 171, 142–154. [Google Scholar] [CrossRef] [PubMed]
- Gress-Smith, J.L.; Luecken, L.J.; Lemery-Chalfant, K.; Howe, R. Postpartum depression prevalence and impact on infant health, weight, and sleep in low-income and ethnic minority women and infants. Matern. Child Health J. 2012, 16, 887–893. [Google Scholar] [CrossRef]
- Netsi, E.; Pearson, R.M.; Murray, L.; Cooper, P.; Craske, M.G.; Stein, A. Association of persistent and severe postnatal depression with child outcomes. JAMA Psychiatry 2018, 75, 247–253. [Google Scholar] [CrossRef]
- Brennan, P.A.; Hammen, C.; Andersen, M.J.; Bor, W.; Najman, J.M.; Williams, G.M. Chronicity, severity, and timing of maternal depressive symptoms: Relationships with child outcomes at age 5. Dev. Psychol. 2000, 36, 759–766. [Google Scholar] [CrossRef] [PubMed]
- Stein, A.; Pearson, R.M.; Goodman, S.H.; Rapa, E.; Rahman, A.; McCallum, M.; Howard, L.M.; Pariante, C.M. Effects of perinatal mental disorders on the fetus and child. Lancet 2014, 384, 1800–1819. [Google Scholar] [CrossRef]
- Sciberras, E.; Ukoumunne, O.C.; Efron, D. Predictors of parent-reported attention-deficit / hyperactivity disorder in children aged 6–7 years: A national longitudinal study. J. Abnorm. Child Psychol. 2011, 39, 1025–1034. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Cho, S.-C.; Kim, J.-W.; Shin, M.-S.; Yoo, H.-J.; Min, S.; Hyun, D.H.; Cheong, J.H.; Kim, B.-N. Differential perinatal risk factors in children with attention-deficit/ hyperactivity disorder by subtype. Psychiatry Res. 2014, 219, 609–616. [Google Scholar] [CrossRef] [PubMed]
- Kingston, D.; Kehler, H.; Austin, M.; Mughal, M.K.; Wajid, A.; Vermeyden, L.; Benzies, K.; Brown, S.; Stuart, S.; Giallo, R. Trajectories of maternal depressive symptoms during pregnancy and the first 12 months postpartum and child externalizing and internalizing behavior at three years. PLoS ONE 2018, 13, e0195365. [Google Scholar] [CrossRef] [PubMed]
- Pearson, R.M.; Bornstein, M.H.; Cordero, M.; Scerif, G.; Mahedy, L.; Evans, J.; Abioye, A.; Stein, A. Maternal perinatal mental health and offspring academic achievement at age 16: The mediating role of childhood executive function. J. Child Psychol. Psychiatry 2016, 57, 491–501. [Google Scholar] [CrossRef] [PubMed]
- Letourneau, N.L.; Tramonte, L.; Willms, J.D. Maternal depression, family functioning and children’s longitudinal development. J. Pediatr. Nurs. 2013, 28, 223–234. [Google Scholar] [CrossRef] [PubMed]
- Dennis, C.L.; Dowswell, T. Psychosocial and psychological interventions for preventing postpartum depression (Review). Cochrane Database Syst. Rev. 2013, 1–206. [Google Scholar] [CrossRef]
- European Network for Workplace Health Promotion. Luxembourg Declaration on Workplace Health Promotion in the European Union; ENWHP: Perugia, Italy, 2018. [Google Scholar]
- Popli, S.; Rizvi, I.A. Drivers of employee engagement: The role of leadership style. Glob. Bus. Rev. 2016, 17, 965–979. [Google Scholar] [CrossRef]
- Walton, M. In consideration of a toxic workplace: A suitable place for treatment. In Employee Well-Being Support a Workplace Resource; Kinder, A., Hughes, R., Cooper, C.L., Eds.; John Wiley & Sons, Ltd.: Chichester, UK, 2008; pp. 9–24. [Google Scholar]
- Jurviste, U.; Prpic, M.; Sabbati, G. Maternity and Paternity Leave in the EU; European Parliament: Brussels, Belgium, 2019.
- Jahoda, M. Employment and Unemployment: A Social-Psychology Analysis; Cambridge University Press: Cambridge, UK, 1982. [Google Scholar]
- Nichols, M.R.; Roux, G.M. Maternal perspectives on postpartum return to the workplace. J. Obstet. Gynecol. Neonatal Nurs. 2004, 33, 463–471. [Google Scholar] [CrossRef] [PubMed]
- Greenhaus, J.H.; Beutell, N.J. Sources of conflict between work and family roles. Acad. Manag. Rev. 1989, 10, 76–88. [Google Scholar] [CrossRef]
- PORDATA. Idade Média da Mãe ao Nascimento do Primeiro Filho. Available online: https://www.pordata.pt/Portugal/Idade+média+da+mãe+ao+nascimento+do+primeiro+filho-805 (accessed on 18 March 2020).
- Gilbreath, B.; Benson, P.G. The contribution of supervisor behaviour to employee psychological well-being. Work Stress 2004, 18, 255–266. [Google Scholar] [CrossRef]
- Skakon, J.; Nielsen, K.; Borg, V.; Guzman, J. Are leaders’ well-being, behaviours and style associated with the affective well-being of their employees? A systematic review of three decades of research. Work Stress 2010, 24, 107–139. [Google Scholar] [CrossRef]
- European Union. Directive of the European Parliament and of the Council on Work-Life Balance for Parents and Carers and Repealing; Coucil Directive 2010/18/EU; European Parliament: Brussels, Belgium, 2019.
- Werner, E.; Miller, M.; Osborne, L.M.; Kuzava, S.; Monk, C. Preventing postpartum depression: Review and recommendations. Arch. Womens Ment. Health 2015, 18, 41–60. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, E.; Senger, C.A.; Henninger, M.L.; Coppola, E.; Gaynes, B.N. Interventions to prevent perinatal depression—Evidence report and systematic review for the US Preventive Services Task Force. JAMA 2019, 321, 588–601. [Google Scholar] [CrossRef] [PubMed]
- Sangsawang, B.; Wacharasin, C.; Sangsawang, N. Interventions for the prevention of postpartum depression in adolescent mothers: A systematic review. Arch. Womens Ment. Health 2019, 22, 215–228. [Google Scholar] [CrossRef] [PubMed]
- De Witte, N.A.J.; Buyck, I.; Van Daele, T. Combining biofeedback with stress management interventions: A systematic review of physiological and psychological effects. Appl. Psychophysiol. Biofeedback 2019, 44, 71–82. [Google Scholar] [CrossRef] [PubMed]
- Association for Applied Psychophysiology and Biofeedback. About Biofeedback—AAPB. Available online: https://www.aapb.org/i4a/pages/index.cfm?pageid=3463 (accessed on 24 September 2019).
- Beckham, A.J.; Greene, T.B.; Meltzer-Brody, S. A pilot study of heart rate variability biofeedback therapy in the treatment of perinatal depression on a specialized perinatal psychiatry inpatient unit. Arch. Womens Ment. Health 2013, 16, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Kudo, N.; Shinohara, H.; Kodama, H. Heart rate variability biofeedback intervention for reduction of psychological stress during the early postpartum period. Appl. Psychophysiol. Biofeedback 2014, 39, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Dhillon, A.; Sparkes, E.; Duarte, R.V. Mindfulness-based interventions during pregnancy: A systematic review and meta-analysis. Mindfulness 2017, 8, 1421–1437. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; MacBeth, A. The effectiveness of mindfulness-based interventions on maternal perinatal mental health outcomes: A systematic review. Mindfulness 2017, 8, 823–847. [Google Scholar] [CrossRef] [PubMed]
- Kabat-Zinn, J. Mindfulness-based interventions in context: Past, present, and future. Clin. Psychol. Sci. Pract. 2003, 10, 144–156. [Google Scholar] [CrossRef]
- Howarth, A.; Smith, J.G.; Perkins-Porras, L.; Ussher, M. Effects of brief mindfulness-based interventions on health-related outcomes: A systematic review. Mindfulness 2019, 10, 1957–1968. [Google Scholar] [CrossRef]
- Hofmann, S.G.; Sawyer, A.T.; Witt, A.A.; Oh, D. The effect of mindfulness-based therapy on anxiety and depression: A meta-analytic review. J. Consult. Clin. Psychol. 2010, 78, 169–183. [Google Scholar] [CrossRef] [PubMed]
- Pan, W.-L.; Chang, C.-W.; Chen, S.-M.; Gau, M.-L. Assessing the effectiveness of mindfulness-based programs on mental health during pregnancy and early motherhood—A randomized control trial. BMC Pregnancy Childbirth 2019, 19, 346. [Google Scholar] [CrossRef]
- Strazdins, L.; Shipley, M.; Broom, D.H. What does family-friendly really mean? Wellbeing, time, and the quality of parents’ jobs. Aust. Bull. Labour 2007, 33, 202–225. [Google Scholar]
- Leigh, B.; Milgrom, J. Risk factors for antenatal depression, postnatal depression and parenting stress. BMC Psychiatry 2008, 8, 24. [Google Scholar] [CrossRef] [PubMed]
- O’Hara, M.W.; Swain, A.M. Rates and risk of postpartum depression—A meta-analysis. Int. Rev. Psychiatry 1996, 8, 37–54. [Google Scholar] [CrossRef]
- Pocock, S.J.; Simon, R. Sequential treatment assignment with balancing for prognostic factors in the controlled clinical trial. Biometrics 1975, 31, 103–115. [Google Scholar] [CrossRef] [PubMed]
- Glasgow, R.E.; Klesges, L.M.; Dzewaltowski, D.A.; Estabrooks, P.A.; Vogt, T.M. Evaluating the impact of health promotion programs: Using the RE-AIM framework to form summary measures for decision making involving complex issues. Health Educ. Res. 2006, 21, 688–694. [Google Scholar] [CrossRef]
- Robiner, W.N. Enhancing adherence in clinical research. Contemp. Clin. Trials 2005, 26, 59–77. [Google Scholar] [CrossRef]
- IBM Corporation. IBM SPSS Statistics for Windows, Version 26.0; IBM: Armonk, NY, USA, 2019. [Google Scholar]
- R Core Development Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2014. [Google Scholar]
- O’Connor, M.; Casey, L. The Mental Health Literacy Scale (MHLS): A new scale-based measure of mental health literacy. Psychiatry Res. 2015, 229, 511–516. [Google Scholar] [CrossRef] [PubMed]
- Rocha, I. Adaptação e Validação da Escala Mental Health Literacy para a População Portuguesa [Adaptation and Validation of the Mental Health Literacy Scale for the Portuguese Population]; Instituto Piaget: Almada Portugal, 2016. [Google Scholar]
- Zigmond, A.; Snalth, R. The Hospital Anxiety and Depression Scale. Acta Psychiatr. Scand. 1983, 67, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Pais-Ribeiro, J.; Silva, I.; Ferreira, T.; Martins, A.; Meneses, R.; Baltar, M. Validation study of a Portuguese version of the Hospital Anxiety and Depression Scale. Psychol. Health Med. 2007, 12, 225–237. [Google Scholar] [CrossRef] [PubMed]
- Cox, J.L.; Holden, J.M.; Sagovsky, R. Detection of postnatal depression: Development of the 10-item Edinburgh Postnatal Depression Scale. Br. J. Psychiatry 1987, 150, 782–786. [Google Scholar] [CrossRef] [PubMed]
- Areias, M.E.G.; Kumar, R.; Barros, H.; Figueiredo, E. Comparative incidence of depression in women and men, during pregnancy and after childbirth: Validation of the Edinburgh Postnatal Depression Scale in Portuguese mothers. Br. J. Psychiatry 1996, 169, 30–35. [Google Scholar] [CrossRef]
- Connor, K.M.; Davidson, J.R.T. Development of a new resilience scale: The Connor-Davidson Resilience scale (CD-RISC). Depress. Anxiety 2003, 18, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Anjos, J.F.; Heitor Dos Santos, M.; Ribeiro, M.T.; Moreira, S. Connor-Davidson Resilience Scale: Validation study in a Portuguese sample. BMJ Open 2019, 9, e026836. [Google Scholar] [CrossRef]
- Heitor, M.J. Promoção da Saúde Mental no Trabalho: Estudo Observacional de Determinantes Biopsicossociais e Presentismo; Universidade de Lisboa: Lisbon, Portugal, 2019. [Google Scholar]
- Carver, C.S. You want to measure coping but your protocol’s too long: Consider the brief COPE. Int. J. Behav. Med. 1997, 4, 92–100. [Google Scholar] [CrossRef]
- Lazarus, R.S.; Folkman, S. Stress, Appraisal, and Coping.; Springer: New York, NY, USA, 1984. [Google Scholar]
- Veenhoven, R. Measures of Happiness, World Database of Happiness. Available online: http://worlddatabaseofhappiness.eur.nl/hap_quer/hqi_fp.htm (accessed on 12 January 2021).
- Logsdon, M.C.; Usui, W.; Birkimer, J.C.; McBride, A.B. The Postpartum Support Questionnaire: Reliability and validity. J. Nurs. Meas. 1996, 4, 129–142. [Google Scholar] [CrossRef] [PubMed]
- Narciso, I.; Costa, M.E. Amores satisfeitos, mas não perfeitos. Cad. Consult. Psicológica 1996, 12, 115–130. [Google Scholar]
- Ainsworth, M.D.S.; Blehar, M.C.; Waters, E.; Wall, S. Patterns of Attachment: A Psychological Study of the Strange Situation; Lawrence Erlbaum: Hillsdale, NJ, USA, 1978. [Google Scholar]
- Brockington, I.F.; Fraser, C.; Wilson, D. The Postpartum Bonding Questionnaire: A validation. Arch. Womens Ment. Health 2006, 9, 233–242. [Google Scholar] [CrossRef]
- Nazaré, B.; Fonseca, A.; Canavarro, M.C. Avaliação da ligação parental ao bebé após o nascimento: Análise fatorial confirmatória da versão portuguesa do Postpartum Bonding Questionnaire (PBQ). Laboratório Psicol. 2012, 10, 47–61. [Google Scholar] [CrossRef][Green Version]
- Chagas, C.S.; Maltez, P.M.L.; Miranda, S.I.S.; Justo, J.M.R.M. The “Questionnaire on the Difference Imaginary Baby Vs. Real Baby”: A new instrument for the evaluation of differences between perinatal and postnatal maternal perceptions after delivery. Int. J. Dev. Educ. Psychol. 2015, 1, 29–38. [Google Scholar] [CrossRef]
- Bellman, M.; Lingam, S.; Aukett, A. Schedule of Growing Skills II: Escala de Avaliação das Competências no Desenvolvimento Infantil II—Dos 0 aos 5 Anos—Manual do Utilizador, 3rd ed.; CEGOC-TEA: Lisboa, Portugal, 2012. [Google Scholar]
- Varajidás, C.A.; Machado, M.; Mota, M.P.; Martins, R.; Lisboa, M.C.; Soares, I.; Sousa, S.; Leitão, J.C. Psychometric properties of the Schedule of Growing Skills II: Portuguese version. Psychologica 2017, 60, 7–18. [Google Scholar] [CrossRef]
- Stifter, C.A.; Braungart, J.M. The Regulation of negative reactivity in infancy: Function and development. Dev. Psychol. 1995, 31, 448–455. [Google Scholar] [CrossRef]
- Crittenden, P.M. CARE-Index Manual; Family Relations Institute: Miami, FL, USA, 2003; unpublished manuscript. [Google Scholar]
- Schaufeli, W.; Bakker, A. UWES—Utrecht Work Engagement Scale; Occupational Health Psychology Unit: Utrecht, The Netherlands, 2003. [Google Scholar]
- Teles, H.; Ramalho, N.; Ramalho, V.; Ribeiro, S. Adaptação e Validação da Utrecht Work Engagement Scale (UWES) aplicada a Assistentes Sociais em Portugal. Rev. Port. Investig. Comport. Soc. 2017, 3, 10–20. [Google Scholar] [CrossRef]
- Hill, E.J.; Hawkins, A.J.; Ferris, M.; Weitzman, M. Finding an extra day a week: The positive influence of perceived job flexibility on work and family life balance. Fam. Relat. 2001, 50, 49–58. [Google Scholar] [CrossRef]
- Kessler, R.C.; Barber, C.; Beck, A.L.; Berglund, P.A.; Cleary, P.D.; McKenas, D.; Pronk, N.P.; Simon, G.E.; Stang, P.E.; Üstün, T.B.; et al. The World Health Organization Health and Work Performance Questionnaire (HPQ). J. Occup. Environ. Med. 2003, 45, 156–174. [Google Scholar] [CrossRef]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences; Academic Press: New York, NY, USA, 1977; ISBN 978-0-12-179060-8. [Google Scholar]
- World Medical Association. World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA 2013, 310, 2191–2194. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, A.; Pereira, M.; Araújo-Pedrosa, A.; Gorayeb, R.; Ramos, M.M.; Canavarro, M.C. Be a Mom: Formative evaluation of a web-based psychological intervention to prevent postpartum depression. Cogn. Behav. Pract. 2018, 25, 473–495. [Google Scholar] [CrossRef]
- Marvin, R.; Cooper, G.; Hoffman, K.; Powell, B. The Circle of Security project: Attachment-based intervention with caregiver-pre-school child dyads. Attach. Hum. Dev. 2002, 4, 107–124. [Google Scholar] [CrossRef] [PubMed]
- Comissão Técnica de Acompanhamento da Reforma da Saúde Mental. Relatório da Avaliação do Plano Nacional de Saúde Mental 2007–2016 e Propostas Prioritárias para a Extensão a 2020; SNS: Lisboa, Portugal, 2017. [Google Scholar]
- Nogueira, J.R.; Moreira, S. Programa Nacional de Saúde Ocupacional (PNSOC)—Extensão 2018/2020; Direção-Geral da Saúde: Lisbon, Portugal, 2018; p. 43. [Google Scholar]
- European Commission. Europe 2020—A strategy for Smart, Sustainable and Inclusive Growth; European Commission: Brussels, Belgium, 2010; p. 37. [Google Scholar]
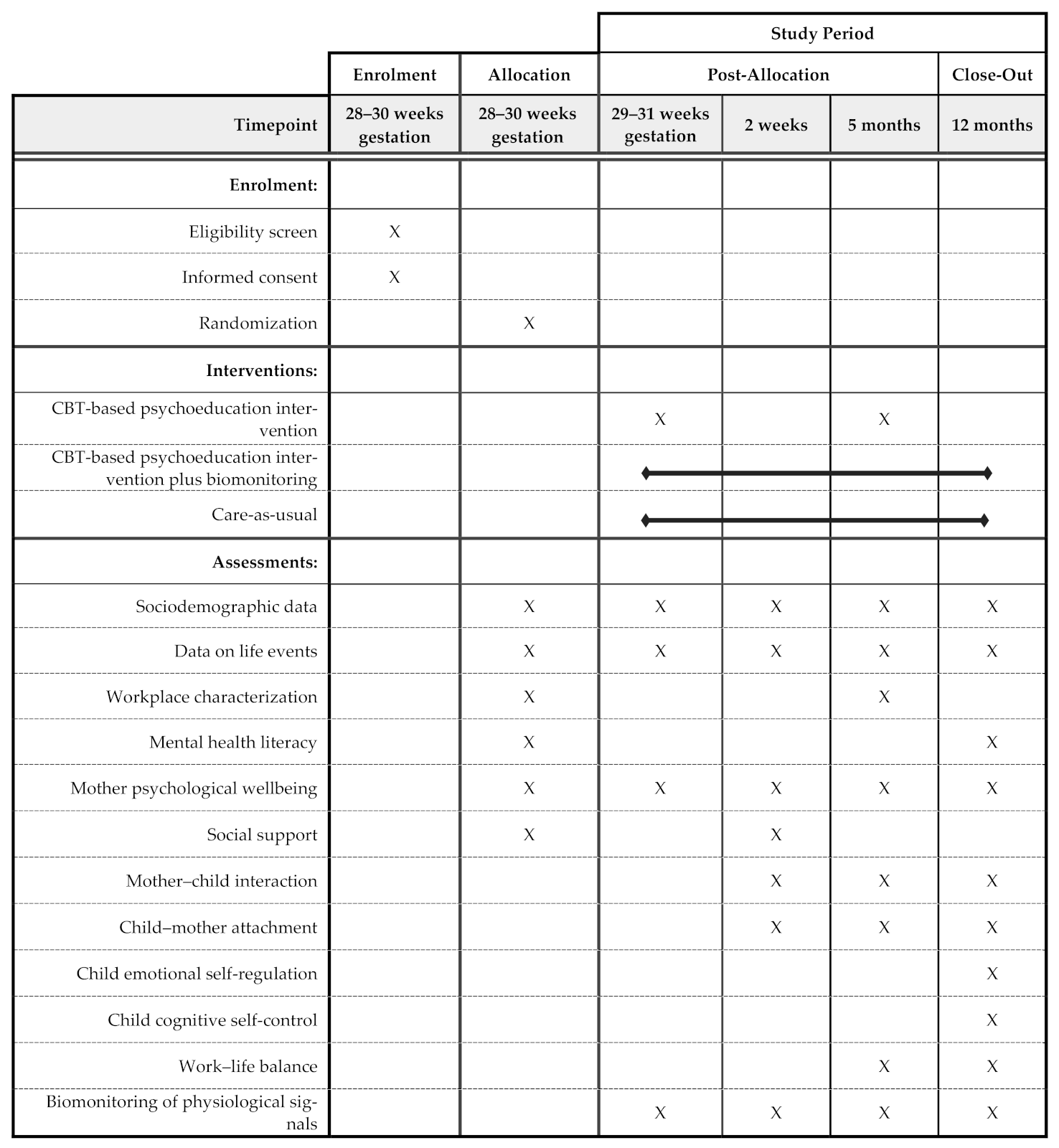
| Questionnaire/Measure Scale | Description | Timing of Assessment |
|---|---|---|
| Mental Health Literacy | ||
|
| Pregnancy Postpartum |
| Psychological Wellbeing, Resilience and Stress-related Coping Style | ||
|
| Pregnancy |
|
| Postpartum |
|
| Postpartum |
|
| Pregnancy Postpartum |
|
| Pregnancy Postpartum |
|
| Pregnancy Postpartum |
| Social Support and Marital Satisfaction | ||
|
| Postpartum |
|
| Pregnancy Postpartum |
| Child-mother Attachment, Child Emotional Self-regulation and Cognitive Self-control | ||
|
| Postpartum |
|
| Postpartum |
|
| Postpartum |
|
| Postpartum |
|
| Postpartum |
|
| Postpartum |
|
| Postpartum |
| Work–life Balance | ||
|
| Postpartum |
|
| Postpartum |
|
| Postpartum |
|
| Postpartum |
| Biomonitoring of Physiological Signals | ||
|
| Pregnancy Postpartum |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Costa, J.; Santos, O.; Virgolino, A.; Pereira, M.E.; Stefanovska-Petkovska, M.; Silva, H.; Navarro-Costa, P.; Barbosa, M.; das Neves, R.C.; Duarte e Silva, I.; et al. MAternal Mental Health in the WORKplace (MAMH@WORK): A Protocol for Promoting Perinatal Maternal Mental Health and Wellbeing. Int. J. Environ. Res. Public Health 2021, 18, 2558. https://doi.org/10.3390/ijerph18052558
Costa J, Santos O, Virgolino A, Pereira ME, Stefanovska-Petkovska M, Silva H, Navarro-Costa P, Barbosa M, das Neves RC, Duarte e Silva I, et al. MAternal Mental Health in the WORKplace (MAMH@WORK): A Protocol for Promoting Perinatal Maternal Mental Health and Wellbeing. International Journal of Environmental Research and Public Health. 2021; 18(5):2558. https://doi.org/10.3390/ijerph18052558
Chicago/Turabian StyleCosta, Joana, Osvaldo Santos, Ana Virgolino, M. Emília Pereira, Miodraga Stefanovska-Petkovska, Henrique Silva, Paulo Navarro-Costa, Miguel Barbosa, Rui César das Neves, Inês Duarte e Silva, and et al. 2021. "MAternal Mental Health in the WORKplace (MAMH@WORK): A Protocol for Promoting Perinatal Maternal Mental Health and Wellbeing" International Journal of Environmental Research and Public Health 18, no. 5: 2558. https://doi.org/10.3390/ijerph18052558
APA StyleCosta, J., Santos, O., Virgolino, A., Pereira, M. E., Stefanovska-Petkovska, M., Silva, H., Navarro-Costa, P., Barbosa, M., das Neves, R. C., Duarte e Silva, I., Alarcão, V., Vargas, R., & Heitor, M. J. (2021). MAternal Mental Health in the WORKplace (MAMH@WORK): A Protocol for Promoting Perinatal Maternal Mental Health and Wellbeing. International Journal of Environmental Research and Public Health, 18(5), 2558. https://doi.org/10.3390/ijerph18052558








